Abstract
Introduction
Growing evidence suggests that long non-coding RNAs (lncRNAs) could function as important regulators in carcinogenesis and cancer progression. Nicotinamide nucleotide transhydrogenase antisense RNA 1 (lncRNA NNT-AS1) is up-regulated in some human tumors and functions as a tumor promoter. This study aimed to detect the effect of NNT-AS1 on cholangiocarcinoma (CCA) prognosis.
Materials and methods
In this study, we detected NNT-AS1 expression in CCA tissue samples and cell lines, and analyzed the association between NNT-AS1 expression levels and clinical parameters of CCA patients. Moreover, we conducted loss-of-function studies in CCA cancer cells to explore the biological function and molecular mechanism of NNT-AS1. NNT-AS1 was downregulated by using RNAi technology. Cell proliferation was examined by CCK8 and clone formation assays. Cell migration and invasion were determined by wound healing and transwell assays. Western blot assays were used to explore protein expression.
Results
In this study, NNT-AS1 was expressed at high levels in CCA and closely associated with poor prognosis of patients with CCA. NNT-AS1 knockdown impaired cell proliferation, suppressed CCA cell migration and invasion, and restrained tumor growth in vitro. Moreover, NNT-AS1 directly bounded to miR-485 and further regulated BCL9. Finally, rescue assays verified that NNT-AS1 modulated the tumorigenesis of CCA by regulating miR-485.
Conclusion
Taken together, NNT-AS1 played a critical biological role in the development of CCA. Our results elucidated NNT-AS1/miR-485/BCL9 axis might lead to a further understanding of the molecular mechanism of CCA.
Keywords:
Introduction
Cholangiocarcinoma (CCA) is the most common biliary tract cancer which represents 3% of all gastrointestinal malignancies, described as a malignancy originating from the ductal epithelial cells lining the intrahepatic and extrahepatic biliary ducts.Citation1–Citation3 Over the past few decades, the overall incidence and mortality rates of CCA have increased worldwide, especially in China and Thailand. Currently, there is no effective chemoprevention or radiotherapy for CCA. Surgical resection and liver transplantation are curative means for treatment, but more than 70% of patients missed surgery opportunities due to early diagnosis failures. Therefore, novel diagnosis and treatment approaches of CCA are urgently demanded.Citation4,Citation5
Long non-coding RNAs (lncRNAs), which are over 200 nucleotides (nt) in length, have no significant protein-coding potential.Citation6,Citation7 Owing to the recent developments in deep-sequencing technologies, lncRNAs have been demonstrated to be capable of serving as pivotal regulators in numerous biological processes, including cellular proliferation, invasion, and so on. RNA transcripts contain miRNA response elements that share miRNAs to communicate with and coregulate each other by titrating the availability of miRNAs, which function as competing endogenous RNAs (ceRNAs). Increasing evidences have shown that lncRNAs could act as endogenous miRNA sponges to modulate targets.Citation8,Citation9 It is worth noting that abnormal expression of lncRNAs have been confirmed in many malignant tumors,Citation10 such as CCA.
Homo sapiens nicotinamide nucleotide transhydrogenase antisense RNA 1 (lncRNA NNT-AS1), locating in 5p12 with 3 exons, is a novel tumor-related lncRNA.Citation11 It has been verified that NNT-AS1 is a cancer-promoting gene in gastric cancer,Citation12 cervical cancer.Citation13 However, the functions of NNT-AS1 in CCA are still largely uncertain. Thus, in the present study, we measured the expression of NNT-AS1 in human CCA tissues and adjacent normal bile duct tissues. Furthermore, the correlation between the expression and clinicopathological parameters of CCA patients was examined. Additionally, the effects of NNT-AS1 on CCA malignant biological behaviors were also detected in vitro and vivo, including cell proliferation, migration and invasion.
