Abstract
Background
Preoperative high blood glucose levels are closely associated with poor performance and high mortality in cancer patients. This study was designed to investigate the relationship between preoperative fasting hyperglycemia and the prognosis of patients with gallbladder cancer (GBC) after undergoing GBC radical surgery.
Patients and methods
A retrospective analysis of 83 eligible patients who underwent GBC radical surgery between 2007 and 2016 was performed. Factors affecting overall survival (OS) and recurrence-free survival (RFS) were analyzed by univariate and multivariate analyses.
Results
Of the 83 patients, 35 (42.2%) had preoperative fasting hyperglycemia. The median OS of the enrolled patients was 12 months. The median OS in patients with fasting hyperglycemia before surgery was 18 months, which was shorter than for patients with normal fasting blood glucose levels before surgery (47 months, P<0.001). Preoperative fasting hyperglycemia was associated with shorter survival times in univariate analyses (HR, 3.215; 95% CI, 1.846–5.601; P<0.001). Multivariate analysis showed that patients with preoperative fasting hyperglycemia had a lower OS (HR, 2.832; 95% CI, 1.480–5.418; P=0.002) and RFS (HR, 2.051; 95% CI, 1.127–3.733; P=0.019) than patients with normal preoperative fasting blood glucose levels.
Conclusion
Preoperative fasting hyperglycemia is an independent indicator of poor prognosis in GBC patients after GBC radical surgery.
Introduction
Gallbladder cancer (GBC) is the most common malignant tumor of the biliary tract, and it accounts for 80%–95% of biliary cancer cases.Citation1 GBC is the fifth most common malignancy of the gastrointestinal tract, and it accounts for ~1% of all cancers in China.Citation2 The prognosis of GBC is still poor because of atypical symptoms, late diagnosis, single treatment choices, and lack of effective diagnostic markers.Citation3 Currently, the prognosis of GBC depends mainly on the pathologic detection of cancer tissue and distinguishing the stage, classification, and pathologic type of cancer to judge the prognosis of the patients. Epidemiologic studies have reported that the 3- and 5-year survival rates for patients with this disease are 30% and 5%.Citation4 Therefore, given the poor prognosis for GBC in general, efforts to identify other factors that influence recurrence and patient survival are needed for this disease.
The relationship between diabetes and cancers has always been studied. A pooled analysis of a prospective cohort based on Asian populations shows that diabetes is closely associated with increased risk of multiple digestive tract tumors including esophageal cancer, gastric cancer, colon cancer, rectal cancer, liver cancer, cholangiocarcinoma, and pancreatic cancer.Citation5 Another population-based case–control study in Shanghai, China, shows that diabetes is a risk factor for gallbladder cancer independent of body mass index (BMI), and its effect is mediated in part by biliary stones and serum high-density lipoprotein levels.Citation6 The possible biologic links between diabetes and cancer are reported to be hyperinsulinemia, inflammation, and hyperglycemia.Citation7
Positron emission tomography imaging using 18F-fluoro-deoxyglucose has shown that tumor cells have higher blood glucose absorption than normal cells,Citation8 and the plasma glucose levels in cancer patients may be an important prognostic indicator.Citation9 Park et al found that stomach and lung cancer patients with a fasting serum glucose level above 126 mg/dL had higher mortality rates.Citation10 Others have shown that the median survival period is significantly shorter for gastric adenocarcinoma patients who are GLUT-1-positive than for those who are GLUT-1-negative.Citation11 The biologic cause of these clinical outcomes may be that glycolysis, as a biochemical feature of cancer, is primarily mediated by GLUT-1. GLUT-1 is a transmembrane transport protein that facilitates glucose transport into cells, and its expression is increased in epithelium-derived tumors, such as head and neck cancers and gastric and colorectal carcinomas.Citation12,Citation13 Regardless of whether GLUT-1 immunoreactivity was detected in GBC cases, patients with strong GLUT-1 expression had significantly shorter survival times after surgery than patients with negative or weak expression.Citation14
Therefore, considering these results, we collected preoperative fasting blood glucose levels in patients who underwent gallbladder cancer surgery and their follow-up survival times. We hypothesized that preoperative fasting blood glucose levels are related to the prognosis of GBC and that higher preoperative blood glucose levels may have greater effects. Analyzing the prognosis of patients with GBC can improve the survival rate of patients by targeted improvements to postoperative patient care.
