Abstract
Objective
Cholangiocarcinoma (CCA) is a devastating disease. Interferon α-inducible protein 27 (IFI27), originally known to involve in innate immunity, is later found to intervene in cell proliferation, leading to inventive studies regarding the role of IFI27 in cancer treatment. We aimed to investigate the role of IFI27 in CCA.
Materials and methods
Cell proliferation, migration, and invasion assays, Western blot, gene transfection and knockdown, immunofluorescent and immunohistochemical stains, and xenograft animal model were applied.
Results
IFI27 knockdown in CCA cells induced cell cycle arrest in S phase, resulting in lower cell proliferative rate in vitro and in vivo. IFI27 knockdown attenuated CCA cell migration and invasion through inhibition of epithelial–mesenchymal transition, which was supported by increased E-cadherin and decreased N-cadherin and fibronectin. Filamentous actin level was also reduced. IFI27 knockdown further repressed expression and secretion of vascular endothelial growth factor (VEGF-A), a strong stimulator of angiogenesis, through downregulation of c-jun and c-fos, which was supported in vitro by the finding that human vascular endothelial cells grew more slowly in conditioned medium of IFI27 knockdown on CCA cells and in vivo by the lower erythropoietin concentration found in the xenografted tumors derived from IFI27 knockdown on CCA cells. In addition, anti-VEGF-A antibody treatment was able to repress CCA cell growth. To the contrary, IFI27 overexpression could increase CCA cell proliferation, migration, and invasion. Clinically, higher IFI27 expression was linked to inferior overall survival of CCA patients.
Conclusion
Our data strongly suggest that IFI27 could be deemed as a potential target for CCA treatment.
Introduction
Cholangiocarcinoma (CCA) is a biliary tract cancer and ranks as second most liver primary malignancy, after hepatoma. CCA accounts for 20% of primary liver cancersCitation1 and mainly happens in patients aged over 50 years. Citation2 Due to the asymptomatic nature during the early stage of CCA, most CCA patients are diagnosed with advanced disease, which excludes the feasibility of the most effective CCA treatment, radical surgery. Citation3 The median survival of non-resectable CCA is reported to be around 3–6 months due to resistance to conventional chemotherapy and radiotherapy. Citation4 Thus, developing new therapeutic targets for CCA should be urgently prioritized.
Table S1 Clinicopathological features and IFI27 expression in patients with CCA
Table S2 Univariate and multivariate analyses of factors influencing the OS of patients with CCA
Interferons activate the interferon-inducible genes to exert the biological functions. Interferon α-inducible protein 27 (IFI27) is coded by one kind of small genes induced highly by interferon α and with basal expression in almost all cell types. Primarily, IFI27 is deemed as a key factor for interferon-mediated antivirus treatment. Citation5 The cDNA for IFI27 was first cloned in the human breast cancer cell line, MCF-7, after estrogen exposure. Citation6 Suomela et al showed that IFI27 mRNA expressed highly in inflammatory epidermis and squamous cell cancers, and IFI27 could be deemed as a cell proliferative marker for epithelium and cancer. Citation7 IFI27 expression has also been found to be upregulated in various cancers with underlying mechanism not fully understood. Citation7 In ovarian cancer, IFI27 was shown to promote epithelial–mesenchymal transition (EMT) and stemness. Citation8 Regarding CCA, there is no IFI27 relevant study available at present.
Angiogenesis plays a crucial role during cancer growth and metastasis, and thus targeting angiogenesis has emerged as a new therapeutic trend for cancer treatment. Citation9 Vascular endothelial growth factor (VEGF), usually known as VEGF-A, is an endothelial cell-specific mitogen and is a strong stimulator of angiogenesis. Citation10 VEGF-A-targeting drugs (like Bevacizumab and Aflibercept) are widely applied in cancer treatment. Citation11 In CCA, higher VEGF expression was shown to imply high hematogenic metastasis. Citation12 Recent in vitro study demonstrated that apatinib, a VEGF receptor inhibitor, could inhibit CCA proliferation through VEGF signaling repression,Citation13 further strongly suggesting the important role of VEGF signaling activation in CCA.
In this study, we investigated the role of IFI27 in CCA in vitro and in vivo. The influence and regulatory mechanism of IFI27 on VEGF-A expression in CCA cells were also studied. In addition, we examined IFI27 expression of human CCA specimen by immunohistochemical staining to evaluate the clinical meaning of IFI27 on CCA patients’ survival. We aimed to develop a new therapeutic target for CCA.
