Abstract
Purpose
The incidence of cervical cancer in young women is increasing. This study aimed to analyze the clinicopathological characteristics, treatment, and prognoses of women aged ≤25 years with cervical cancer.
Patients and methods
Medical record data of 60 cervical cancer patients aged ≤25 years treated at Peking Union Medical College Hospital between January 1986 and December 2017 were reviewed. The overall survival rate was estimated using the Kaplan–Meier method. Prognosis-related risk factors were analyzed using univariate and multivariate analyses.
Results
Among the 60 patients, 44 (73.3%) were diagnosed with cervical carcinoma and 16 (26.7%) with cervical sarcoma. In the cervical carcinoma group, the most common histology was squamous cell carcinoma (n=22, 50.0%) followed by adenocarcinoma (n=18, 40.9%). Notably, clear cell carcinoma dominated cervical adenocarcinomas at 61.1% (11/18). In the cervical sarcoma group, embryonal rhabdomyosarcoma comprised 50% of the cases (8/16). A total of eleven patients with cervical carcinoma underwent fertility-sparing surgeries, and the live birth rate approached 66.7%. The estimated 5-year overall survival rate of the entire cohort was 79.8% with no statistically significant difference between the carcinoma and sarcoma groups (74.3% vs 93.3%, P=0.14). Stage (RR 6.71, 95% CI 1.366–32.970, P=0.019) and lymph node metastasis (RR 9.09, 95% CI 1.050–78.732, P=0.045) were independent risk factors for poor prognosis in those young patients with cervical carcinoma.
Conclusion
Adenocarcinoma and sarcoma of the cervix comprise the majority of cervical cancer in young women; their overall prognoses are not worse than older patients; the survival rates tend to vary widely according to histologic subtypes.
Introduction
Cervical cancer is the fourth most common cancer and the fourth leading cause of cancer-related mortality in women worldwide. Approximately 570,000 new patients were estimated to be diagnosed in 2018, with 311,000 deaths projected. Citation1 Over the last few decades, the incidence and mortality rates of cervical cancer have declined owing to population-based cervical cancer screening programs and anti-human papillomavirus (HPV) vaccines. Citation2 However, according to epidemiological studies, the incidence of cervical cancer in young women is increasing. Patel et alCitation3 reported that the incidence of cervical cancer in women aged 20–29 years increased annually by 10.3% between 2000 and 2009. The mean age at cervical cancer diagnosis was 5–10 years younger than that reported before 2000 in the People’s Republic of China, and patients aged 35 years or younger accounted for 16%. Citation4 Another study also showed that the cervical cancer mortality rate increased from 0.1 to 0.2/100,000 population in Japan. Citation5 Some previous studies reported that young cervical cancer patients had worse prognoses and lower survival rates than older patients,Citation6,Citation7 while other investigations found that age was not associated with poor prognosis. Citation8,Citation9 Moreover, previous studies have shown that cervical adenocarcinoma appears to be increasing rapidly in young women. Citation10,Citation11 The reason why adenocarcinoma is more likely to be found in young women is that HPV types associated with adenocarcinoma are more prevalent in younger women, including HPV-18 and HPV-45. Citation12 Several studies have demonstrated that adenocarcinoma has a worse prognosis than squamous cell carcinoma because it is more likely to progress rapidly and often escapes detection. Citation6,Citation13–Citation18 Furthermore, most young patients with cervical cancer desire to preserve their fertility, which complicates treatment options. Consequently, it is critical to attain a better understanding of cervical cancer in women aged ≤25 years. However, data in this age group are limited. Additionally, invasive cervical cancer develops over 5–10 years in 20%–30% of patients with cancer precursors. Citation2 However, among cervical cancer patients ≤25 years, the majority of them probably do not have a >5–10 years history of HPV infection or cancer precursors. Hence, we postulate that cervical cancer in young women aged ≤25 years is likely to be more aggressive.
Therefore, we conducted this retrospective study to analyze the clinicopathological characteristics, treatments, and prognoses of young women with cervical cancer (defined as patients 25 years old or younger). To the best of our knowledge, this study comprised the largest sample size of cervical cancer patients aged ≤25 years and reveals the prognoses of cervical cancer on this age group for the first time.
