Abstract
Background:
Toll-like receptor 4 (TLR4), a member of the pattern recognition receptors, has been reported to be involved in carcinogenesis. However, the clinical impact of TLR4 in peripheral T-cell lymphomas (PTCL) remains unclear.
Methods:
The current study, using immunohistochemical staining, first examined TLR4 and programmed cell death-ligand 1 (PD-L1) expression in patients with PTCL, to correlate TLR4 and PD-L1 expression with clinicopathological parameters.
Results:
It was found that the rates of high expression of TLR4 and PD-L1 were 41.7% and 45.8%, respectively. TLR4 expression was closely associated with PD-L1 expression. The expression of TLR4 was closely related to primary extranodal site involvement, increased Ann Arbor stage, and low hemoglobin expression, while the expression of PD-L1 was closely related to a low platelet count and multiple extranodal organ involvements (>1). High expression of either TLR4 or PD-L1 indicated a poor survival rate for patients with PTCL. Multivariate analyses further confirmed that increased expression levels of TLR4 and PD-L1 are unfavorable prognostic factors for PTCL.
Conclusion:
This study demonstrates that the expressions of TLR4 and PD-L1 are independent predictors of survival time for patients with PTCL. Thus, TLR4 and PD-L1 may serve as potential therapeutic targets in PTCL patients.
Introduction
Peripheral T-cell lymphomas (PTCL) are a heterogeneous group of mature T-cell neoplasms whose aggressiveness may vary depending on the subtype.Citation1 PTCL display a wide geographical heterogeneity that accounts for 22–25% of all aggressive lymphomas and 10–15% of all the non-Hodgkin lymphomas (NHLs) in China, which is a significantly higher proportion than that of European countries.Citation2 Although targeted therapies and comprehensive treatments have made rapid progress, the outcome of PTCL still remains poor. With the notable exception of ALK+ anaplastic large cell lymphoma (ALCL), the prognosis of most PTCL subtypes is extremely poor, with a 5-year overall survival (OS) of approximately 15–30% reported in most studies.Citation3 Thus, the treatment of PTCL is becoming the most challenging area for clinicians.
As the first identified human Toll homolog, toll-like receptor 4 (TLR4) is a critical player in both innate and adaptive immunity.Citation4,Citation5 Initially, TLR4 expression was thought to be restricted to immune cells, but recent studies have shown that TLR4 is also highly expressed in some types of cancer cells, including colon cancer,Citation6,Citation7 pancreatic ductal adenocarcinoma,Citation8 non-small cell lung cancer,Citation9 and hepatocyte carcinoma.Citation10 Some researchers have shown that high TLR4 expression is related to poor prognosis in malignant diseases,Citation6,Citation9,Citation11 while others have failed to come to a similar conclusion.Citation8 For PTCL, the significance of TLR4 expression has not been evaluated.
Programmed cell death ligand 1 (PD-L1), a ligand for the T-cell inhibitory receptor PD-1, is a key immunosuppressive molecule by which cancer avoids eradication by the immune system.Citation12,Citation13 PD-L1 expression has been found to be positive in 5–40% of tumor cells,Citation14,Citation15 and increased expression of PD-L1 is closely related to poor survival rate in some solid cancer patients.Citation16,Citation17 We and others also demonstrated that overexpression of PD-L1 indicate a worse survival rate for patients with DLBCL.Citation18,Citation19 However, the significance of PD-L1 expression in PTCL remains unclear.
In the current study, using immunohistochemical staining, we analysed TLR4 and PD-L1 expression in patients with PTCL and compared these data with the data regarding clinicopathological features and survival of these patients. The relationship between TLR4 and PD-L1 was also evaluated in this study. To our knowledge, the current study is the first to investigate the relationship between TLR4 and PD-L1 expression in a relatively large population with PTCL.
Materials and methods
Patients and tissue specimens
From January 2006 to October 2015, a total of 144 patients diagnosed with PTCL at the Harbin Medical University Cancer Hospital were enrolled in this study. As nasal NK/T-cell lymphoma (NKTCL) is considered a distinct disease entity associated with specific viral infections, the recommended first-line treatment strategies are different from those of other T-cell lymphomas.Citation20 Therefore, this study focused on PTCL excluding NKTCL. All cases were reviewed according to present World Health Organization criteria (WHO 2016).Citation21 Each enrolled patient had complete clinicopathological and follow-up data, including patients’ age, sex, histological type, and international prognostic index (IPI) score. The research was approved by the ethics committee of the Harbin Medical University Cancer Hospital. The patients provided written informed consent, and this study was conducted in accordance with the Declaration of Helsinki.
mRNA expression profiles
Gene expression datasets (GSE6338) were downloaded from Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) (Affymetrix GPL570 platform, Affymetrix Human Genome U133 Plus 2.0 Array).Citation22 We analyzed fivePTCL samples (GSM146143–GSM146147) and fivenormal T-cell samples (GSM146182–GSM146186) using the R Statistical Package (R version 3.4.3), which differentially expressed genes (DEGs) in PTCL.