Materials and methods
Cell culture
Human CCA cell lines were purchased from Korean Cell Line Bank (Seoul, Korea). Cells were grown in RPMI 1640 medium supplemented with 10% FBS and 1% antibiotic-antimycotic agents. Culture medium was changed thrice per week. Human vascular endothelial cells (HUVECs) were purchased from Bioresource Collection and Research Center (Hsinchu, Taiwan R.O.C.) and maintained as previously described. Citation14
Knockdown of IFI27 in SNU308 cells
SNU308 cells were transduced with lentiviral particles containing control small hairpin (sh)RNA (sc-108080; Santa Cruz Biotechnology Inc., Dallas, TX, USA) or IFI27 shRNA (sc-105551-V; Santa Cruz Biotechnology Inc.) according to the manufacturer’s instructions. One day after transduction, SNU308-COLsi (with control shRNA) and SNU308-IFI27si (with IFI27 shRNA) were selected by incubation with 2 µg/mL puromycin dihydrochloride for another three generations.
IFI27 overexpression in YSCCC cells
YSCCC cells were transduced with control lentiviral activation particles (sc-437282; Santa Cruz Biotechnology Inc.) or IFI27 lentiviral activation particles (sc-416981-LAC; Santa Cruz Biotechnology Inc.) according to the manufacturer’s instructions. Three days after transduction, the cells (YSCCC-DNA and YSCCC-IFI27) were selected by incubation with 10 µg/mL puromycin dihydrochloride (sc-108071; Santa Cruz Biotechnology Inc.), 500 µg/mL hygromycin B (sc-29067; Santa Cruz Biotechnology Inc.), and 10 µg/mL Blasticidin S HCl (sc-495389; Santa Cruz Biotechnology Inc.) for at least four generations.
Cell cycle analysis
The analysis procedure was performed as previously described. Citation15,Citation16 Cell cycle analysis was performed using a FACSCalibur cytometer and CellQuest Pro software (BD Biosciences, San Jose, CA, USA).
Matrigel invasion assay
The matrigel invasion assay was conducted as previously described. Citation17 It was carried out for 48 hours and the invading cells were fixed with 4% paraformaldehyde in 1× PBS, stained, digitally photographed, and counted under the microscope (IX71; Olympus Corporation, Tokyo, Japan). The experiments were performed in triplicate.
Transwell filter migration assay
The migration assay was conducted as previously described. Citation18 It was carried out for 24 hours and the migrating cells were stained and counted under four random high-power microscopic fields (100×) per filter. The experiments were performed in triplicate.
Real-time quantitative-PCR (RT-qPCR)
Total RNA was isolated using Trizol reagent purchased from Thermo Fisher Scientific (Waltham, MA, USA). RT-qPCR was performed using the Mx3000P™ QPCR system (Stratagene, San Diego, CA, USA) with EvaGreen® (TOOLS Biotechnology Co., Ltd., New Taipei City, Taiwan R.O.C.) as fluorescent dye. The sequences of specific PCR primers were described in the supplemental data.
Western blotting
Western blots were performed as described previously. Citation15 The antibodies used are listed in the supplementary data.
Filamentous actin (F-actin) staining
The detailed procedures were as described previously. Citation19 The F-actin expression was shown by incubation with FITC-conjugated phalloidin and mounted with ProLongR Gold reagent as instructed by the manufacturer (Thermo Fisher Scientific). Fluorescence representing the distribution of F-actin was checked using confocal microscope (LSM 510 Meta; Zeiss, Oberkochen, Germany).
VEGF promoter activity assay
Cells (5×104 cells/well) were seeded in 24-well plate, 24 hours prior to transfection with mixture containing 1 µg VEGF promoter plasmid DNA (S721026; Active Motif, Carlsbad, CA, USA), 3 µL transfection reagent FuGENE® HD (Active Motif), and Opti-MEM. Promoter activity was quantified 24 hours post-transfection using the LightSwitch™ Luciferase Assay Kit (Active Motif).
VEGF-A ELISA
VEGF-A concentration in conditioned media was measured by VEGF-A ELISA according to the methods described by the manufacturer (DY293B; R&D Systems Inc., Minneapolis, MN, USA).
Tumor xenografts
This study was approved by the Chang Gung University Animal Research Committee (Permit Number: 2014022601). All methods were performed in accordance with the “Animal Welfare Law and Policy” (LAW3ANI). Equal volumes of tumor cells and matrigel were mixed (total 100 µL, containing 5×106 cells) and injected into the dorsal region of nude mice (BALB/cAnN-Foxn1, 4 weeks old). The weight, volume, and erythropoietin (EPO) concentrations of the xenografts were measured after 4 weeks. The EPO concentration was quantified using Mouse EPO Quantikine ELISA Kit (R&D Systems Inc.).
Patient demographics for IHC analysis and bile collection
Patients with CCA who underwent hepatectomy between 1989 and 2006 at the Department of Surgery, Chang Gung Memorial Hospital were enrolled (N=96). The study was approved by the local institutional review board of Chang Gung Memorial Hospital (clinical study numbers 99-2886B, 99-3810B, and 102-5813B). Written informed consents for participants were obtained. All methods were performed in accordance with the Declaration of Helsinki.