Materials and methods
Patient samples
A total of 48 CCA tissues and adjacent non-tumorous tissues were collected from CCA patients who was hospitalized in the Department of HPB Surgery, The 2nd Affiliated Hospital of Harbin Medical University, from August 2014 to July 2015. All the patients did not receive preoperative chemotherapy before enrollment, and their tissue specimens were immediately stored and kept in liquid nitrogen until analysis. Pathological examination was performed immediately after resection of samples. This study was approved by The 2nd Affiliated Hospital of Harbin Medical University. All of the written informed consents were obtained from each patient before recruitment, and the local medical ethics committee approved the study protocol.
Cell culture and subcellular fractionation address
HCCC-9810 and RBE cells used in the study were purchased from the Cell Bank of Type Culture of Chinese Academy of Sciences (Shanghai, People’s Republic of China). The other CCA cells including QBC939, Huh-28, HuCCT1, KMBC and CCLP-1 and human intrahepatic biliary epithelial cell (HIBEC) were preserved in our laboratory. Cells were maintained in RPMI-1640 (Gibco, Grand Island, NY, USA) or DMEM (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (Invitrogen Life Technologies, Carlsbad, CA, USA) in a humidified atmosphere at 37 °C and 5% CO2. All cell lines were passaged for less than 6 months. The division of nuclear as well as cytosolic fractions was constructed with the PARIS Kit (Life Technologies, Carlsbad, CA, USA) following the producer’s guides.
Transfection
CCA cells were planted in six-well plates till growth to half confluence, then transfected with particular siRNA (100 nM) or scramble negative control siRNA (100 nM) by using Lipofectamine 3000 (Thermo Fisher Scientific, USA) on the basis of the producer’s protocol. Human NNT-AS1-specific sequences were used, sh-NNT-AS1: 5ʹ-AATTCGAGGTGTCTGTGAGGGTGTTTCTGTATTCAAGAGATACAGAAACACCCTCACAGACACCTTTTTTTG-3ʹ. Small interfering RNA (siRNA) sequences: si-NNT-AS1-1, 5ʹ-CGACAGUGCUIGUGAACUUTT-3ʹ; si-NNT-AS1-2, 5ʹ-ACUGACGCUGACCAUGUGATT-3ʹ; si-NNT-AS1-3, 5ʹ-CCAAGGCUGGAACUGAUATT-3ʹ. And si-NC was obtained from GenePharma (Shanghai, China). MiR-485 mimic and miR-485 inhibitor were purchased from Ribobio (Guangzhou, China).
qRT-PCR analysis
Total RNA was extracted from CCA tissue specimens and cells by using Trizol (Thermo Fisher Scientific, Waltham, MA, USA). The Transcriptor First Strand cDNA Synthesis Kit (Roche, Germany) was used to reverse transcribe RNA into cDNA. FastStart Universal SYBR Green Master Kit (Roche, Germany) and a BIO-RAD C1000 Thermal Cycler were used for qRT-PCR experiments. GAPDH was used as the internal control. The primers for PCR were as follows: NNT-AS1, forward: 5ʹ-AGTTCCACCAAGTTTCTTCA-3ʹ, reverse: 5ʹ-AGGTTTTGCCAGCATAGAC-3ʹ; GAPDH, forward: 5′-GGGAGCCAAAAGGGTCAT-3′, reverse: 5′-GAGTCCTTCCACGATACCAA-3′. Specifically, the primers for miR-485 were purchased from RiboBio (Guangzhou, China) with the small nuclear RNA (snRNA) U6 as an endogenous control.
CCK-8 assays and colony formation assays
Cell viability was monitored by cell counting kit-8 (CCK8) kit (Houston TX, USA) following the producer’s suggestions. The CCLP1 and QBC-939 cells transfected with si-NNT-AS1 or si-NC (3000 cells/well) were cultivated in five 96-well plates with six replicate wells. In terms of the colony formation assay, altogether 200 transfected cells were addressed in a 6-well plate and kept in a medium with 10% FBS for 2 weeks with the replacement of the medium every 4 days. Then, colonies were confirmed with paraformaldehyde and dyed with 0.1% crystal violet (Beyotime, Beijing, China) for 30 min. The quantity of visibly stained colonies was counted in colony formation.