Functional and pathway enrichment analyses
To analyze the function of DEGs, biological analyses were performed using DAVID online database (https://david.ncifcrf.gov/),Citation23 which consisted of the Gene Ontology (GO) database and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. The protein–protein interaction (PPI) network was predicted using the Search Tool for the Retrieval of Interacting Genes (STRING; https://string-db.org/) (version 10.5) online database and constructed PPI network using the Cytoscape (version 3.6.1).
Determination of TLR4 and PD-L1
For immunohistochemistry (IHC) analysis, 3-μm-thick formalin-fixed paraffin-embedded sections were submitted. Briefly, one representative section of the tissue was cut and placed on poly-L-lysine coated slides. The slides were deparaffinized, dehydrated, immersed in 10 mM sodium citrate buffer (pH 6.0), and pretreated in a microwave oven for 10 minutes. After blocking with 3% hydrogen peroxide for 10 minutes at room temperature, the slides were incubated at 4°C overnight with primary antibodies for anti-TLR4 (ab-22048, dilution 1:100, Abcam, Cambridge, UK) and anti-PD-L1 (ab-205921, dilution 1:100, Abcam). Afterwards, the slides were stained using the Mouse and Rabbit Specific HRP/DAB Detection IHC Kit (ab80436, Abcam,) and IHC detection kit HRP/DAB (ab209101, Abcam). After visualization of the reaction with DAB chromogen, the slides were counterstained with hematoxylin and covered with a glycerin gel. For negative controls, the primary antibody was substituted with PBS to confirm the specificity of the primary antibody.
Evaluation of immunohistochemical staining
Tumor sections were evaluated to determine a consensus diagnosis by two pathologists who were blinded to the identity of the tissue. IHC score was acquired by the semi-quantitative method of taking both the staining intensity and the proportion of cells stained into account. For TLR4, the staining intensity was scored into four categories according to the color of the immune reaction: no color, 0; light brown, 1; brown, 2; and dark brown, 3. The proportion of positively stained cells was determined and scored as: ≤5%, 0; 6–25%, 1; 26–50%, 2; 51–75%, 3; and 76–100%, 4. The expression level of TLR4 was obtained by multiplying the intensity and proportion score. A final score of 0–2 was considered negative for TLR4 expression (−, noted as 0); 3–4: weak positive expression (+, noted as 1); 6–8: moderate positive expression (++, noted as 2); and 9–12 was strong positive expression (+++, noted as 3).Citation24 Scores 0 and 1 were defined as low expression, and scores 2 and 3 were defined as high expression. For PD-L1, based on previous studies, the samples with <5% stained tumor cells were defined as negative (−, scored as 0).Citation25 For >5% positive PD-L1 stained samples, the expression levels were further classified into three groups: weak staining (light brown, 1+, noted as 1), moderate staining (brown, 2+, noted as 2), and intense staining (dark brown, 3+, noted as 3) according to staining intensity.Citation26 Samples with negative and weak staining were defined as low expression, and samples with moderate and intense staining were defined as high expression for PD-L1.
Statistics
The statistical analyses were performed using SPSS version 23.0 (IBM Corporation, New York, NY, USA). The relationships or correlations between the groups were assessed using Fisher’s exact test or Spearman’s correlation test. Cox-regression analyses, both univariate and multivariate, were used to identify the independency of the expression status of these two proteins. Progression-free survival (PFS) was measured from the start of therapy to the first observation of objective disease relapse, progression, or death due to any cause. OS was measured from the date of diagnosis to the date of death from any cause. PFS and OS were determined by the Kaplan-Meier method and compared using log-rank test. A two-tailed P-value <0.05 was considered statistically significant.
Results
Identification of differentially expressed genes (DEGs) in PTCL
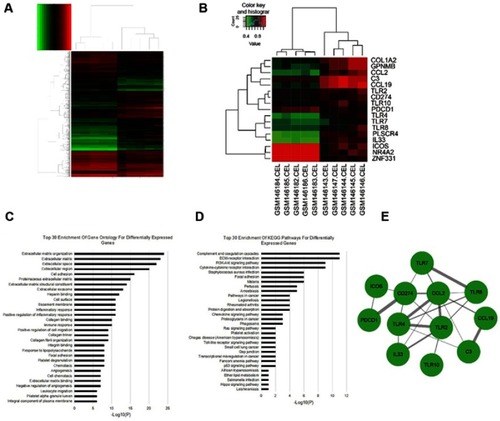
We analyzed DEGs in PTCL compared with normal T-cell controls from the GEO database by bioinformatics analysis. A total of 2009 DEGs were identified, consisting of 993 downregulated genes and 1,016 upregulated genes (fold change ﹥2, FDR ﹤0.01, P﹤0.001) (). We found CD274 (PD-L1), PDCD1 (PD-1), and multiple TLRs (TLR2, TLR4, TLR8, TLR10) were upregulated (). To analyze the biological classifcation of those DEGs, functional and pathway enrichment analyses were performed using DAVID. GO analysis results showed that DEGs were signifcantly enriched in positive regulation of inflammatory response, immune response, cell adhesion, cell migration, and chemotaxis (). KEGG pathway analysis revealed that the upregulated DEGs were mainly enriched in ECM-receptor interaction, PI3K-Akt signaling pathway, chemokine signaling pathway, Ras signaling pathway, TLR signaling pathway, transcriptional misregulation, and proteoglycans in cancer (). The PPI network of DEGs was constructed, in which we found TLR4 had interactions with CD274 (PD-L1) (combined score=0.496) ().