IFI27 immunohistochemistry
IFI27 expression levels in the aforementioned 96 CCA patients were examined by immunohistochemical staining. IFI27 primary antibody (NBP1-84745, 1:50 dilution; Novus Biologicals, LLC, Centennial, CO, USA) was used. Bound antibody signal was visualized using Labelled Streptavidin-Biotin2 System horseradish peroxidase (No. K0675; Dako Denmark A/S, Glostrup, Denmark). Staining intensities were scored as 1 (mild), 2 (moderate), or 3 (strong). H-scores were calculated as the percentage of positive staining (0–100) × the corresponding staining intensity (0–3). Specimens with H-scores <160 or ≥160 were classified as having low or high expression, respectively.
Statistical analyses
Differences between the experimental and control groups were calculated using the Student’s t-test. Differences in tumor weights between the experimental and control animals were compared by the Mann–Whitney U test. Overall survival (OS) rate was evaluated using the Kaplan–Meier method. Several clinicopathological variables were considered for the initial single-variable analysis, which was performed with the log-rank test. The Cox proportional hazards model was applied for multivariate regression. A value of P≤0.05 derived from a two-tailed test was considered statistically significant.
Result
IFI27 knockdown reduced the proliferation of CCA cells
Eight kinds of CCA cells, including RBE, TFK-1, SSP-25, SNU308, SNU1079, TGBC-24, YSCCC, and HUCCT1 cells, were evaluated for IFI27 mRNA expressions by RT-qPCR. shows that HUCCT1 expressed the most IFI27 mRNA expression, while YSCCC expressed the least.
Figure 1 Evaluation of IFI27 effect on SNU308 cell growth.
Notes: (A) IFI27 mRNA expressions were determined in eight kinds of CCA cells with YSCCC and HUCCT1cells having the lowest and highest IFI27 mRNA expressions, respectively. (B) Knockdown of IFI27 in SNU308 cells. Western blot of IFI27 expression in SNU308 IFI27 and SNU308-COLsi cells (upper panel) (cropped). Quantitative result of Western blot. Each value was a mean ± SD of the three independent determinations relative to the SNU308-COLsi cell group (lower panel) (SNU308-COLsi group was set as 1). (C) Doubling time of SNU308 IFI27 and SNU308-COLsi cells. (D) MTT assay for SNU308 IFI27 and SNU308-COLsi cells at days 1, 2, and 3 were performed. Data were presented as mean percentage relative to the SNU308-COLsi group (± SD, n=6). (E) Histogram of cell cycle distribution (upper panel) and quantitative result of cell cycle distribution (lower panel) of SNU308 IFI27 and SNU308-COLsi cells (*P<0.05, **P<0.01).
Abbreviations: CCA, cholangiocarcinoma; IFI27, interferon α-inducible protein 27.
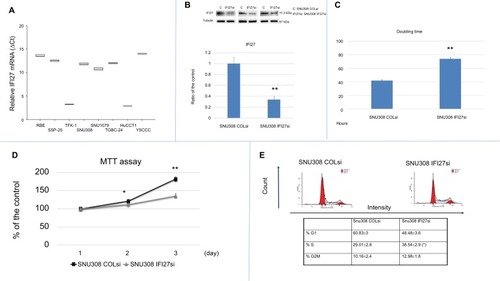
To investigate the potential role of IFI27 in CCA cell proliferation, IFI27 was knocked down in SNU308 cells (SNU308-IFI27si cells). As shown in , upper panel, the expression of IFI27 protein was knocked down significantly in SNU308-IFI27si cells to <35% of that in SNU308-COLsi cells (, lower panel). The doubling time of SNU308-IFI27si cells was increased to 72 hours compared to 44 hours of SNU308-COLsi cells (). We further evaluated cell proliferation rate by MTT assay. As shown in , SNU308-IFI27si cells had a decreased cell proliferation rate compared to SNU308-COLsi cells after 2 and 3 days. The cell cycle distribution as analyzed by flow cytometry revealed an S-phase arrest in SNU308-IFI27si cells, which was similar to the IFI27 knockdown effect in epidermal keratinocytes reported in our previous study. Citation20 The percentage of S-phase cells increased from 29.01% ±2.8% in SNU308-COLsi cells to 38.5%±2.9% SNU308-IFI27si cells (). Taken together, our results indicated that the functional role of IFI27 was indeed involved in the cell proliferation and cell cycle progression of SNU308 cells.