Patients and methods
This study was a retrospective study conducted in accordance with the Helsinki Declaration and approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University. All data were anonymized; therefore, patient consent was waived by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University. A total of 105 people were included in the study, and those without preoperative fasting blood glucose data, with evidence of other infectious diseases or who were lost to follow-up were excluded. Clinical data within 30 days before surgery for 83 of the patients were included. All clinical information and laboratory parameters were collected from the patients’ hospital medical records. All patients underwent radical operation for GBC during 2007–2016 at the Second Affiliated Hospital, Wenzhou Medical University. All final diagnoses were confirmed by pathologic examination. It is recommended that postoperative GBC patients receive corresponding adjuvant treatment according to their GBC stage and performance status. Abdominal computed tomography and blood indexes should be reviewed every 3 months for the first year after operation. Once each year, the patients were contacted by phone, and their recent physical condition was obtained. The date of follow-up was from the date of surgery to the date of death or last contact, or the end of September 2018. Overall survival (OS) was defined as the date from the surgery to the date of death or the last follow-up. Recurrence-free survival (RFS) was calculated from the date of surgery until the first relapse or death for any reason, or to the date of last follow-up.
Clinical information
The following medical data were retrospectively collected from the patients in the hospital: gender, age, BMI, hemoglobin, serum albumin, neutrophil-to-lymphocyte ratio (NLR), serum CA 19-9, serum carcinoembryonic antigen (CEA), pathologic data (tumor differentiation, tumor size, tumor lymph node metastasis), and TNM staging (The United States Joint Committee on Cancer [7th edition], staging of gallbladder cancer). Serum CA 19-9 and CEA levels were detected by electrochemiluminescence immunoassays (Cobas; Roche Diagnostics, Germany) performed at the Clinical Laboratory Department, Second Affiliated Hospital of Wenzhou Medical University, China. The normal reference values according to a previous study are as follows: CEA ≤5 µg/L, CA 19-9 ≤37 U/mL, NLR <2.6.Citation15 The definition of radical resection is that the primary tumor is removed together with the metastatic lymph nodes and the affected tissue. (For the T1a GBC, we performed a simple cholecystectomy using a laparotomy or laparoscopic surgery. For the T1b GBC, we used an extended cholecystectomy. For patients with GBC in stage T2 or above, we usually perform an extended cholecystectomy. In the extended cholecystectomy, the gallbladder bed wedge resection or IVb/V liver resection can be performed according to the intraoperative condition. The scope of lymph node dissection should include the cystic duct lymph node, the common bile duct lymph node, the lymph nodes around the hepatoduodenal ligament [the hepatic artery and portal vein lymph nodes], and the posterior superior pancreatico-duodenal lymph node.) The tumor pathology shows that there is no tumor tissue at the margin.
Plasma glucose
Fasting plasma glucose (fasting for >8 hours) was determined from 5 to 6 am on any day before surgery as part of the preoperative laboratory tests. Normal fasting blood glucose levels range from 70 to 99 mg/dL (American Diabetes Association). Levels starting at 100 mg/dL indicate high blood glucose levels on an empty stomach, so fasting hyperglycemia is defined according to this threshold value. Accordingly, the patients were assigned to the fasting hyperglycemia group and the normal fasting blood glucose group. Among the fasting hyperglycemia group, only two patients were diagnosed with diabetes before enrolment and took insulin daily to control blood glucose.
Statistical method
For categorical variables, significant differences were assessed using the chi-squared test or Fisher’s exact test. For continuous data, the mean difference was compared using the Mann–Whitney test or independent-sample t-test. Univariate and multivariate Cox proportional hazard models were used to test the association between variables and OS. OS was calculated as the time from the date of surgery to the date of death from any cause or the last follow-up. Survival curves were analyzed by the Kaplan–Meier method and compared using the log-rank test. A P-value <0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 22 (SPSS, Lnc., Chicago, IL, USA).