Figure 1 Gene expression profiling analysis in PTCL. (A) Hierarchical clustering of gene expression profiles generated from five AITL samples and five normal T-cell samples. DEGs were selected with a fold change >2 and P-value < 0.001 among the mRNA expression profiling; (B) Ten samples are clustered according to the expression of 17 differentially expressed genes; (C and D) Top 30 enrichment of GO terms and KEGG pathways for differentially expressed genes; (E) The PPI network of 17 DEGs was constructed. Red cycle nodes represent genes, and the edges represent interaction between genes, the width of edges represents the combined score.
Clinical results
To verify the relationship between TLR4 and PD-L1 expression, we first examined the expression of TLR4 and PD-L1 in PTCL. A total of 144 PTCL tissue specimens were collected in this study (). There were 93 (64.6%) males and 51 (35.4%) females, with a median age of 58 years (range=12–82 years). According to the 2016 revision of the WHO classification of lymphoid neoplasms, 17 patients (11.8%) were diagnosed with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 88 patients (61.1%) with angioimmunoblastic T-cell lymphoma (AITL), 20 patients (13.9%) with ALK+ anaplastic large cell lymphoma (ALCL), and 19 patients (13.2%) with ALK− ALCL. According to the Ann Arbor classification system, 39 (27.1%) patients had limited-stage disease (Ann Arbor stage I/II), whereas 105 patients (72.9%) had advanced stage disease (stage III/IV). At the time of diagnosis, 81 patients (56.3%) had B symptoms, and 69 patients (47.9%) had increased levels of lactate dehydrogenase (LDH). A total of 126 patients (87.6%) received systemic chemotherapy, including anthracycline-containing regimens. Sixteen patients (11.1%) received no treatment, and no treatment data were available for two patients (1.3%).
Table 1 Correlation between TLR4 and PD-L1 expression and various clinicopathological features
TLR4 and PD-L1 expression in PTCL
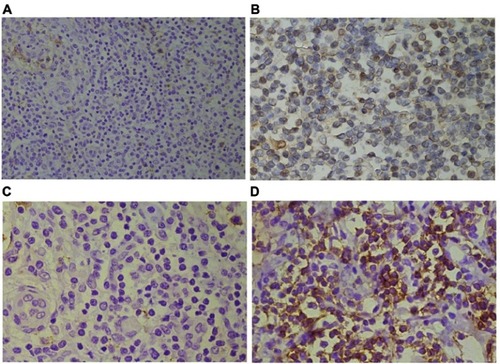
Immunohistochemical staining was performed to determine the expression level of TLR4 and PD-L1 in PTCL. TLR4 is a specific exogenous receptor for LPS, which plays a vital role in pathogen recognition and activation of the innate immune system. TLR4 is normally expressed in immune cells, such as dendritic cells and macrophage, but not on normal T-cells. On the other hand, activated T-cells can express PD-1 rather than its ligand, PD-L1. As shown in , TLR4 positive staining was localized to the cytomembrane of the carcinoma. Of 144 PTCL specimens, samples from 84 patients were classified as TLR4 low expression (58.3%) (), and the rest (n=60) were classified as TLR4 high expression (). Immunohistochemistry with PD-L1 showed positive staining was mainly located on the cytomembrane of the tumor cells. Seventy-eight (54.2%) were classified as being PD-L1 low expression (), and the rest (n=66) were classified as PD-L1 high expression ().
Correlations between TLR4 and PD-L1 expression and various clinicopathological features
Correlations between TLR4 expression and various clinicopathological features are summarized in . TLR4 expression was associated with a primary site (extranodal more than nodal, P=0.007), increased Ann Arbor stage (P=0.039), and lower HGB level (P=0.005). No statistical associations were observed between TLR4 expression and age, gender, histological type, tumor size, number of extranodal involvements, B symptoms, ECOG score, IPI score, LDH level, PLT count, leukocyte count, or Ki-67 expression. The relationships between PD-L1 expression and various clinicopathological features were also analyzed in this study. We found no significant correlations between PD-L1 expression and age, gender, primary site, tumor size, histological type, Ann Arbor stage, ECOG score, IPI score, B symptom, LDH level, HGB level, leukocyte count, or Ki-67. PD-L1 expression, however, was significantly associated with the number of extranodal involvements (P=0.034) and increased PLT count (P=0.031).