Patients and Methods
Between January 1986 and December 2017, we identified all cervical cancer patients aged ≤25 years who were treated at Peking Union Medical College Hospital (PUMCH) – a class A tertiary comprehensive hospital in Beijing, People’s Republic of China. Patients with cervical carcinoma or cervical sarcoma confirmed by pathological diagnosis were included. We reviewed the database and medical records to extract demographic and clinicopathologic data including age at diagnosis, age at first intercourse, number of sexual partners, marital status, symptoms (post-coital bleeding, abnormal vaginal bleeding, cervical polypoid mass, abnormal cervical cancer screening tests and others), stage, histologic subtype, differentiation, tumor size, surgical treatment, parametrial involvement, lymphovascular space involvement (LVSI), deep stromal invasion (DSI), lymph node (LN) metastasis, surgical margin status, and any radiotherapy and/or chemotherapy treatments. Surgical procedures included conization, radical trachelectomy, radical hysterectomy for cervical carcinoma, and polypoid mass resection for sarcoma. This study was approved by the Ethics Committee of PUMCH. Owing to the retrospective study design and analysis of clinical data, informed consent was formally waived by the Ethics Committee of PUMCH. All patient information is ensured to be confidential. All the procedures in this study are in accordance with the Helsinki Declaration.
All the patients were followed either through telephone interviews or at outpatient clinics until November 2018. The survival time was defined as the interval between the date of diagnosis and that of death or last follow-up. The overall survival rate was estimated using the Kaplan–Meier method, and rates were compared using the log-rank test with univariate analysis to identify prognostic risk factors related to survival. Factors found to be associated with survival on univariate analysis were subjected to a Cox proportional hazards model. P-values (two-tailed) of <0.05 were considered statistically significant. Statistical analyses were performed using the SPSS Version 25.0 statistical software (IBM Corporation, Armonk, NY, USA).
Results
Between January 1986 and December 2017, 5,833 patients with cervical cancer who were treated at PUMCH were identified. Sixty of these patients were 25 years or younger (1.0% of the cervical cancer patient population). The patients’ demographic and clinical characteristics are summarized in . The median age at the diagnosis of cervical cancer was 23 years old. Among the 60 young patients, 44 (73.3%) were diagnosed with cervical carcinoma and 16 (26.7%) with sarcoma. In the cervical carcinoma group, the most common histology was squamous cell carcinoma (50.0%) followed by adenocarcinoma (40.9%). Notably, clear cell carcinoma of the cervix (CCCC) comprised 61.1% (11/18) of adenocarcinomas; 8 (72.7%) of which were pre-sexual debut. In the cervical sarcoma group, embryonal rhabdomyosarcoma (ERMS) comprised half this patient subpopulation (8/16) while endometrial stromal sarcoma afflicted 25%. Nearly 77% of cervical carcinoma patients had engaged in sexual activity before diagnosis; the median age at first intercourse was 19 years, and eleven patients had three or more sexual partners. In contrast, the majority of cervical sarcoma patients had never been sexually active. More than 80% of patients with cervical carcinoma presented with post-coital or abnor mal vaginal bleeding, and only three patients had their cancers detected by cervical cancer screening. However, abnormal vaginal bleeding and cervical polypoid mass were the common symptoms in patients with cervical sarcoma. Over 88% of patients with cervical cancer had stage I–II disease; only seven patients had stage III–IV.