Wound-healing assay
4×105 cells were cultured into 12-well plates and grew until 80–90% confluence. The cell monolayer was scratched with a sterile pipette tip. At different times (0, 24 h), the images of cell morphology were captured under an Olympus microscope (10×10). The results were analyzed by software IPP Image-Pro Plus 6.0.
Transwell assay
The migration and invasion of CCA were examined by transwell assay. After 24 hrs of transfection, cells (5×104 cells/well) were detached with serum free RPMI-1640 and loaded in the upper section of a 24-well transwell unit with 8 μm polycarbonate nucleopore filters (Corning, NY, USA), while medium with 10% FBS was added to the lower chamber. The transwell unit was incubated for 24 hrs before the cells on the lower surface of the membrane were fixed and stained with crystal violet. The experiment was performed in triplicate. For the invasion assay, 40 μL Matrigel (BD Biosciences, San Jose, CA, USA) was coated in the top filter of the transwell unit and placed in an incubator at 37 °C for 4 h to form a reconstructed basement membrane. The methods used were identical to those applied to the migration assay.
Plasmid construction and luciferase reporter assay
The sequence of NNT-AS1 contained the predicted binding site with miR-485; then we cloned the putative sequences of the binding site into a psi-CHECK-2 vector to shape the reporter vector psi-CHECK-2-NNT-AS1-wild type (NNT-AS1-WT). NNT-AS1-mutation (NNT-AS1-MUT) psi-ChECK-2 plasmid was constructed by using the QuikChange® Site-Directed Mutagenesis kit (Stratagene, Wilmington, USA). BCL9-WT and BCL9-MUT reporter plasmid were constructed by the same method. HEK-293T cells were seeded into 6-well plates and co-transfected with the appropriate plasmids (NNT-AS1-WT, NNT-AS1-MUT; Bcl9-WT, Bcl9-MUT) and miR-485 mimics. After 24 h, cells were collected, firefly as well as Renilla luciferase activities were measured by using the Luciferase Reporter Gene Assay kit from Beyotime Institute of Biotechnology (Nantong, China). Renilla luciferase was regarded as the internal control and the RLU (relative light units) ratio of firefly luciferase relative to Renilla luciferase was calculated.
Western blot assay
Whole-cell lysate preparation and Western blot analysis were performed as previously described.Citation14 Cells’ protein lysates were separated by 10% SDS-PAGE, then transferred to a 0.45 μm polyvinylidene fluoride (PVDF) membrane (GE, Piscataway, NJ, USA), and cultivated with specific antibodies. Densitometry (Quantity One software; Bio-Rad) was used to quantify the protein band density. The GAPDH antibody was employed as a control. Anti-BCL9 was obtained from Abcam (Cambridge, UK).
Xenograft mice model
To evaluate the in vivo tumorigenic effects, CCLP1 cells (5×106) transfected with sh-NNT-AS1 and sh-NC were inoculated subcutaneously in the left and right posterior flank of female BALB/c nude mice (6 weeks) separately. Xenograft were measured every 3 days and tumor volume was calculated. 21 days after CCLP1 inoculation, the mice were sacrificed and the samples were collected. All animal experiments were approved and performed in accordance with the guidelines by the Animal Care and Use Committee of The 2nd Affiliated Hospital of Harbin Medical University. All animal experiments were con-ducted according to the Principles of Laboratory Animal Care (National Society for Medical Research). All the experimental procedures were in accordance with the Declaration of Helsinki.
Statistical analysis
An independent t-test was performed to analyze the comparison of continuous data and chi-square test was applied into categorical data. Kaplan-Meier survival analysis and log-rank tests were used to evaluate the correlation betweenNNT-AS1 expression and prognosis of patients with CCA. All statistical analyses were performed by SPSS for Windows v.19.0 (Chicago, IL, USA) and p<0.05 was considered statistically significant for all results.