IFI27 knockdown inhibited the migration and invasion of CCA cells
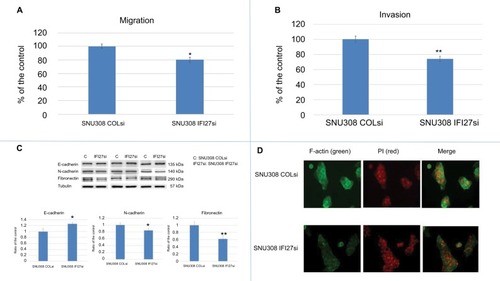
Since cancer metastasis is one of the main causes of cancer-related death, we evaluated the potential role of IFI27 in CCA cell metastasis. As shown in , SNU308-IFI27si cells had only 80%±4% cell migration ability compared to that of SNU308-COLsi cells. The invasion ability of SNU308-IFI27si cells was also reduced to 74%±3.8% of SNU308-COLsi cells (). E-and N-cadherins were next evaluated. demonstrated that SNU308-IFI27si cells had higher E-cadherin but lower N-cadherin expression than SNU308-COLsi cells. Fibronectin, one of the mesenchymal cell markers, was also found to be expressed in lower level in SNU308-IFI27si cells (). The fluorescent stain by FITC-conjugated phalloidin showed that the formation of F-actin in SNU308-IFI27si was also decreased compared to that in SNU308-COLsi cells (). Collectively, our data indicated that knockdown of IFI27 in SNU308 cells would inhibit cell metastasis through inhibition of EMT and F-actin formation.
Figure 2 The effect of IFI27 knockdown on SNU308 cell metastasis.
Notes: (A) The migration ability of SNU308 IFI27 and SNU308-COLsi cells. Data were presented as mean percentage relative to the SNU308-COLsi group (± SD). Experiments were performed in triplicate and repeated at least thrice. (B) The invasion ability of SNU308 IFI27 and SNU308-COLsi cells. Data were presented as mean percentage relative to the SNU308-COLsi group (± SD). Experiments were performed in triplicate and repeated at least thrice. (C) The Western blot of E-cadherin, N-cadherin, and fibronectin expressions in SNU308 IFI27 and SNU308-COLsi cells (upper panel) (cropped). Quantitative result of the Western blot (lower panel). Each value was a mean ± SD of three independent determinations relative to the SNU308-COLsi group (SNU308-COLsi group was set as 1). (D) Immunofluorescence staining of F-actin (green) expression of SNU308 IFI27 and SNU308-COLsi cells. DAPI (red) was applied for nucleus stain (*P<0.05, **P<0.01).
Abbreviations: F-actin, filamentous actin; IFI27, interferon α-inducible protein 27; P, propidium iodine.
IFI27-regulated VEGF-A expression in CCA cells
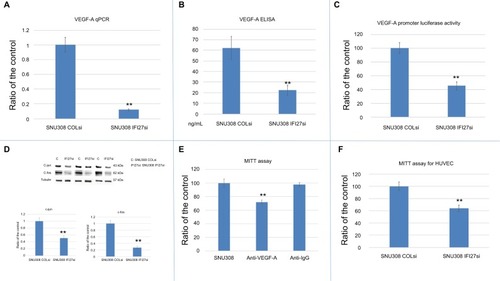
Since VEGF-A is a strong stimulator of angiogenesis, which plays a vital role during cancer growth and metastasis, we evaluated whether IFI27 exerted any effect on the regulation of VEGF-A expression in CCA cells. and showed that IFI27 knockdown in SNU308 cells attenuated the expressions of VEGF-A at the mRNA and secreted protein levels. The luciferase activity of VEGF-A promoter was also decreased to 45%±6% in SNU308-IFI27si cells (). Two VEGF-A-inducing transcriptional factors, c-jun and c-fos, were also downregulated by IFI27 knockdown in SNU308 cells (). To evaluate the role of VEGF-A in CCA cell growth, SNU308 cells were then treated by anti-VEGF-A antibodies for 1 day. As shown in , the cell growth was inhibited to 72% ±3% by anti-VEGF-A antibody treatment, indicating the stimulatory effect of VEGF-A on the proliferation of CCA cells. Conditioned medium taken from SNU308-IFI27si cells and SNU308-COLsi cells was further used for the growth of HUVECs culture. showed that HUVECs cultured with SNU308-IFI27si cell medium had lower cell proliferation rate as determined by MTT assay. Our results showed, for the first time, that IFI27 knockdown in SNU308 cells could inhibit the production and secretion of VEGF-A, thus repressing the cell growth of both CCA cells and vascular endothelial cells, and therefore, involved in the control of angiogenesis.
Figure 3 The effect of IFI27 knockdown on VEGF-A in SNU308 cells.
Notes: (A) The mRNA expressions of VEGF-A were analyzed in SNU308 IFI27 and SNU308-COLsi cells by RT-qPCR. Data were presented as mean ± SD relative to SNU308-COLsi group (SNU308-COLsi cells group was set as 1). Experiments were performed in triplicate and repeated at least thrice. (B) The amounts of secreted VEGF-A in SNU308 IFI27 and SNU308-COLsi cells culture medium were determined by ELISA. Experiments were performed in triplicate and repeated at least thrice. (C) Luciferase activity of VEGF-A reporter vector-transfected SNU308 IFI27 or SNU308-COLsi cells. Data were presented as mean percentage relative to the control group (SNU308-COLsi group) (± SE, n=6). (D) Western blot showing c-jun and c-fos expressions in SNU308 IFI27 and SNU308-COLsi cells (upper panel) (cropped). Quantitative result of the Western blot (lower panel). Each value was a mean ± SD of three independent determinations relative to the SNU308-COLsi group (SNU308-COLsi group was set as 1). (E) Cell viability of SNU308 cells with or without anti-VEGF-A antibodies treatment for one day as determined by MTT assay. Anti-IgG treatment group was served as the negative control. Data were present as the mean percentage relative to the SU308 cell group (± SD). Experiments were performed in triplicate and repeated at least thrice. (F) Cell viability of HUVECs cultured with mediums from SNU308 IFI27 or SNU308-COLsi cells for one day. Data were presented as mean percentage relative to the SNU308-COLsi group (± SD, n=6) (**P<0.01).