In addition, we further analyzed the expression differences of TLR4 and PD-L1 in different histological types of PTCL. As shown in , the proportion of TLR4 high expression in PTCL-NOS was 58.8%, 39.8% in AITL, 50% in ALK+ ALCL, and 26.3% in ALK− ALCL. The rate of TLR4 high expression seemed higher in PTCL-NOS and ALK+ ALCL groups. However, no statistical difference was found between any two groups. The proportion of PD-L1 high expression in PTCL-NOS was 58.8%, 50% in AITL, 30% in ALK+ ALCL, and 31.6% in ALK− ALCL. The rate of PD-L1 high expression was higher in PTCL-NOS and AITL groups. Similarly, there was no statistical difference in PD-L1 expression between any two groups (data not shown).
Correlation between TLR4 and PD-L1 expression
We further discussed the relationship between TLR4 and PD-L1 expression. Among the TLR4 high expression group (n=60), 45 samples were PD-L1 high expression (75%), and 15 were PD-L1 low expression (25%). In contrast, among the TLR4 low expression group (n=84), only 21 were PD-L1 high expression (25%), and 63 were PD-L1 low expression (75%). The expression of TLR4 in PTCL tissue was significantly associated with PD-L1 expression (P<0.001) ().
Table 2 Correlation between TLR4 expression and PD-L1 expression
Correlations between TLR4 and PD-L1 expression and survival in patients with PTCL
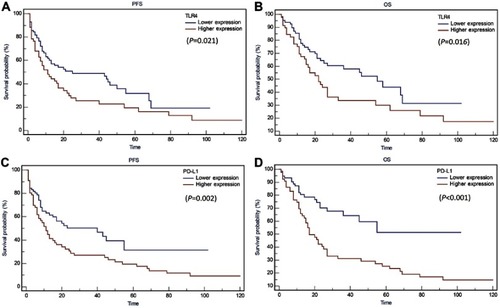
Kaplan-Meier survival curves are shown in . High TLR4 expression was significantly associated with worse progression-free survival (PFS) (10 vs. 8 months, P=0.021) and overall survival (OS) (21 vs. 15 months, P=0.016) (). Similarly, we found that the prognosis of PD-L1 high expression cancer patients was significantly poorer than that of PD-L1 low expression cancer patients with regard to PFS (11 vs. 7.5 months, P=0.002) and OS (19.5 vs. 15.5 months, P<0.001) ().
Figure 3 Kaplan Meier analysis for PFS and OS based on TLR4 and PD-L1 expression in PTCL patients. (A) Kaplan-Meier analysis for PFS based on TLR4 expression in patients with PTCL (log-rank test, P=0.021); (B) Kaplan-Meier analysis for OS based on TLR4 expression in patients with PTCL (log-rank test, P=0.016); (C) Kaplan-Meier analysis for PFS based on PD-L1 expression in patients with PTCL (log-rank test, P=0.002); (D) Kaplan-Meier analysis for OS based on PD-L1 expression in patients with PTCL (log-rank test, P<0.001).
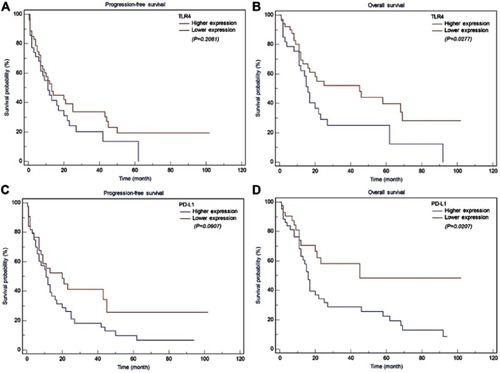
PTCL represent a heterogeneous group of diseases, including more than 20 subtypes according to WHO classification. Each subtype may have a different physiopathology, including TLR4 and PD-L1 expression. We further explored the effect of TLR4 expression on the prognosis of PTCL with different pathological types. As shown in , we found that, for patients with AITL, the prognosis of TLR4 and PD-L1 high expression was significantly poorer with regard to OS (P=0.027 and P=0.021, respectively), but not to PFS. For other pathological types, we found that the survival time of patients with low TLR4 and PD-L1 expression also tended to be prolonged. However, there was no statistical difference found for the small sample size (data not shown).
Figure 4 Kaplan Meier analysis for PFS and OS based on TLR4 and PD-L1 expression in AITL patients. (A) Kaplan-Meier analysis for PFS based on TLR4 expression in patients with AITL (log-rank test, P=0.208); (B) Kaplan-Meier analysis for OS based on TLR4 expression in patients with AITL (log-rank test, P=0.027); (C) Kaplan-Meier analysis for PFS based on PD-L1 expression in patients with AITL (log-rank test, P=0.091); (D) Kaplan-Meier analysis for OS based on PD-L1 expression in patients with AITL (log-rank test, P=0.021).