Table 1 Demographic and clinical characteristics of cervical cancer patients aged ≤25 years
Of the 44 patients with cervical carcinoma, 3 were unable to tolerate any treatment owing to extensive metastases and multiple organ failure. Twenty patients underwent laparoscopic or abdominal radical hysterectomy, among whom 14 had ovarian conservation surgery. Radical trachelectomy was performed in eight patients with stage IB1 tumors <2 cm, and three patients with stage IA1 tumors underwent conization. Among the eleven patients who underwent fertility-sparing surgeries, one experienced a recurrence and got remission after chemoradiotherapy. Seven patients attempted to become pregnant and four (57.1%) were able to conceive. A total of six pregnancies occurred, resulting in four live births (66.7%) and two miscarriages (33.3%) owing to premature membrane ruptures during the second trimester (). Additionally, 14 patients with postoperative pathological risk factors received adjuvant pelvic radiotherapy following radical surgery, and 10 received concurrent chemoradiotherapy as their primary treatment. Among the 31 patients who underwent surgeries, LN metastasis was diagnosed in 7 patients; moreover, LVSI was observed in 6 patients and DSI in 11 patients. Among patients with sarcoma, nine with ERMS or alveolar soft part sarcoma underwent polypoid mass resection with adjuvant chemotherapy consisting of vincristine, actinomycin, and cyclophosphamide/ifosfamide. Six patients with endometrial stromal sarcoma or adenosarcoma underwent conization, followed by adjuvant chemotherapy consisting of cisplatin, epirubicin, and ifosfamide. Laparoscopic radical hysterectomy and pelvic LN dissection was performed in one patient with endometrial stromal sarcoma who had lung metastasis.
Table 2 Fertility results and pregnancy outcomes of eleven cervical carcinoma patients with fertility-sparing surgeries
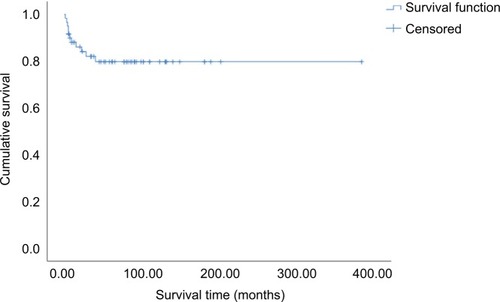
All the patients were followed either through telephone interviews or at outpatient clinics. The median followup time was 60 months (range 1–384 months) and four patients were lost to follow up. Forty-five patients (75%) have remained tumor-free to date, while seven (11.7%) experienced recurrence between 2 months and 3 years after completion of treatment. Eleven patients died, including nine of extensive metastases and multiple organ failure and two of septic shock. The estimated 5-year overall survival rate of the entire cohort was 79.8% (). There was no statistically significant difference in 5-year survival between patients with cervical carcinoma and those with sarcoma (74.3% vs 93.3%, P=0.14, ). Furthermore, univariate analysis of prognostic risk factors revealed that non-squamous histology (53.0% vs 94.4%, P=0.003), stage IIB–IV disease (36.4% vs 86.0%, P=0.001), and pelvic LN metastasis (37.3% vs 94.7%, P=0.001) were significantly associated with poorer disease-specific survival (). Multivariate analysis with Cox proportional regression was used to evaluate risk factors identified on univariate analysis. The data demonstrated that stage IIB–IV disease (RR 6.71, 95% CI 1.366–32.970, P=0.019) and LN metastasis (RR 9.09, 95% CI 1.050–78.732, P=0.045) were independent risk factors for poor prognosis in those young patients with cervical carcinoma, whereas histologic subtype was excluded (RR 3.07, 95% CI 0.314–29.978, P=0.335, ).
Figure 2 Kaplan-Meier curve showing overall survival among women ≤25 years with cervical carcinoma and cervical sarcoma.