Abbreviations: IFI27, interferon α-inducible protein 27; HUVEC, human vascular endothelial cells; SE, standard error; RT-qPCR, real-time quantitative-PCR; VEGF, vascular endothelial growth factor.
The in vivo functional role of IFI27 on CCA cell growth and angiogenesis
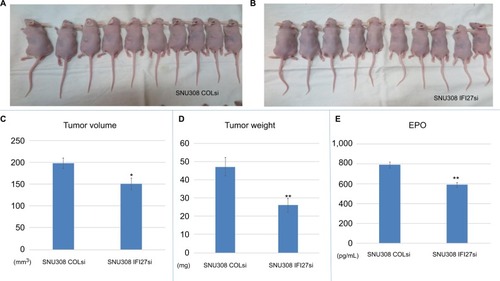
To determine the cell growth of SNU308-IFI27si and SNU308-COLsi cells in vivo, two cells were xenografted into two groups of nude mice (). After 4 weeks, the tumor volume, weight, and EPO concentration were measured. As shown in and , the tumors derived from SNU308-IFI27si cells had much smaller tumor volume and tumor weight than tumors from SNU308-COLsi cells. The EPO concentration in tumors from SNU308-IFI27si cells was around 587 pg/mL compared to 786 pg/mL of tumors from SNU308-COLsi cells (), indicating a less amount of vessel formation inside the tumor mass. These results demonstrated that IFI27 knockdown could also attenuate in vivo CCA cell growth and angiogenesis.
Figure 4 IFI27 knockdown repressed SNU308 cell growth and angiogenesis in vivo.
Notes: (A and B) SNU308-COLsi and SNU308-IFI27si cells were xenografted into the nude mice. (C–E) Four weeks later, the tumors were harvested. The volume, weight, and EPO concentration of tumors are measured (*P<0.05, **P<0.01).
Abbreviations: EPO, erythropoietin; IFI27, interferon α-inducible protein 27.
IFI27 overexpression in CCA cells promoted proliferation, migration, and matrix invasion
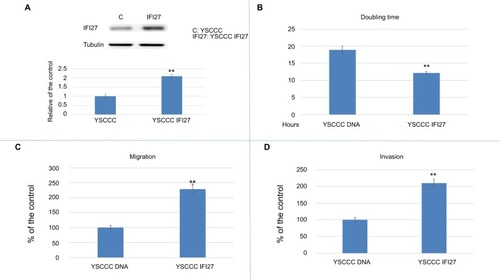
To evaluate the effect of IFI27 overexpression in CCA cells, YSCCC cells, like the other CCA cells expressing lower level of IFI27 compared to that of SNU308 cells were transfected with recombinant IFI27 gene, and cells with increased IFI27 protein expression () were selected to perform the following experiments. As expected, IFI27 overexpression promoted the cell proliferation rate of YSCCC cells (). The doubling time of YSCCC IFI27 cells (YSCCC with IFI27 overexpression) was 12.1 hours, while the doubling time of YSCCC DNA cells (YSCCC with IFI27 mock overexpression) was 18.9 hours. IFI27 overexpression also markedly increased the cell migration and matrix invasion of YSCCC IFI27 cells to 229% and 210%, respectively, comparted to YSCCC DNA cells ( and ).
Figure 5 The effect of IFI27 overexpression in YSCCC cell proliferation and metastasis.
Notes: (A) Western blot to show IFI27 expression in YSCCC DNA and YSCCC IFI27 cells (upper panel) (cropped). Quantitative result of Western blot. Each value was a mean ± SD of three independent determinations relative to the YSCCC DNA cell group (YSCCC DNA cell group was set as 1). (B) The doubling time of YSCCC DNA and YSCCC IFI27 cells. (C) The migration ability (24 hours) of YSCCC DNA and YSCCC IFI27 cells. Data were presented as mean percentage relative to the YSCCC DNA cell group (± SD). Experiments were performed in triplicate and repeated at least thrice. (D) The invasion ability (48 hours) of YSCCC DNA and YSCCC IFI27 cells. Data were presented as mean percentage relative to the YSCCC DNA cell group (± SD). Experiments were performed in triplicate and repeated at least thrice (*P<0.05, **P<0.01).