Univariate and multivariate analyses of TLR4 and PD-L1 expression with clinicopathological variables
Univariate analysis of PFS identified TLR4-positive expression (P=0.035), PD-L1 high expression (P=0.023), increased Ann Arbor stage (P=0.006), presenting with B symptoms (P=0.026), and lower leukocyte count (P=0.036) as significantly unfavorable prognostic predictors. Age, gender, primary site, tumor size, number of extranodal involvement, ECOG score, IPI score, LDH and HGB levels, PLT count, and Ki-67 had no prognostic value. Multivariate analysis was performed on the same set of patients. The result indicated that TLR4 and PD-L1 status, B symptoms, and Leukocyte count were independent poor prognostic factors ().
Table 3 Prognostic factors affecting the progression free survival of patients with PTCLs
Univariate analysis of OS identified TLR4 expression (P=0.033), PD-L1 expression (P<0.001), age (P=0.031), gender (P=0.026), increased Ann Arbor stage (P=0.013), ECOG score (P=0.016), IPI score (P=0.009), and leukocyte count (P=0.021) as significant prognostic predictors. Multivariate analysis indicated that TLR4 and PD-L1 status, gender, IPI score, and leukocyte count were independent unfavorable prognostic factors ().
Table 4 Prognostic factors affecting the overall survival of patients with PTCLs
Discussion
The expression of TLR4 and PD-L1 in PTCL has been largely unstudied, and, to our knowledge, there are no studies that specifically investigate the expression of TLR4 and PD-L1 in PTCL. Our study is the first to examine the expression of these two molecules in PTCL by immunohistochemical staining. The results showed that the TLR4 expression level in PTCL tissue was positively correlated with the PD-L1 expression. Elevated TLR4 expression was significantly associated with primary site, increased Ann Arbor stage, and lower HGB levels, whereas PD-L1 expression was significantly associated with the number of extranodal involvements and the higher PLT count. Multivariate analysis further confirmed that both TLR4 and PD-L1 were independent unfavorable prognostic factors and that overexpression of either TLR4 or PD-L1 indicated a poor OS and PFS for PTCL patients.
Currently, the role of inflammation in tumorigenesis and cancer progression has become evident, and it is generally accepted that an inflammatory microenvironment is an essential component of all tumors.Citation27 TLR4, the receptor for lipopolysaccharide (LPS), primarily induces inflammatory cytokines in immune cells,Citation28 but it is also involved in carcinogenesis and cancer cell survival. Although TLR4 expression has been reported in a variety of tumors,Citation8,Citation9,Citation11,Citation29 TLR4 expression in patients with PTCL had not been studied. In the present study, we found that high expression of TLR4 was observed in 41.7% of all PTCL patients (60/144), and most frequently in patients with PTCL-NOS (58.8%) and ALK+ ALCL (50.0%). More specifically, we found that the expression of TLR4 in primary extranodal PTCL (64.3%) was significantly higher than that in primary nodal PTCL (36.2%). Extranodal NHLs account for approximately one-third of all NHLs. Data from large studies reported in the literature have shown that the gastrointestinal (GI) tract, skin, nasopharynx, and bone are the most common sites of ENHL.Citation30,Citation31 These sites are in contact with the outside world and are more susceptible to infection by pathogenic microorganisms, resulting in the production of inflammation. The GI tract is the most frequently involved site of extranodal localization, accounting for 30–40% of extranodal lymphomaCitation32,Citation33 and from 4–20% of all NHL cases,Citation34 which are associated with chronic Helicobacter pylori (H. pylori) infection in two-thirds of cases.Citation35 The GI tract is not the most common site of extranodal involvement in PTCL. In this study, five patients were diagnosed as primary gastrointestinal lymphoma, of which three were PTCL-NOS, one was ALK− ALCL and one was ALK+ ALCL. All of the above five patients had high expression of TLR4. In addition, Epstein-Barr virus (EBV) infection was observed in 50–60% of nasopharyngeal lymphoma.Citation36,Citation37 We speculate that infection can lead to chronic inflammation, which is one of the important reasons to promote the expression of TLR4.