Results
shows the clinical and pathologic features of 83 patients. Of the 83 patients, 35 had preoperative fasting hyperglycemia (42.2%), most were female (60, 72.2%) and the average age was 66.1 years (SD =10.4). Gender (P=0.02), low hemoglobin (P=0.006), high NLR (P<0.001), and high CEA (P=0.002) were significantly different between the hyperglycemic patients and the normal blood glucose patients, which may affect patient preoperative glucose levels. Regarding tumor characteristics, advanced infiltration depth T (P=0.001), large tumor size (P=0.002), advanced TNM stage (P=0.002), and lymph node metastasis (P<0.001) had great influences in high fasting blood glucose levels. Other patient-related factors, such as age, serum albumin, BMI, serum CA 19-9 levels, the presence of stones, and tumor differentiation, were not associated with preoperative high blood glucose levels.
Table 1 Clinical and pathologic characteristics of the 83 study patients
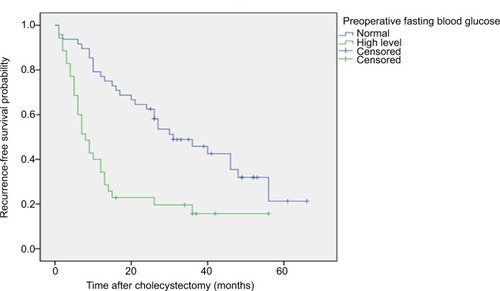
Of the 83 patients, 53 died, 4 were lost to follow-up, and 26 survived. For the OS (P<0.001) () and RFS (P<0.001) (), the prognosis was significantly worse in patients with high fasting blood glucose levels than in patients with normal fasting blood glucose levels. Patients in the normal blood glucose level group had a median OS time of 47 months (95% CI, 31.69–62.30), and those in the preoperative fasting hyperglycemia group had a median OS of 18 months (95% CI, 15.183–20.817). The recurrence rate during follow-up in this study was 71.1% (59 patients), and the median disease-free survival in our series was 17 months. The median RFS was significantly shorter in the hyperglycemic group than in the normal blood glucose group (8 months and 31 months, respectively; P<0.001).
Figure 1 The overall survival rate of GBC patients after GBC radical surgery.
Notes: The Kaplan–Meier curve showed significant differences in the probability of total survival after GBC radical surgery in patients with preoperative fasting hyperglycemia and preoperative fasting normal blood glucose levels. P<0.001 (log-rank test).
Abbreviation: GC, gallbladder carcinoma.
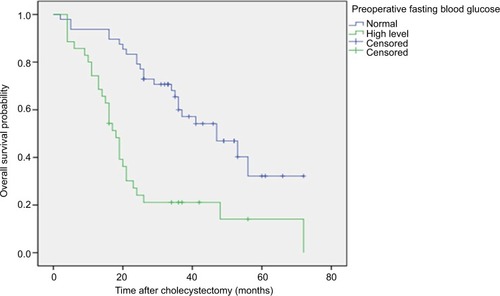
Figure 2 RFS of patients with GBC after GBC radical surgery.
Notes: Kaplan–Meier curves show significant differences in RFS probability after GBC radical surgery in patients with preoperative fasting hyperglycemia and preoperative fasting normal blood glucose levels. P<0.001 (log-rank test).
Abbreviations: GC, gallbladder carcinoma; RFS, recurrence-free survival.