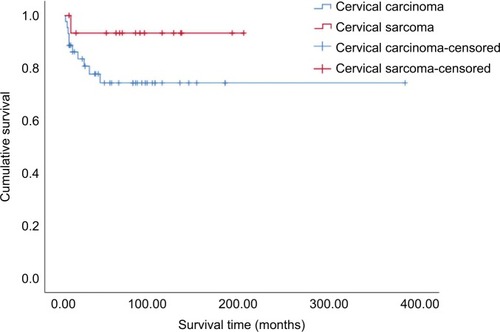
Table 3 Univariate analysis of prognostic risk factors in 44 young cervical carcinoma patients
Table 4 Multivariate analysis of prognostic risk factors in 44 young cervical carcinoma patients
Discussion
Cervical cancer is almost always caused by a persistent HPV infection. There are four steps in cervical cancer development: infection with HPV, viral persistence, precancerous changes, and invasive cervical cancer. Generally, precancerous changes often develop within 5 years of HPV infection, while invasive cervical cancer typically arises over 5–10 years in 20%–30% of patients with precancerous growths. Citation2 However, among cervical cancer patients ≤25 years, the majority of them do not have a long history of sexual activity, even without sexual debut. As a result, some of the cervical cancer patients aged ≤25 years probably do not have a more than 5–10 years history of HPV infection or precancerous changes. Therefore, we postulate that cervical cancer develops more aggressively in young women ≤25 years than older patients. In addition, most previous studies defined young cervical cancer patients as those ≤30 or ≤35 years, and conflicting prognoses were reported for these age groups,Citation6–Citation9,Citation18 in which much heterogeneity also exists. Hence, we focused on very young women with cervical cancer (≤25 years) for our study.
The distribution of cervical cancer histologic subtypes in women ≤25 years differs from that of patients of all ages. According to the Surveillance, Epidemiology, and End Result Program Cancer Statistics Review,Citation19 squamous cell carcinoma is dominant among cervical carcinomas in patients of all ages (65.5%), followed by adenocarcinoma (28.5%). However, our data revealed that adenocarcinoma accounted for ~40% of cervical carcinomas afflicting young women, which is much higher than the proportion of adenocarcinomas among all age groups and indicates that adenocarcinoma is more likely to be found in younger women. Indeed, the proportion of adenocarcinoma is reportedly rising in young women;Citation10,Citation11,Citation20,Citation21 moreover, patients with adenocarcinoma reportedly have poorer prognoses and lower survival rates than those with squamous cell carcinoma. Citation6,Citation13–Citation18
Interestingly, >60% of the cervical adenocarcinomas were CCCCs, which is a rare disease usually accounting for 4%–9% of adenocarcinomas of the cervix. Citation22 Previous studies found that CCCC is related to intrauterine exposure to diethylstilbestrol; however, this agent was banned in the 1970s,Citation23 and none of our patients with CCCC had been exposed to it in utero. Although HPV infection is associated with almost all cervical squamous cell carcinomas and 85%–90% of cervical adenocarcinomas, there appears to be no association between high-risk HPV infection and CCCC. Citation24,Citation25 Consistent with previous studies, the majority of patients with CCCC had not been sexually active, further indicating that CCCC is unlikely to be caused by HPV. Instead, genetic factors, instability of microsatellite repeat sequences, and exogenous risk factors may play an important role in the carcinogenesis of CCCC. Citation26,Citation27 The 5-year overall survival rate of patients with CCCC was 67%–72%;Citation22,Citation23 in our cohort of patients ≤25 years, seven of eleven (63.6%) have remained tumor-free, which is similar to the tumor-free rate of CCCC patients of all ages.
Additionally, 26.7% of cervical cancers among the very young women in our study were sarcomas. Cervical sarcoma is a rare gynecological neoplasm, accounting for 0.4% of cervical cancers overall. Citation19 Furthermore, the distribution of the histologic subtypes of cervical sarcoma in very young women is different from that in patients overall. Bansal et alCitation28 found that carcinosarcoma is the most common histologic subtype in patients of all ages, followed by leiomyosarcoma and adenosarcomas. In our cohort, however, ERMS comprised 50% of cervical sarcomas in very young women. Rhabdomyosarcoma is the most common childhood soft-tissue sarcoma, and includes ERMS, botryoid rhabdomyosarcoma, alveolar rhabdomyosarcoma, spindle cell rhabdomyosarcoma, and undifferentiated sarcoma. Citation29 ERMS comprises up to 58% of all rhabdomyosarcomas; cervical ERMS most commonly occurs in the second and third decades of life. Citation30,Citation31 Consistent with previous studies, the age at ERMS diagnosis ranged from 13 to 20 years in our cohort.