As a ligand on tumor cells for the checkpoint receptor PD-1 on the surface of T-cells, tumor-associated PD-L1 has been shown to inhibit tumor-specific T-cell-mediated immunity and cytotoxic T lymphocytes (CTL)-mediated lysis by interacting with PD-1 and other potential unknown receptors on T-cells to induce T-cell apoptosis and impair cytokine production.Citation38–Citation40 Moreover, it has been reported that overexpression of PD-L1 is closely associated with the poor prognosis in several tumors, including renal cell carcinoma, esophageal cancer, and malignant melanoma.Citation41–Citation45 However, clinical significance of PD-L1 expression in PTCL is still unknown. In the current study, we found that PD-L1 expression was observed in 45.8% of all PTCL patients (66/144), most frequently in patients with PTCL-NOS (58.8%) and AITL (50.0%). Moreover, we found that the expression of PD-L1 in TLR4-positive PTCL was significantly higher than that in TLR4-negative PTCL. In TLR4-positive specimens, 75.0% (45/60) of patients were PD-L1-positive, and 25.0% (15/60) of patients were PD-L1-negative. In contrast, in TLR4-negative specimens, only 25.0% (21/84) of patients were PD-L1-positive, and 75.0% (63/84) patients were PD-L1-negative (). The data shown here clearly demonstrates that PD-L1 expression is closely associated with TLR4 expression in PTCL (P<0.001). TLR4 activation in Langerhans cells has recently been shown to induce PD-L1 and favor tolerogenic properties in the oral cavity. Beswick et alCitation46 demonstrated that the stimulation of TLR4 on CD90-positive myofibroblasts/fibroblasts (CMF) leads to the NF-ĸB-dependent upregulation of PD-L1, with reinforced CMF-mediated suppression of CD4-positive effector T-cell responses. Wang et alCitation47 also found that activation of TLR4 signaling in bladder cancer cells could induce tumor-associated PD-L1 expression via the activation of Erk and c-Jun N-terminal kinase pathways and that TLR4 signaling protects tumor cells from CTL-mediated killing. PD-L1 is an inducible molecule, in which inflammatory factors, such as IFN-γ, TNF-α, and GM-CSF, are important factors to induce the expression of PD-L1. Our finding is consistent with previous studies in indicating that TLR4 may contribute to PD-L1 expression, by which inhibiting the function of immune effector cells in tumor stroma. We speculate that activation of TLR4 may induce tumor cells to release inflammatory factors and promote the expression of PD-L1 by autocrine pathway. Continuous support of TLR4 for PD-L1 expression may be one of the potential reasons for resistance to immune checkpoint inhibitors targeting the PD-1/PD-L1 pathway. This theory needs to be further validated in future research.
In addition, we found that PD-L1 expression in patients with normal platelet counts (48.5%) was significantly higher than that in patients with thrombocytopenia (11.1%). Recently, Nakayama et al reported that lower platelet counts were associated with worse OS for patients with NHL. Moreover, Piccaluga et alCitation48 suggested that platelet-derived growth factor alpha (PDGFɑ), which was initially found in platelets and is released from platelet alpha particles at the early stages of injury, could promote PTCL cell proliferation. Whether PLT derived liquid factors, such as PDGFɑ, promote the expression of PD-L1 remains to be explored in the future.
In this study, we also explored the effects of TLR4 and PD-L1 expression on the prognosis of PTCL. We found that TLR4 expression in PTCL tissue was negatively associated with the PFS and OS in enrolled patients. Similarly, PD-L1 high expression patients showed significantly lower PFS rates and OS rates when compared with PD-L1 low expression patients. Our observations confirmed that TLR4 and PD-L1 are poor prognostic marker for patients with PTCL. Yu et alCitation49 demonstrated that Fusobacterium nucleatum can activate the TLR4/MyD88 pathway in colorectal cancer cells, promoting resistance to chemotherapy. It has also been reported that triggering of TLR4 on metastatic breast cancer cells could reciprocally regulate the expression of αvβ3 and promote αvβ3-mediated adhesion and invasive migration of the cells.Citation50 These studies are consistent with our experimental results, suggesting that the expression of TLR4 in PTCL affects the proliferation, invasion, and drug resistance of tumor cells, and targeted therapy for TLR4 may become a promising potential therapeutic target for PTCL. Recently, immunotherapy with PD-1 and PD-L1 targeted monoclonal antibodies has dramatically changed the therapeutic and prognostic landscape for several types of malignancy, including metastatic melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma, bladder cancer, head and neck cancer, Hodgkin lymphoma, etc. The detection of PD-L1 expression by IHC has become the most widely used clinical method to predict the efficacy of PD-1/PD-L1 inhibitors. Many studies have shown that both the objective response rate (ORR) and the OS of PD-L1-positive patients after PD-1/PD-L1 inhibitors treatment were higher than those of PD-L1-negative patients. Currently, several clinical trials are going on to estimate the efficacy and safety of PD-1/PD-L1 inhibitors in the treatment of PTCL.Citation51 Our results shown here suggest the potential effectiveness of this therapy.
In conclusion, our study is the first to explore the expression of TLR4 in the context of PTCL and suggests that high TLR4 expression is associated with worse PFS and OS in patients with PTCL. We further demonstrated that PD-L1 expression was negatively correlated with PFS and OS. Furthermore, both highly expressed TLR4 and PD-L1 are correlated with poor prognosis. Therefore, based on the current results, clinical trials will be needed to test combination therapy with multiple checkpoint blockades to enhance antitumor activity. Thus, the potential differential therapeutic implications of these immune checkpoint molecules should be further investigated.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
The study was supported by the Natural Science Foundation of Heilongjiang Province (H2018045, JJ2018ZR167 & ZD2016018).