Results
NNT-AS1 was up-regulated in CCA tissues and cells
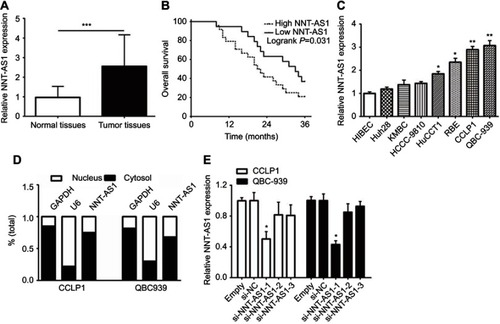
To explore whether NNT-AS1 was related to CCA, we firstly tested the expression in tumor and non-tumor tissues. NNT-AS1 expression was relatively high in tumor tissues (n=48) ( ). In addition, the 48 patients were categorized into low (n=21) group and high (n=27) group according to the median expression level of NNT- AS1. Kaplan Meier analysis revealed that higher expression of NNT-AS1 causes lower overall survival rate. ( ). The clinicopathologic data of CCA patients were presented in . The high expression level of NNT-AS1 was significantly associated with tumor size (p =0.013), lymph node invasion (p =0.017), and distant metastasis (p =0.009). Meanwhile, NNT-AS1 was present at higher expression in CCA cells (CCLP-1, HCCC- 9810, HuCCT1, KMBC, Huh-28, QBC-939 and RBE) compared with HIBEC ( ). Since cellular location could dictate function of lncRNA, cellular fractionation was performed to identify the subcellular localization of NNT-AS1 in CCLP1 and QBC-939 cells. The results showed that NNT-AS1 was mainly expressed in the cytoplasm ( ). All these data confirmed that overexpressed NNT-AS1 was closely associated with poor prognosis in CCA.
Table 1 Association between NNT-AS1 expression and clinicopathological characteristics of CCA
Figure 1 NNT-AS1 was highly expressed in CCA tissues and cells.
Notes: (A) The qRT-PCR results revealed the expression of NNT-AS1 in 48 CCA samples collected in the research cohort. (B) Kaplan-Meier curves and log-rank test revealed the overall survival of CCA patients with high/low NNT-AS1 expression levels. (C) The differential expression levels of NNT-AS1 in CCA cell lines (Huh28, KMBC, HCCC-9810, HuCCT1, RBE, CCLP1, QBC-939) and HIBEC cell were examined by qRT-PCR. (D) The expression of NNT-AS1 in the nucleus and cytoplasm of CCA cells (CCLP1, QBC-939). U6 (nuclear retained) and GAPDH (exported to cytoplasm) were served as controls. (E) qRT-PCR was used to determine the expression of NNT-AS1 after siRNA transfection in the CCLP1 and QBC-939. The error bars indicate the means ± SD, and each experiment was repeated three times. *p<0.05, **p<0.01, ***p<0.001.
Abbreviations: CCA, Cholangiocarcinoma; NNT-AS1, nicotinamide nucleotide transhydrogenase antisense RNA 1; qRT-PCR, quantitative real time polymerase chain reaction; HIBEC, human intrahepatic biliary epithelial cell.
NNT-AS1 inhibition suppressed CCA cell proliferation in vitro
To explore the biological functions of NNT-AS1 in CCA progression, three siRNAs targeting the coding region of NNT-AS1 were tested for their knockdown efficiency ( ). Being the most efficient one, si-NNT-AS1-1 was selected for further experiments. CCK-8 assay showed that NNT-AS1 knockdown suppressed the proliferation ability of CCA cells (CCLP1, QBC-939) compared with control group ( ). In accordance with proliferation, the data of colony formation were also conspicuous. The clonogenic ability of si-NNT-AS1 transfected CCLP1 and QBC-939 cells was more subdued ().
Figure 2 Knockdown of NNT-AS1 inhibited cell proliferation, migration and invasion in vitro.
Notes: (A) CCK-8 assays were used to determine the viability of si-NNT-AS1-transfected cholangiocarcinoma cells. (B) Colony-forming ability was measured in CCLP1 and QBC-939 cells after transfected with si-NNT-AS1 or si-NC by clonogenic assays. (C) The migration capacities were detected in CCLP1 and QBC-939 cells transfected with si-NNT-AS1 or si-NC by wound healing assays. (D) The invasive and migration capacities were detected in CCLP1 and QBC-939 cells transfected with si-NNT-AS1 or si-NC by transwell assays. The error bars indicate the means ± SD. *p<0.05, **p<0.01.