shows the OS-related variables after GBC radical surgery according to univariate and multivariate Cox proportional hazard models. In the univariate analysis, preoperative fasting hyperglycemia, invasive depth III + IV, TNM III + IV, lymph node metastasis, poor differentiation, serum albumin levels, NLR ≥2.6, and CA 19-9 >37 U/mL were correlated with poor OS. Multivariate analysis identified that serum albumin (HR, 0.942; 95% CI, 0.895–0.992; P=0.022) and poor differentiation (HR, 0.233; 95% CI, 0.107–0.508; P<0.001) positively affected OS. However, TNM stage III + IV (HR, 8.024; 95% CI, 2.635–24.439; P<0.001) and hyperglycemia (HR, 2.832; 95% CI, 1.480–5.418; P=0.002) negatively impacted OS. shows a univariate and multivariate Cox proportional hazard regression model for RFS. According to the univariate analysis, significant prognostic factors for RFS were preoperative fasting hyperglycemia, invasive depth III + IV, TNM III + IV, lymph node metastasis, poor differentiation, decreased serum albumin levels, NLR <2.6, and CA 19-9 >37 U/mL. Multivariate analysis identified two adverse prognostic factors, high blood glucose levels (HR, 2.051; 95% CI, 1.127–3.733; P=0.019) and TNM III + IV (HR, 3.140; 95% CI, 1.444–6.828; P=0.004), which affect RFS. Poor differentiation positively affected RFS (HR, 0.277; 95% CI, 0.137–0.560; P<0.001).
Table 2 Univariate and multivariate analyses of prognostic factors associated with overall survival
Table 3 Univariate and multivariate analyses of prognostic factors associated with RFS
Discussion
GBC is a rare disease that is the most common invasive tumor in the biliary system.Citation1,Citation16 However, GBC progresses quickly and has few early symptoms.Citation17 Therefore, early detection and early surgical treatmentCitation16 are advocated, but the prognosis for GBC remains poor. It is, thus, necessary to find new biomarkers that could be used for GBC prognosis. We found that preoperative fasting hyperglycemia is a poor predictor of OS and RFS in GBC patients after GBC radical surgery.
In our study, women with preoperative fasting hyperglycemia had a higher prevalence than those with normal blood glucose levels. However, according to univariate and multivariate analyses, gender is not an important predictor of OS difference. There was no correlation between preoperative fasting hyperglycemia levels and age, BMI, serum albumin levels, serum CA 19-9 levels, gallstones, or histopathologic differentiation. Although previous studies have shown that high preoperative fasting blood glucose levels are associated with lower serum albumin levels,Citation18 we did not find a significant correlation between these parameters in our study. Some studies have also shown that the preoperative BMI level is associated with hyperglycemia in non-small-cell lung cancer patients.Citation19 However, our study and Cai et al’s study confirmed that preoperative fasting hyperglycemia was not associated with BMI.Citation18 However, our sample size is small, and the association between high preoperative fasting blood glucose levels and BMI should be further clarified with a larger sample size.
Gallstones are the main cause of GBC,Citation20,Citation21 but we did not find an association between gallbladder stones and overall OS or RFS in GBC patients undergoing GBC radical surgery. Preoperative fasting hyperglycemia was a poor predictor of overall OS and RFS in GBC patients who underwent GBC radical surgery. Compared to patients with normal fasting blood glucose levels before surgery, patients with preoperative fasting hyperglycemia had poorer GBC differentiation (P=0.005), higher possibilities to present lymph node metastasis (P<0.001), more advanced TNM staging (P=0.002), lower hemoglobin levels (P=0.006), higher NLR (≥2.6; P<0.001), higher serum CEA levels (P=0.002), greater tissue infiltration (P=0.001), and larger tumor size (P=0.002). However, in the study by Cai et al,Citation18 hemoglobin levels were not associated with preoperative fasting hyperglycemia. The difference between our experiments may be related to the lymphoma itself, and a larger sample size is needed for verification. We found no correlation between the NLR and the long-term prognosis of gallbladder cancer, which is also different from Pang et al’s study.Citation15