Fertility preservation is an important factor in young women with cervical cancer. The cornerstone of conservative surgery is radical trachelectomy, which may be performed either vaginally, abdominally, laparoscopically, or robotically. The safety and feasibility of radical trachelectomy have been well described in a series of studies. Citation32–Citation34 All eight patients in our study who underwent radical trachelectomy conformed to the universal selection criteria, which are 1) clinical stage between IA1 (with LVSI) and IB1 (tumor <2 cm); 2) squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma; 3) tumor limited to the cervix; 4) and no evidence of clinical pelvic LN metastasis. Citation35 Since radical trachelectomy is not the standard surgical treatment, both its oncological and obstetrical outcomes should be taken into consideration when this procedure is performed. A recent review of the published literature revealed an overall recurrence rate of <5% and death rate of <3% following radical trachelectomy, which are comparable to the rates of radical hysterectomy. Citation36–Citation39 Moreover, Bentivegna et alCitation40 reported that the pregnancy rates following vaginal, abdominal, and minimally invasive radical trachelectomy were 57%, 44%, and 65%, respectively. Furthermore, the live birth rates following these three trachelectomy procedures ranged from 67% to 78%. In our cohort, only one patient who underwent radical trachelectomy (12.5%) experienced a recurrence and got remission after chemoradiotherapy. Moreover, 57% of patients who attempted to conceive succeeded, and the live birth rate among them was 66%, which is similar to previously published rates. Therefore, radical trachelectomy should be regarded as an optional surgical approach in the management of early cervical cancer in young women to preserve their fertility.
The 5-year overall survival rate of cervical cancer patients of all ages varied from 55% to 75% worldwide. Citation41–Citation43 In our study, the 5-year overall survival rate was 79.8%, which indicates that the prognosis of cervical cancer in very young women is not inferior to that in older patients. However, the survival rates varied widely according to the cervical cancer histologic subtypes in our young cohort. The 5-year survival rate among patients with cervical carcinoma was much lower than that among patients with cervical sarcoma (74.3% vs 93.3%, P=0.14), although this difference was not statistically significant owing to the small cervical sarcoma patient sample size. Further analysis found a statistically significant difference of survival rate between squamous cell and non-squamous cell carcinoma of the cervix (94.4% vs 53.0%, P=0.003). Among the ten patients who died of cervical carcinoma, nine had adenocarcinoma, adenosquamous carcinoma, or neuroendocrine carcinoma. These results indicate that histology substantially affects the survival rate, and that non-squamous cell carcinoma has a poorer prognosis than squamous cell carcinoma, which is consistent with previous studies. Citation6,Citation13–Citation18 We also found that histologic subtype, stage, and LN metastasis status all had significant impacts on survival based on univariate analysis. However, the histologic subtype was not found to be a statistically significant factor on multivariate analysis. Moreover, although stage and LN metastasis achieved to be statistically significant risk factors on multivariate analysis, the CIs are quite wide. Perhaps this is due to the small sample size of our cohort.
Given the rarity of cervical cancer in women ≤25 years, we were only able to conduct a retrospective study on this younger population; therefore, it was difficult to rule out selection bias. In addition, it is hard to reach a statistical significance in the univariate and multivariate analysis due to the small sample size of our cohort. Moreover, this study did not include a comparison group of older patients; hence, we only compared clinicopathological characteristics and prognoses with patients reported in previous studies. However, ours is the largest study focusing on cervical cancer patients aged ≤25 years, and we were able to identify the histologic subtype distributions and prognoses in this specific age group. These data provide valuable information pertaining to the treatment of cervical cancer in young women as well as to cervical cancer screening programs.
Conclusion
In conclusion, adenocarcinoma and sarcoma of cervix, rather than squamous cell carcinoma, comprise the majority of cervical cancer subtypes afflicting very young women. The overall prognoses of cervical cancer in this age group are not inferior to those of older patients. However, survival rates tend to vary widely according to histologic subtypes; the survival rate of patients with non-squamous cell carcinoma is much lower than that of patients with squamous cell carcinoma. Moreover, greater attention should be paid to cervical carcinoma patients with higher disease stages and LN metastasis, as these factors predict significantly poorer prognoses.