Abbreviation: IFI27, interferon α-inducible protein 27.
Survival and prognostic analysis of CCA patients
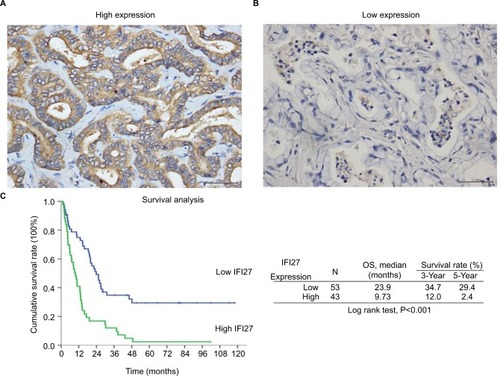
The expression of IFI27 was then analyzed in 96 CCA patient specimens (all intrahepatic CCA) and 43 of them (45%) revealed high cytoplasmic immunostaining for IFI27 (H-score ≥160, ). Less intense staining of IFI27 expression was found in other specimens (). Interestingly, overexpression of IFI27 was found to be independently associated with symptoms and tumor size >5 cm (). Univariate log-rank analysis identified the following factors as adverse influences on OS: presence of symptoms, elevated ALP, decreased albumin, CAE >5, tumor size >5 cm, positive surgical margin and lymph node status, and high IFI27 immunostaining (). Multivariate Cox proportional hazard analysis demonstrated that elevated ALP, non-curative hepatectomy, and high IFI27 immunostaining independently predicted an inferior OS rate for MF mass-forming-CCA patients after hepatectomy (, ).
Figure 6 High IFI27 expression correlated with worse OS in MF-CCA patients.
Notes: (A) The representative picture of high IFI27 expression. (B) The representative picture of low IFI27 expression. (C) The Kaplan–Meier plot of OS in patients with CCA after surgery based on IFI27 expression levels (scale bar =50 µm).
Abbreviations: CCA, cholangiocarcinoma; IFI27, interferon α-inducible protein 27; MF, mass-forming; OS, overall survival.
Discussion
In this current study, we show for the first time that IFI27 functions as on oncogene in CCA. Knockdown of IFI27 in CCA cells would lead to cell growth attenuation in vitro through cell cycle arrest in S-phase. Further, the migration and invasion ability of CCA cells would be decreased with IFI27 knockdown, which was found to repress EMT and F-actin synthesis. VEGF-A expression and secretion were also downregulated by IFI27 knockdown. Furthermore, in vivo xenograft animal study demonstrated that tumor growth and vascularity were less in CCA cells with IFI27 knock-down. Higher IFI27 expression indicated poorer prognosis of CCA patients as determined by IHC staining. Our results clearly show that IFI27-targeting therapy could be deemed as a promising direction for CCA treatment.
To grow cell needs reproduction entailing DNA replication and division of the cytoplasm and nucleus, which is now known as cell cycle consisting of G0, G1, S, G2, and M phases. Cell cycle is under strict control in normal circumstance to maintain homeostasis. Once on deregulation the cells may sustain uncontrolled proliferation. Due to excess mitogenic signaling stimulation, cancer cells tend to have cell cycle disturbance, leading to the unlimited growth. Citation21 Previously, Hsieh et al demonstrated that IFI27 knockdown in keratinocytes could slow cell cycle progression at S-phase,Citation20 which is in line with our result shown in indicating that IFI27 knockdown could induce a S-phase cell cycle arrest in SNU308 cells. The lower cell growth rate of SNU308-IFI27si cells was further supported by the longer cell doubling time and lower cell proliferative rate shown in and . Similar results are found in and , demonstrating that after we xenografted SNU308-IFI27si cells into the nude mice, tumors derived from SNU308-IFI27si cells also had lower tumor volume and lighter tumor weight. In addition, overexpression of IFI27 in SNU308 cells could increase the cell growth rate ().
Cancer metastasis accounts for 90% of cancer-related death. Citation22 To initiate metastasis, cancer cells need to get abilities of migration and invasion, which let cancer cells move from one place to another place. SNU308-IFI27si cells had lower migration and invasion ability compared to IFI27 COLsi cells ( and ), indicating IFI27 knockdown could attenuate SNU308 cell metastatic potential. To the contrary, overexpression of IFI27 could increase CCA cell metastasis ( and ). EMT is a key process during embryonic development. It is also found to be involved in wound healing and tumor cell migration. Citation23 After EMT, cancer cells lose epithelial cell characteristics and gain mesenchymal cell features, which makes cancer cells lose cell–cell adhesion and endows them with the ability to migrate, leading to the higher metastatic potential. Thus, targeting EMT process has emerged as a new therapeutic trend against cancer progression. Citation24 EMT could induce cadherin switch, which means increase of N-cadherin and decrease of E-cadherin. Citation25 At the same time, the mesenchymal cell marker, fibronectin, would increase. Citation26 As shown in , SNU308-IFI27si cells had higher E-cadherin expression and lower N-cadherin and fibronectin expressions than SNU308 cells, indicating that IFI27 knockdown could block EMT process in SNU308 cells, leading to the attenuation of metastatic ability shown in and .