Abbreviations: NC, negative control; NNT-AS1, nicotinamide nucleotide transhydrogenase antisense RNA 1.
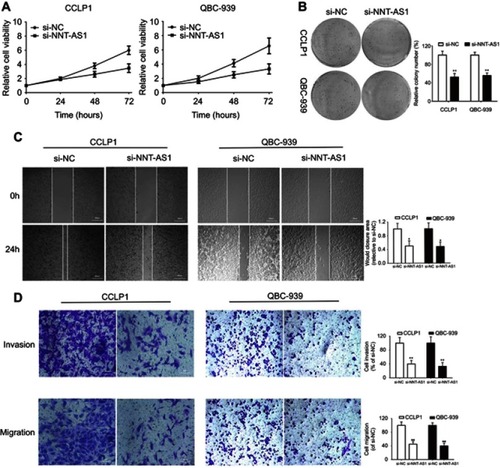
Knockdown of NNT-AS1 in CCA cell lines inhibited cell invasion and migration
Further investigated about the potential impacts of NNT-AS1 on the migration and invasion of CCA cells were conducted with wound healing and transwell assays. Knockdown of NNT-AS1 with siRNA significantly reduced wound closure area (). In line with our predictions, as revealed by the transwell assays, knockdown of NNT-AS1 decreased the migration and invasion of CCLP1 and QBC-939 ().
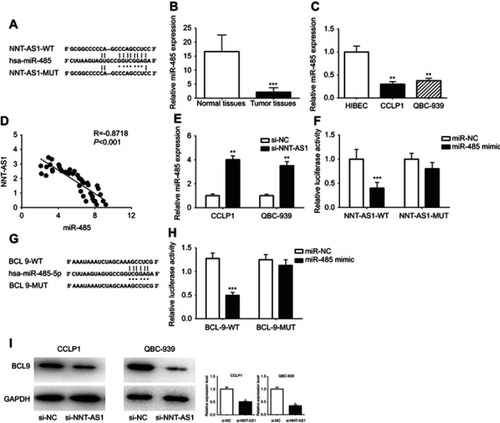
NNT-AS1 acted as a ceRNA by sponging miR-485 and indirectly regulated BCL9 expression
It is well known that lncRNA, as the molecular sponge mechanism of miRNA, is the most common molecular mechanism of lncRNA. NNT-AS1 is mainly located in cytoplasm. Therefore, we verified whether NNt-AS1 played a role as a “sponge” of miRNA in the process of CCA tumorigenesis. Bioinformatics online program (StarBase, http://starbase.sysu.edu.cn ) was used to predict the NNT-AS1 and miR-485 potential binding sites ( ). MiR-485 expression level was much lower than that in CCA tissues than in normal tissues ( ). MiR- 485 was also less expressed in CCLP1 and QBC- 939, compared with that in HEBIC ( ). Spearman’s correlation analysis suggested a significant negative correlation between NNT-AS1 and miR-485 in CCA patients’ tissues (R=−0.8717, p<0.0001, ). In CCLP1 and QBC-939 transfected with si-NNT-AS1, the expression level of miR-485 was up-regulated (). To investigate the binding ability between miR-485 and NNT-AS1, a luciferase reporter assay was conducted using wild-type and mutant versions of NNT-AS1. The results of luciferase reporter assay showed that ectopic miR-485 expression evidently repressed the luciferase activity (). Moreover, we predicted a target by TargetScan (http://www.targetscan.org) and considered that miR-485 might regulate BCL9 (). By luciferase reporter assay, we confirmed miR-485 could bind to BCL9 mRNA (). Western blot analysis indicated that BCL9 expression was decreased after knockdown NNT-AS1 ().
Figure 3 NNT-AS1 acted as a ceRNA by sponging miR-485 and regulated BCL9 expression indirectly.