Acknowledgments
We thank the medical record room staff for their assistance in retrieving the medical records of the patients. This work was supported by grants from the National Natural Science Foundation of China (No. 81772783 and No. 81472446) and the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (CAMS-2017-I2M-1–002) to Y Xiang.
Disclosure
The authors report no conflicts of interest in this work.
References
- BrayFFerlayJSoerjomataramIGlobal cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countriesCA Cancer J Clin201868639442430207593
- SchiffmanMCastlePEJeronimoJRodriguezACWacholderSHuman papillomavirus and cervical cancerLancet2007370959089090717826171
- PatelAGalaalKBurnleyCCervical cancer incidence in young women: a historical and geographic controlled UK regional population studyBr J Cancer2012106111753175922531636
- LiSHuTLvWChanges in prevalence and clinical characteristics of cervical cancer in the People’s Republic of China: a study of 10,012 cases from a nationwide Working GroupOncologist201318101101110724043599
- MotokiYMizushimaSTaguriMIncreasing trends in cervical cancer mortality among young Japanese women below the age of 50 years: an analysis using the Kanagawa population-based cancer Registry, 1975–2012Cancer Epidemiol201539570070626277329
- LauHYJuangCMChenYJAggressive characteristics of cervical cancer in young women in TaiwanInt J Gynaecol Obstet2009107322022319716131
- RutledgeFNMitchellMFMunsellMYouth as a prognostic factor in carcinoma of the cervix: a matched analysisGynecol Oncol19924421231301544591
- MeanwellCAKellyKAWilsonSYoung age as a prognostic factor in cervical cancer: analysis of population based data from 10,022 casesBr Med J198829666193863913125911
- SpanosWJJrKingAKeeneyEWagnerRSlaterJMAge as a prognostic factor in carcinoma of the cervixGynecol Oncol198935166682792904
- BulkSVisserORozendaalLVerheijenRHMeijerCJCervical cancer in the Netherlands 1989–1998: decrease of squamous cell carcinoma in older women, increase of adenocarcinoma in younger womenInt J Cancer200511361005100915515017
- LiuSSemenciwRMaoYCervical cancer: the increasing incidence of adenocarcinoma and adenosquamous carcinoma in younger womenCMAJ200116481151115211338801
- SeoudMTjalmaWARonsseVCervical adenocarcinoma: moving towards better preventionVaccine201129499148915821983356
- DavyMLDoddTJLukeCGRoderDMCervical cancer: effect of glandular cell type on prognosis, treatment, and survivalObstet Gynecol20031011384512517643
- LeeKBLeeJMParkCYWhat is the difference between squamous cell carcinoma and adenocarcinoma of the cervix? A matched case-control studyInt J Gynecol Cancer20061641569157316884367
- NakanishiTIshikawaHSuzukiYA comparison of prognoses of pathologic stage IB adenocarcinoma and squamous cell carcinoma of the uterine cervixGynecol Oncol200079228929311063659
- Vinh-HungVBourgainCVlastosGPrognostic value of histopathology and trends in cervical cancer: a SEER population studyBMC Cancer20077116417718897
- GrisaruDCovensAChapmanBDoes histology influence prognosis in patients with early-stage cervical carcinoma?Cancer200192122999300411753977
- YangLJiaXLiNComprehensive clinic-pathological characteristics of cervical cancer in southwestern China and the clinical significance of histological type and lymph node metastases in young patientsPLoS One2013810e7584924130747
- Surveillance, epidemiology, and end results program, SEER Cancer statistics review, 1975–20152018 cervix uteri, table 5.16. Available from: https://seer.cancer.gov/csr/1975_2015/results_merged/sect_05_cervix_uteri.pdfAccessed September 10, 2018
- SmithHOTiffanyMFQuallsCRKeyCRThe rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States—a 24-year population-based studyGynecol Oncol20007829710510926787