References
- Laribi K, Alani M, Truong C, Baugier de Materre A. Recent advances in the treatment of peripheral T-cell lymphoma. Oncologist. 2018;23(9):1039–1053. doi:10.1634/theoncologist.2017-052429674443
- Armitage JO. The aggressive peripheral T-cell lymphomas: 2017. Am J Hematol. 2017;92(7):706–715. doi:10.1002/ajh.2479128516671
- Marchi E, Raufi AG, O’Connor OA. Novel agents in the treatment of relapsed or refractory peripheral T-cell lymphoma. Hematol Oncol Clin North Am. 2017;31(2):359–375. doi:10.1016/j.hoc.2016.11.00228340883
- Vijay K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol. 2018;59:391–412. doi:10.1016/j.intimp.2018.03.00229730580
- Wang Z, Yu G, Liu Z, et al. Paeoniflorin inhibits glioblastoma growth in vivo and in vitro: a role for the Triad3A-dependent ubiquitin proteasome pathway in TLR4 degradation. Cancer Manag Res. 2018;10:887–897. doi:10.2147/CMAR.S16029229740218
- Chung YH, Kim D. Enhanced TLR4 expression on colon cancer cells after chemotherapy promotes cell survival and epithelial-mesenchymal transition through phosphorylation of GSK3beta. Anticancer Res. 2016;36(7):3383–3394.27354597
- Hsu RY, Chan CH, Spicer JD, et al. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011;71(5):1989–1998. doi:10.1158/0008-5472.CAN-10-283321363926
- Lanki MA, Seppanen HE, Mustonen HK, et al. Toll-like receptor 2 and Toll-like receptor 4 predict favorable prognosis in local pancreatic cancer. Tumour Biol. 2018;40(9):1010428318801188. doi:10.1177/101042831880118830246618
- Wang K, Wang J, Wei F, Zhao N, Yang F, Ren X. Expression of TLR4 in non-small cell lung cancer is associated with PD-L1 and poor prognosis in patients receiving pulmonectomy. Front Immunol. 2017;8:456. doi:10.3389/fimmu.2017.0045628484456
- Hsiao CC, Chen PH, Cheng CI, et al. Toll-like receptor-4 is a target for suppression of proliferation and chemoresistance in HepG2 hepatoblastoma cells. Cancer Lett. 2015;368(1):144–152. doi:10.1016/j.canlet.2015.08.00426276725
- Hao B, Chen Z, Bi B, et al. Role of TLR4 as a prognostic factor for survival in various cancers: a meta-analysis. Oncotarget. 2018;9(16):13088–13099. doi:10.18632/oncotarget.2417829560134
- Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi:10.1038/s41586-018-0392-830089911
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982. doi:10.1200/JCO.2014.59.435825605845
- Xiang X, Yu PC, Long D, et al. Prognostic value of PD -L1 expression in patients with primary solid tumors. Oncotarget. 2018;9(4):5058–5072. doi:10.18632/oncotarget.2358029435162
- Qu QX, Xie F, Huang Q, Zhang XG. Membranous and cytoplasmic expression of PD-L1 in ovarian cancer cells. Cell Physiol Biochem. 2017;43(5):1893–1906. doi:10.1159/00048410929055949
- Aghajani M, Graham S, McCafferty C, et al. Clinicopathologic and prognostic significance of programmed cell death ligand 1 expression in patients with non-medullary thyroid cancer: a systematic review and meta-analysis. Thyroid. 2018;28(3):349–361. doi:10.1089/thy.2017.044129455638
- Li J, Wang P, Xu Y, Lee JW. Prognostic value of programmed cell death ligand 1 expression in patients with head and neck cancer: a systematic review and meta-analysis. PLoS One. 2017;12(6):e0179536. doi:10.1371/journal.pone.017953628604812
- Zhao S, Zhang M, Zhang Y, et al. The prognostic value of programmed cell death ligand 1 expression in non-Hodgkin lymphoma: a meta-analysis. Cancer Biol Med. 2018;15(3):290–298. doi:10.20892/j.issn.2095-3941.2018.004730197796
- Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126(19):2193–2201. doi:10.1182/blood-2015-02-62960026239088
- Makita S, Tobinai K. Clinical features and current optimal management of natural killer/T-cell lymphoma. Hematol Oncol Clin North Am. 2017;31(2):239–253. doi:10.1016/j.hoc.2016.11.00728340876
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-64356926980727
- Piccaluga PP, Agostinelli C, Califano A, et al. Gene expression analysis of peripheral T cell lymphoma, unspecified, reveals distinct profiles and new potential therapeutic targets. J Clin Invest. 2007;117(3):823–834. doi:10.1172/JCI2683317304354
- Huang Da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi:10.1038/nprot.2008.21119131956
- Fu HY, Li C, Yang W, et al. FOXP3 and TLR4 protein expression are correlated in non-small cell lung cancer: implications for tumor progression and escape. Acta Histochem. 2013;115(2):151–157. doi:10.1016/j.acthis.2012.06.00222749378
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi:10.1056/NEJMoa120069022658127