Notes: (A) The predicted complementary binding sequences of NNT-AS1 3ʹ-UTR and miR-485. (B) The differential expression of miR-485 in CCA tissues and adjacent normal bile duct tissues was analyzed by qRT-PCR. (C) The miR-485 expression levels were investigated in CCA cells (CCLP1, QBC-939) and HIBEC by qRT-PCR. (D) Pearson’s correlation curve revealed the negative relevance between NNT-AS1 and miR-485 expression. (E) The expression levels of miR-485 in both CCLP1 and QBC-939 transfected with si-NNT-AS1 were evaluated by qRT-PCR. (F) Luciferase activity of 293T cells cotransfected with miR-485 mimic and luciferase reporters containing NNT-AS1-WT or NNT-AS1-MUT transcript were analyzed. (G) The predicted binding sites of miR-485 to the BCL9 sequence were shown. (H) Luciferase activity of 293T cells cotransfected with miR-485 mimic and luciferase reporters containing BCL9-WT or BCL9-MUT transcript were performed. (I) The BCL9 expression transfected with si-NNT-AS1 in CCLP1 and QBC-939 were analyzed by Western blot. The error bars in all graphs represented SD. *p<0.05, **p<0.01, ***p<0.001.
Abbreviations: NC, negative control; NNT-AS1, nicotinamide nucleotide transhydrogenase antisense RNA 1; MUT, mutation; WT, wild type.
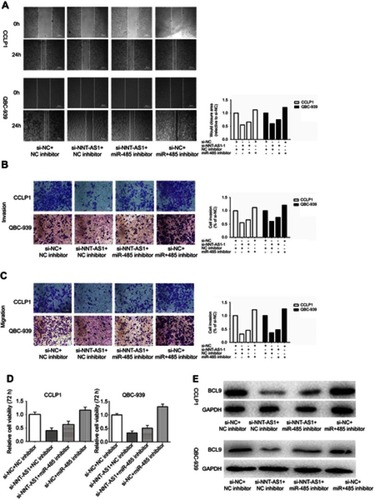
MiR-485 reversed the carcinogenesis of NNT-AS1
Rescue assays were carried out to validate the regulatory mechanism of NNT-AS1 in CCA. Wound-healing assay and transwell assay revealed that silencing of NNT-AS1 repressed the migration and invasion of CCA cells, which could be rescued by suppression of miR-485 (–). Moreover, CCK8 assays elucidated that the inhibitory impact of NNT-AS1 knockdown on proliferation of CCA cells could be abolished by miR-485 suppression (). In addition, the expression of BCL9 in transfected CCA cells were analyzed. The influence of si-NNT-AS1 on the expression was reversed by miR-485 inhibitor (). These data suggested that miR-485 reversed the promoting effect of NNT-AS1 on the proliferation and metastasis of CCA cells.
Figure 4 MiR-485 reversed the carcinogenesis of NNT-AS1 on CCA cells.
Notes: (A–D) Functional assays identified the phenomenon of NNT-AS1 and BCL9 regulated each other to compete for the binding of miR-485 by wound healing assays, transwell invasion, migration assay and CCK8 in CCLP1 and QBC-939. (E) The expression levels of BCL9 in CCLP1 and QBC-939 cotransfected si-NNT-AS1 with miR-485 inhibitor was analyzed by Western blot. The error bars in all graphs represented SD.
Abbreviations: NC, negative control; NNT-AS1, nicotinamide nucleotide transhydrogenase antisense RNA 1.
Ectopic expression of NNT-AS1 promoted CCA growth in vivo
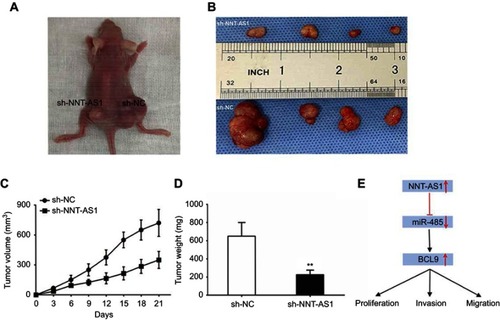
In order to confirm whether NNT-AS1 caused CCA tumorigenesis in vivo, we conducted a subcutaneous tumor formation experiment in nude mice. CCLP1 cells treated with sh-NNT-AS1 or a control vector were injected into nude mice. After 21 days post-injection, the tumors established in the sh- NNT- AS1 group were dramatically smaller than those in sh-NC group ( and ). As shown in and , sh-NNT-AS1 injected mice showed a reduction in tumor weight and volume at the end of the in vivo assay compared to the control group (p<0.05). These findings indicated that silencing NNT-AS1 could repress CCA tumor growth in vivo.