- ShermanMEWangSSCarreonJDevesaSSMortality trends for cervical squamous and adenocarcinoma in the United States. Relation to incidence and survivalCancer200510361258126415693030
- ReichOTamussinoKLahousenMClear cell carcinoma of the uterine cervix: pathology and prognosis in surgically treated stage IB–IIB disease in women not exposed in utero to diethylstilbestrolGynecol Oncol200076333133510684706
- JiangXJinYLiYClear cell carcinoma of the uterine cervix: clinical characteristics and feasibility of fertility-preserving treatmentOnco Targets Ther2014711111624470762
- PirogECKleterBOlgacSPrevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinomaAm J Pathol200015741055106211021808
- UenoSSudoTOkaNAbsence of human papillomavirus infection and activation of PI3K-Akt pathway in cervical clear cell carcinomaInt J Gynecol Canc201323610841091
- BoydJTakahashiHWaggonerSEMolecular genetic analysis of clear cell adenocarcinomas of the vagina and cervix associated and unassociated with diethylstilbestrol exposure in uteroCancer19967735075138630958
- HanselaarAvan LoosbroekMSchuurbiersOClear cell adenocarcinoma of the vagina and cervix. An update of the central Netherlands registry showing twin age incidence peaksCancer19977911222922369179071
- BansalSLewinSNBurkeWMSarcoma of the cervix: natural history and outcomesGynecol Oncol2010118213413820541244
- ParhamDMBarrFGClassification of rhabdomyosarcoma and its molecular basisAdv Anat Pathol201320638739724113309
- DehnerLPJarzembowskiJAHillDAEmbryonal rhabdomyosarcoma of the uterine cervix: a report of 14 cases and a discussion of its unusual clinicopathological associationsMod Pathol201225460261422157934
- NarinMAKaralokABasaranDEmbryonal rhabdomyosarcoma of the cervix in young womenJ Adolesc Young Adult Oncol20165326126627003182
- RamirezPTSchmelerKMSolimanPTFrumovitzMFertility preservation in patients with early cervical cancer: radical trachelectomyGynecol Oncol20081103 Suppl 2S25S2818501409
- GizzoSAnconaESaccardiCRadical trachelectomy: the first step of fertility preservation in young women with cervical cancer (review)Oncol Rep20133062545255424065029
- HalaskaMRobovaHPlutaMRobLThe role of trachelectomy in cervical cancerEcancermedicalscience2015950625729419
- Mejia-GomezJFeigenbergTFeigenberTArbel-AlonSKoganLBenshushanARadical trachelectomy: a fertility-sparing option for early invasive cervical cancerIsr Med Assoc J201214532432822799068
- HertelHKöhlerCGrundDRadical vaginal trachelectomy (RVT) combined with laparoscopic pelvic lymphadenectomy: prospective multicenter study of 100 patients with early cervical cancerGynecol Oncol2006103250651116690104
- HauspyJBeinerMHarleyISentinel lymph nodes in early stage cervical cancerGynecol Oncol2007105228529017368525
- MarchiolePBenchaibMBuenerdAOncological safety of laparoscopic-assisted vaginal radical trachelectomy (LARVT or Dargent’s operation): a comparative study with laparoscopic-assisted vaginal radical hysterectomy (LARVH)Gynecol Oncol2007106113214117493666
- DiazJPSonodaYLeitaoMMOncologic outcome of fertility-sparing radical trachelectomy versus radical hysterectomy for stage IB1 cervical carcinomaGynecol Oncol2008111225526018755500
- BentivegnaEMaulardAPautierPFertility results and pregnancy outcomes after conservative treatment of cervical cancer: a systematic review of the literatureFertil Steril201610651195121127430207
- BenardVBWatsonMSaraiyaMCervical cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD-2 studyCancer2017123Suppl 245119513729205300
- MelanKJankyEMacniJEpidemiology and survival of cervical cancer in the French West-Indies: data from the Martinique cancer registry (2002–2011)Glob Health Action2017101133734128649938
- DiazABaadePDValeryPCComorbidity and cervical cancer survival of Indigenous and non-Indigenous Australian women: a semi-national registry-based cohort Study (2003–2012)PLoS One2018135e019676429738533