- Shen JK, Cote GM, Choy E, et al. Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol Res. 2014;2(7):690–698. doi:10.1158/2326-6066.CIR-13-022424866169
- Zhang S, Yang X, Wang L, Zhang C. Interplay between inflammatory tumor microenvironment and cancer stem cells. Oncol Lett. 2018;16(1):679–686. doi:10.3892/ol.2018.871629963133
- Mitchell JA, Paul-Clark MJ, Clarke GW, McMaster SK, Cartwright N. Critical role of toll-like receptors and nucleotide oligomerisation domain in the regulation of health and disease. J Endocrinol. 2007;193(3):323–330. doi:10.1677/JOE-07-006717535871
- Hu J, Shi B, Liu X, et al. The activation of Toll-like receptor 4 reverses tumor differentiation in human glioma U251 cells via Notch pathway. Int Immunopharmacol. 2018;64:33–41. doi:10.1016/j.intimp.2018.08.01930145468
- Mertsoylu H, Muallaoglu S, Besen AA, et al. Primary extranodal non-Hodgkin’s lymphoma: clinicopathological features, survival and treatment outcome in two cancer centers of southern Turkey. Asian Pac J Cancer Prev. 2014;15(17):7207–7211.25227815
- Muller AM, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non-Hodgkin’s lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol. 2005;84(1):1–12. doi:10.1007/s00277-004-0939-715480663
- Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011;17(6):697–707. doi:10.3748/wjg.v17.i6.69721390139
- Peng JC, Zhong L, Ran ZH. Primary lymphomas in the gastrointestinal tract. J Dig Dis. 2015;16(4):169–176. doi:10.1111/1751-2980.1223425678011
- Herrmann R, Panahon AM, Barcos MP, Walsh D, Stutzman L. Gastrointestinal involvement in non-Hodgkin’s lymphoma. Cancer. 1980;46(1):215–222.7388763
- Diaconu S, Predescu A, Moldoveanu A, Pop CS, Fierbinteanu-Braticevici C. Helicobacter pylori infection: old and new. J Med Life. 2017;10(2):112–117.28616085
- Geng L, Wang X. Epstein-barr virus-associated lymphoproliferative disorders: experimental and clinical developments. Int J Clin Exp Med. 2015;8(9):14656–14671.26628948
- Ferreri AJ, Govi S, Ponzoni M. The role of Helicobacter pylori eradication in the treatment of diffuse large B-cell and marginal zone lymphomas of the stomach. Curr Opin Oncol. 2013;25(5):470–479. doi:10.1097/01.cco.0000432523.24358.1523942292
- Callahan MK, Postow MA, Wolchok JD. Targeting T cell co-receptors for cancer therapy. Immunity. 2016;44(5):1069–1078. doi:10.1016/j.immuni.2016.04.02327192570
- Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD-1/PD-L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol. 2016;21(3):462–473. doi:10.1007/s10147-016-0959-z26899259
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi:10.1038/nature1395425428505
- Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–2953. doi:10.1158/1078-0432.CCR-04-146915837746
- Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116(7):1757–1766. doi:10.1002/cncr.2489920143437
- Abbas M, Steffens S, Bellut M, et al. Intratumoral expression of programmed death ligand 1 (PD-L1) in patients with clear cell renal cell carcinoma (ccRCC). Med Oncol. 2016;33(7):80. doi:10.1007/s12032-016-0794-027317388
- Eto S, Yoshikawa K, Nishi M, et al. Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer. 2016;19(2):466–471. doi:10.1007/s10120-015-0519-726210691
- Zhao YJ, Sun WP, Peng JH, et al. Programmed death-ligand 1 expression correlates with diminished CD8+ T cell infiltration and predicts poor prognosis in anal squamous cell carcinoma patients. Cancer Manag Res. 2018;10:1–11. doi:10.2147/CMAR.S15396529296096
- Allam JP, Peng WM, Appel T, et al. Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J Allergy Clin Immunol. 2008;121(2):368–374.e361. doi:10.1016/j.jaci.2007.09.04518036651
- Wang YH, Cao YW, Yang XC, et al. Effect of TLR4 and B7-H1 on immune escape of urothelial bladder cancer and its clinical significance. Asian Pac J Cancer Prev. 2014;15(3):1321–1326.24606459
- Piccaluga PP, Rossi M, Agostinelli C, et al. Platelet-derived growth factor alpha mediates the proliferation of peripheral T-cell lymphoma cells via an autocrine regulatory pathway. Leukemia. 2014;28(8):1687–1697. doi:10.1038/leu.2014.5024480986
- Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(3):548–563.e516. doi:10.1016/j.cell.2017.07.00828753429
- Liao SJ, Zhou YH, Yuan Y, et al. Triggering of toll-like receptor 4 on metastatic breast cancer cells promotes alphavbeta3-mediated adhesion and invasive migration. Breast Cancer Res Treat. 2012;133(3):853–863. doi:10.1007/s10549-011-1844-022042369
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (London, England). 2017;389(10066):255–265. doi:10.1016/S0140-6736(16)32517-X