Figure 5 NNT-AS1 knockdown suppressed tumor growth in vivo.
Notes: (A) Tumors established with the mice in the sh-NC or sh-NNT-AS1 group. (B) Tumors collected from mice were exhibited. (C) Tumor volume curve of mice injected with CCLP1 cells transfected with sh-NC and sh-NNT-AS1 was analyzed. (D) Tumor weight of mice was measured. (E) Summary of the mechanism of NNT-AS1/miR-485/BCL9 pathway in CCA. The error bars in all graphs represented SD. **p<0.01.
Abbreviations: NC, negative control; NNT-AS1, nicotinamide nucleotide transhydrogenase antisense RNA 1; CCA, cholangiocarcinoma.
Discussion
Cholangiocarcinoma (CCA) is one of the most aggressive malignancies of the digestive system. This disease is difficult to diagnose at its inchoate stage and has poor prognosis. Therefore, a clear understanding of pathogenesis and major influencing factors is the key to develop effective therapeutic methods for CCA. However, the mechanism of CCA progression, including cell proliferation, invasion, metastasis and anti-apoptosis, has not been elaborated. Increasing evidences have indicated that lncRNAs emerge as an important regulator of cancers,Citation15 including CCA. NNT-AS1 has been reported to participate in the tumorigenesis of various cancer.Citation16–Citation18 In agreement with most of current studies of tumors on NNT-AS1, our study found that NNT-AS1 was highly expressed in CCA, and its expression level was correlated with clinical prognosis. We confirmed that NNT-AS1 played an oncogene role in CCA, and the specific molecular mechanism needed to be further explored.
A large number of lncRNAs have been shown to play an important role in the development of cholangiocarcinoma, such as NEAT1Citation19 and LINC01296.Citation20 In the current study, our data showed that the NNT-AS1 expression level was significantly increased of in CCA tissues compared to adjacent non-tumor tissues. High NNT-AS1 expression was associated with advanced clinicopathological features and poor overall survival. Therefore, the evidence proved the oncogenic role of NNT-AS1 in CCA tumorigenesis. Functional experiments results revealed that NNT-AS1 knockdown inhibited the proliferation and invasion ability in vitro, and suppressed the CCA tumor growth in vivo.
Recently, the miRNA ‘sponge’ theoryCitation21 is well known. There was a significant negative correlation between NNT-AS1 and miR-485 in CCA. MiR-485 was reported to be abnormally expressed in many cancers. Kang have reported that miR-485 could suppress gastric cancer cell metastasis and sphere formation,Citation22 miR-485 was also dysregulated in non-small-cell lung cancer (NSCLC) and markedly inhibited NSCLC cell growth and induced cell apoptosis.Citation23 Moreover, miR-485 widely participated in cell proliferation and invasion, as well as autophagy and apoptosis.
Then we identified BCL9 as the potential biological target for miR-485 by using TargetScan Database. Luciferase reporter assay confirmed the molecular binding at 3ʹ-UTR. We hypothesized that there was a ‘ceRNA network’ among NNT-AS1, miR-485 and BCL9. The results of rescue assays showed that miR-485 reversed the promoting effect of NNT-AS1 on the proliferation and metastasis of CCA. NNT-AS1 was up-regulated and competed with BCL9 for binding to miR-485, thereby increasing the protein expression of BCL9 and promoting the proliferation, migration and invasion in CCA (). Published studies have shown that BCL9 was closely related to the development of cancers such as renal cell carcinoma,Citation24 colorectal cancer,Citation25 and gastric cancer.Citation26 Moreover, BCL9 was involved in signal transduction through the Wnt pathway, promoting β-catenin’s transcriptional activity.Citation27 As our knowledge, the Wnt/β-catenin signaling pathway was pathologically activated in CCA, and β-Catenin was a key mediator of the Wnt/β-catenin pathway. In this regard, NNT-AS1 might promote the CCA tumorigenesis by regulating the Wnt signaling pathway.