Glucose is a nutrient required for tumor growth, and the infinite growth characteristics of tumors have attracted great interest in the relationship between tumors and glucose.Citation22 Studies have found that diabetes can increase the risk and mortality of several cancers, such as pancreatic cancer, liver cancer, and rectal cancer.Citation23 Patients with type 2 diabetes often present with insulin resistance. Many studies have found that insulin-like growth factor and insulin resistance increase the prognosis and risk of some cancers.Citation24 At the same time, diabetic patients have increased cardiovascular risk, delayed wound healing, increased risk of wound infection, arrhythmia, and delayed gastric emptying caused by neurologic complications, which affect the prognosis.Citation25 Moreover, although hyperglycemia is a characteristic of metabolic disorders in diabetic patients,Citation26 there are few studies on hyperglycemia and cancer. Recent studies have found that a high-sugar diet is associated with increased cancer cell proliferation,Citation27 and hyperglycemia can synergize with cancer cell proliferation, migration, invasion, and recurrence.Citation28 For example, in JAR cells, a choriocarcinoma cell line, the expression of glucose transporters is altered under hyperglycemic conditions. Increased glucose uptake and glycolysis ratesCitation29,Citation30 have been shown in endometrial and breast tumors, and GLUT1 and GLUT3 expressions are higher in tumors with low differentiation.Citation31 In addition, decreasing blood glucose levels during the remission period in gynecologic tumor patients are currently considered to be related to decreased GLUT1 expression.Citation32 Hyperglycemia is also associated with cell proliferation, and studies have found that hyperglycemia regulates cyclin-dependent kinase 2, E2F, cycln A, and the cell cycle.Citation27,Citation33 In addition, hyperglycemia is important for cancer cells to synthesize DNA, RNA, and protein.Citation34 The blood glucose concentration influences endometrial cancer cell proliferation and is positively correlated with endometrial cancer cell production,Citation35 which is also associated with unrestricted cell growth due to the dysregulation of apoptosis.Citation33 Recently, some studies have found that hyperglycemia and GBC are related.Citation33 GLUT1 expression was also found in GBC and was associated with its prognosis.Citation14 In addition, we found that miR-139-5p was significantly downregulated in GBC tissue and predicted poor prognosis. miR-139-5p overexpression directly inhibits PKM2 expression and regulates glycolysis.Citation36
It is undeniable that our research has some shortcomings. For example, our study is a retrospective study, and the number of patients is not very large. All of the patients included in the study were Asian, and both diabetes and nondiabetes patients were included. Thus, we could not avoid the effects of the diabetes status. However, the patients included in the study were newly diagnosed patients with preoperative data, and we excluded pretreated patients. The blood glucose levels were also calculated using samples collected at 5–6 am after 8 hours of fasting, which reduced the variation caused by eating and the circadian rhythm effect on glucose metabolism.
Conclusion
This study demonstrates that preoperative fasting hyperglycemia is a reliable and objective indicator of GBC mortality and recurrence after GBC radical surgery. Preoperative fasting blood glucose levels can supplement clinical considerations for decision making in preoperative GBC patients. Further investigation is needed to better understand the mechanisms by which blood glucose levels are associated with clinical outcomes. In the future, we need to prospectively and continuously check the fasting blood glucose levels in the months and years after surgery to detect the relationships between the average fasting blood glucose concentration and the survival time and/or RFS.
Author contributions
Peng Zheng and Xiaoqian Wang are co-first authors. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
- Lazcano-PonceECMiquelJFMuñozNEpidemiology and molecular pathology of gallbladder cancerCA Cancer J Clin200151634936411760569
- LeganMCyclooxygenase-2, p53 and glucose transporter-1 as predictors of malignancy in the development of gallbladder carcinomasBosn J Basic Med Sci201010319219620846124
- XuWYZhangHHYangXBPrognostic significance of combined preoperative fibrinogen and CA199 in gallbladder cancer patientsWorld J Gastroenterol201824131451146329632426