F-actin cytoskeleton networks regulate many vital cellular functions, in addition to maintaining and changing cellular shape. The change of F-actin could generate force contributing to cell migration. Citation27 As shown in , knockdown of IFI27 in SNU308 cells could inhibit F-actin synthesis, leading to the weaker migration ability noted in .
Angiogenesis is crucial for cancer growth and metastasis. It is suggested that prevention of neovascularization is an effective way to keep tumor stay in dormancy. Citation28 Thus, antiangiogenesis therapy has been applied clinically, and successfully prolonged progression of free survival in some cancers. Citation29,Citation30 Since VEGF-A is a strong stimulator for angiogenesis, targeting VEGF-A has emerged as an effective way to treat cancers. Citation10 As shown in , IFI27 knock-down in SNU308 cells reduced VEGF-A mRNA expression, secretion, and reporter activity. To further evaluate the effect of VEGF-A on SNU308 cells, anti-VEGF-A antibodies were used to treat SNU308 cells, which effectively repressed SNU308 cells growth (), indicating IFI27 knock-down inhibited SNU308 cell growth partly medicated by downregulation of VEGF-A.
The analysis of VEGF-A promoter sequence revealed several binding sites for transcriptional factors, including AP-1 and AP-2 (c-jun and c-fos). Citation31 showed that c-jun and c-fos were downregulated with IFI27 knockdown in SNU308 cells, implicating that IFI27 knockdown repressed VEGF-A through inhibition of c-jun and c-fos. It has previously been shown that VEGF-A is able to stimulate HUVECs growth. Citation32 The cultured mediums from SNU308-IFI27si cells and SNU308-COLsi cells were then applied to treat HUVECs. It is obvious that HUVECs cultured with the medium from SNU308-IFI27si cells had lower cell proliferative rate (). To further investigate the effect of IFI27 on angiogenesis, EPO concentrations of the xenografted tumors were measured. showed that tumors derived from SNU308-IFI27si cells group had lower EPO concentration than that from SNU308-COLsi cells group.
The finding that 43 out of 96 CCA patients expressed high IFI27 demonstrates the IFI27 expression in CCA. It is interesting to find that high IFI27 expression was associated with symptoms and large tumor size (>5 cm) (). After multivariate Cox proportional hazard analysis, elevated ALP, non-curative hepatectomy, and high IFI27 immunostaining were identified as predictors for inferior OS of CCA patients after surgery ( and ). For the first time it was shown that IFI27 expression could correlate with survival of cancer patients.
Conclusion
Since currently available therapies only have limited effect on CCA, finding new therapeutic method for CCA patients is urgently needed. Our results show that IFI27 could increase tumor growth, angiogenesis, and metastasis. VEGF-A is one of the downstream genes of IFI27. Higher IFI27 expression is associated with poorer OS in CCA patients after surgery. Collectively, our data suggest that IFI27 is a novel oncogene for human CCA. Targeting IFI27 therapy has the potential to become a new CCA therapeutic direction.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by CMRPG2F0111-3 to Kun-Chun Chiang.
Supplementary materials
The primary antibodies used in this study were monoclonal antibodies against cIFI27 (1:1,000, H00003429-D01P; Abnova, Taipei, Taiwan R.O.C.), c-jun (1:1,000, #9165; Cell Signaling Technology, Danvers, MA, USA), c-fos (1:1,000, #2250; Cell Signaling Technology), E-cadherin (1:1,000, #3195; Cell Signaling Technology), N-cadherin (1:1,000, #2189; Cell Signaling Technology), and Fibronectin (1:500, MAB1918; R&D Systems Inc.).
The primer sequences used in this study
5′->3′
IFI27-sense GTGCCCATGGTGCTCAGT
IFI27-antisense CCAGTGACTGCAGAGTAGCC
VEGFA-sense ATTATGCGGATCAAACCT
VEGFA-antisense TTCTTGTCTTGCTCTATCTT
Disclosure
The authors report no conflicts of interest in this work.