- HuMTWangJHYuYTumor suppressor LKB1 inhibits the progression of gallbladder carcinoma and predicts the prognosis of patients with this malignancyInt J Oncol20185331215122630015925
- ChenYWuFSaitoEAssociation between type 2 diabetes and risk of cancer mortality: a pooled analysis of over 771,000 individuals in the Asia cohort ConsortiumDiabetologia20176061022103228265721
- SheblFMAndreottiGRashidADiabetes in relation to biliary tract cancer and stones: a population-based study in Shanghai, ChinaBr J Cancer2010103111511920517308
- GiovannucciEHarlanDMArcherMCDiabetes and cancer: a consensus reportDiabetes Care20103371674168520587728
- DoSKJeongJYLeeSYGlucose transporter 1 gene variants predict the prognosis of patients with early-stage non-small cell lung cancerAnn Surg Oncol201825113396340330062472
- LamkinDMSpitzDRShahzadMMGlucose as a prognostic factor in ovarian carcinomaCancer200911551021102719156916
- ParkSMLimMKShinSAYunYHImpact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation StudyJ Clin Oncol200624315017502417075121
- KawamuraTKusakabeTSuginoTExpression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survivalCancer200192363464111505409
- NoguchiYMaratDSaitoAExpression of facilitative glucose transporters in gastric tumorsHepatogastroenterology199946282683268910522065
- HaberRSRathanAWeiserKRGLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosisCancer199883134409655290
- LeganMTevžičSTolarALuzarBMaroltVFGlucose transporter-1 (GLUT-1) immunoreactivity in benign, premalignant and malignant lesions of the gallbladderPathol Oncol Res2011171616620512538
- PangQZhangLQWangRTPlatelet to lymphocyte ratio as a novel prognostic tool for gallbladder carcinomaWorld J Gastroenterol201521216675668326074706
- KanthanRSengerJLAhmedSKanthanSCGallbladder cancer in the 21st centuryJ Oncol2015201510126
- ZhuAXHongTSHezelAFKoobyDACurrent management of gallbladder carcinomaOncologist201015216818120147507
- CaiQLuoXLiangYFasting blood glucose is a novel prognostic indicator for extranodal natural killer/T-cell lymphoma, nasal typeBr J Cancer2013108238038623299534
- LuoJChenYJChangLJFasting blood glucose level and prognosis in non-small cell lung cancer (NSCLC) patientsLung Cancer201276224224722112292
- HundalRShafferEAGallbladder cancer: epidemiology and outcomeClin Epidemiol201469910924634588
- ShenHXSongHWXuXJClinical epidemiological survey of gallbladder carcinoma in northwestern China, 2009-2013: 2379 cases in 17 centersChronic Dis Transl Med201731606629063057
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- SuhSKimKWDiabetes and cancer: is diabetes causally related to cancer?Diabetes Metab J201135319319821785737
- GiovannucciEInsulinGEInsulin, insulin-like growth factors and colon cancer: a review of the evidenceJ Nutr200113111 Suppl3109S312011694656
- CookKDBorzokJSumreinFOplerDJEvaluation and perioperative management of the diabetic patientClin Podiatr Med Surg20193618310230446046
- HeindelJJBlumbergBCaveMMetabolism disrupting chemicals and metabolic disordersReprod Toxicol20176833327760374
- MasurKVetterCHinzADiabetogenic glucose and insulin concentrations modulate transcriptome and protein levels involved in tumour cell migration, adhesion and proliferationBr J Cancer2011104234535221179032
- DuanWShenXLeiJHyperglycemia, a neglected factor during cancer progressionBiomed Res Int201420144176110
- YoungCDAndersonSMSugar and fat – that’s where it’s at: metabolic changes in tumorsBreast Cancer Res200810120218304378
- RudlowskiCMoserMBeckerAJGLUT1 mRNA and protein expression in ovarian borderline tumors and cancerOncology200466540441015331928
- KrzeslakAWojcik-KrowirandaKFormaEExpression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancersPathol Oncol Res201218372172822270867
- KroneCAElyJTControlling hyperglycemia as an adjunct to cancer therapyIntegr Cancer Ther200541253115695475
- RyuTYParkJSchererPEHyperglycemia as a risk factor for cancer progressionDiabetes Metab J201438533033625349819
- PiątkiewiczPCzechAGlucose metabolism disorders and the risk of cancerArch Immunol Ther Exp (Warsz)201159321523021448680
- HanJZhangLGuoHGlucose promotes cell proliferation, glucose uptake and invasion in endometrial cancer cells via AMPK/mTOR/S6 and MAPK signalingGynecol Oncol2015138366867526135947
- ChenJYuYChenXmiR-139-5p is associated with poor prognosis and regulates glycolysis by repressing PKM2 in gallbladder carcinomaCell Prolif2018516e1251030105813