References
- DeoliveiraMLCunninghamSCCameronJLCholangiocarcinoma: thirty-one-year experience with 564 patients at a single institutionAnn Surg2007245575576217457168
- CharbelHAl-KawasFHCholangiocarcinoma: epidemiology, risk factors, pathogenesis, and diagnosisCurr Gastroenterol Rep201113218218721271364
- DohertyBNambudiriVEPalmerWCUpdate on the diagnosis and treatment of cholangiocarcinomaCurr Gastroenterol Rep2017191228110453
- CunninghamSCChotiMABellavanceECPawlikTMPalliation of hepatic tumorsSurg Oncol200716427729117935975
- CheriyathVLeamanDWBordenECEmerging roles of FAM14 family members (G1P3/ISG 6–16 and ISG12/IFI27) in innate immunity and cancerJ Interferon Cytokine Res201131117318120939681
- RasmussenUBWolfCMatteiMGIdentification of a new interferon-alpha-inducible gene (p27) on human chromosome 14q32 and its expression in breast carcinomaCancer Res19935317409641018358738
- SuomelaSCaoLBowcockASaarialho-KereUInterferon alpha-inducible protein 27 (IFI27) is upregulated in psoriatic skin and certain epithelial cancersJ Invest Dermatol2004122371772115086558
- KuoYYJimWTSuLCCaffeic acid phenethyl ester is a potential therapeutic agent for oral cancerInt J Mol Sci2015165107481076625984601
- AlbiniATosettiFLiVWNoonanDMLiWWCancer prevention by targeting angiogenesisNat Rev Clin Oncol20129949850922850752
- MariottoABYabroffKRShaoYFeuerEJBrownMLProjections of the cost of cancer care in the United States: 2010–2020J Natl Cancer Inst2011103211712821228314
- RibattiDTumor refractoriness to anti-VEGF therapyOncotarget2016729466684667727081695
- YoshikawaDOjimaHIwasakiMClinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinomaBr J Cancer200898241842518087285
- PengHZhangQLiJApatinib inhibits VEGF signaling and promotes apoptosis in intrahepatic cholangiocarcinomaOncotarget2016713172201722926967384
- TsaiMYYangRCWuHTPangJHHuangSTAnti-angiogenic effect of tanshinone IIA involves inhibition of matrix invasion and modification of MMP-2/TIMP-2 secretion in vascular endothelial cellsCancer Lett2011310219820621788102
- ChiangKCYehCNChenHY19-Nor-2α-(3-hydroxypropyl)-1α,25-dihydroxyvitamin D3 (MART-10) is a potent cell growth regulator with enhanced chemotherapeutic potency in liver cancer cellsSteroids201176131513151921888924
- OuyangWMaQLiJCyclin D1 induction through IkappaB kinase beta/nuclear factor-kappaB pathway is responsible for arsenite-induced increased cell cycle G1-S phase transition in human keratinocytesCancer Res200565209287929316230390
- TsuiKHChungLCFengTHChangPLJuangHHUpregulation of prostate-derived Ets factor by luteolin causes inhibition of cell proliferation and cell invasion in prostate carcinoma cellsInt J Cancer2012130122812282321780100
- ChiangKCChenSCYehCNMART-10, a less calcemic vitamin D analog, is more potent than 1α,25-dihydroxyvitamin D3 in inhibiting the metastatic potential of MCF-7 breast cancer cells in vitroJ Steroid Biochem Mol Biol2014139546024125734
- ChiangKCKuoSFChenCHMART-10, the vitamin D analog, is a potent drug to inhibit anaplastic thyroid cancer cell metastatic potentialCancer Lett20153691768526282787
- HsiehWLHuangYHWangTMMingYCTsaiCNPangJHIFI27, a novel epidermal growth factor-stabilized protein, is functionally involved in proliferation and cell cycling of human epidermal keratinocytesCell Prolif201548218719725664647
- VermeulenKVan BockstaeleDRBernemanZNThe cell cycle: a review of regulation, deregulation and therapeutic targets in cancerCell Prolif200336313114912814430
- GuptaGPMassaguéJCancer metastasis: building a frameworkCell2006127467969517110329
- GuarinoMRubinoBBallabioGThe role of epithelial-mesenchymal transition in cancer pathologyPathology200739330531817558857
- PalenaCFernandoRIHamiltonDHAn immunotherapeutic intervention against tumor progression: targeting a driver of the epithelial-to-mesenchymal transitionOncoimmunology201431e2722024575384
- WheelockMJShintaniYMaedaMFukumotoYJohnsonKRCadherin switchingJ Cell Sci2008121672773518322269
- ChristiansenJJRajasekaranAKReassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasisCancer Res200666178319832616951136
- StrickerJFalzoneTGardelMLMechanics of the F-actin cytoskeletonJ Biomech201043191419913792
- FolkmanJTumor angiogenesis: therapeutic implicationsN Engl J Med197128521118211864938153
- EbosJMKerbelRSAntiangiogenic therapy: impact on invasion, disease progression, and metastasisNat Rev Clin Oncol20118421022121364524
- JaysonGCHicklinDJEllisLMAntiangiogenic therapy--evolving view based on clinical trial resultsNat Rev Clin Oncol20129529730322330688
- TischerEMitchellRHartmanTThe human gene for vascular endothelial growth factor. multiple protein forms are encoded through alternative exon splicingJ Biol Chem19912661811947119541711045
- TsuiKHLinYHChungLCProstate-derived Ets factor represses tumorigenesis and modulates epithelial-to-mesenchymal transition in bladder carcinoma cellsCancer Lett2016375114215126965996