Conclusion
We have a conclusion that NNT-AS1 overexpression is associated with tumorigenesis and poor prognosis in CCA, NNT-AS1 promotes proliferation, migration and invasion of CCA via miR-485/BCL9 axis.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (No. 81602088), China Postdoctoral Science Foundation (No. 2017M621305), Heilongjiang Postdoctoral Science Foundation (No. LBH-Z16096), Health and Family Planning Commission Research Project of Heilongjiang Province (No. 2016-049), HMU College Students’ Innovation and Entrepreneurship Training Program (No. 201810226105) and Innovative Science Foundation of Harbin Medical University (No. 2016LCZX09).
References
- Wang WT, Ye H, Wei PP, et al. LncRNAs H19 and HULC, activated by oxidative stress, promote cell migration and invasion in cholangiocarcinoma through a ceRNA manner. J Hematol Oncol. 2016;9:117. doi:10.1186/s13045-016-0348-027809873
- Blechacz B. Cholangiocarcinoma: current knowledge and new developments. Gut and Liver. 2017;11:13–26. doi:10.5009/gnl1556827928095
- Afshar M, Khanom K, Ma YT, Punia P. Biliary stenting in advanced malignancy: an analysis of predictive factors for survival. Cancer Manag Res. 2014;6:475–479. doi:10.2147/CMAR.S7111125525389
- Doherty B, Nambudiri VE, Palmer WC. Update on the diagnosis and treatment of cholangiocarcinoma. Current Gastroenterology Reports. 2017;19:2. doi:10.1007/s11894-017-0542-428110453
- Gatto M, Bragazzi MC, Semeraro R, et al. Cholangiocarcinoma: update and future perspectives. Dig Liver Dis. 2010;42:253–260. doi:10.1016/j.dld.2009.12.00820097142
- Zhang Y, Tang L. The application of lncRNAs in cancer treatment and diagnosis. Recent Pat Anticancer Drug Discov. 2018;13:292–301. doi:10.2174/157489281366618022612181929485010
- Chen X, Yang Y, Cao Y, et al. lncRNA PVT1 identified as an independent biomarker for prognosis surveillance of solid tumors based on transcriptome data and meta-analysis. Cancer Manag Res. 2018;10:2711–2727. doi:10.2147/CMAR.S16626030147369
- Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi:10.1038/nm.398126540387
- Li X, Du N, Zhang Q, et al. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 2014;5:e1479. doi:10.1038/cddis.2014.43025341033
- Li ZL, Li JL, Ji DL, et al. Overexpressed long noncoding RNA Sox2ot predicts poor prognosis for cholangiocarcinoma and promotes cell proliferation and invasion. Gene. 2018;645:131–136. doi:10.1016/j.gene.2017.12.01729246536
- Fagerberg L, Hallstrom BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397–406. doi:10.1074/mcp.M113.03560024309898
- Wang X, Ren M, Li Y, et al. Long noncoding RNA NNT-AS1 promotes gastric cancer proliferation and invasion by regulating microRNA-363 expression. J Cell Biochem. 2018;120(4):5704–5712. doi:10.1002/jcb.2785530324628
- Hua F, Liu S, Zhu L, Ma N, Jiang S, Yang J. Highly expressed long non-coding RNA NNT-AS1 promotes cell proliferation and invasion through Wnt/beta-catenin signaling pathway in cervical cancer. Biomed Pharmacother. 2017;92:1128–1134. doi:10.1016/j.biopha.2017.03.05728628975
- Li X, Edwards M, Swaney KF, et al. Mutually inhibitory Ras-PI(3,4)P2 feedback loops mediate cell migration. Proc Natl Acad Sci U S A. 2018;115:E9125–E9134. doi:10.1073/pnas.180903911530194235
- Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi:10.1038/onc.2017.18428604750
- Shen Q, Jiang Y. LncRNA NNT-AS1 promotes the proliferation, and invasion of lung cancer cells via regulating miR-129-5p expression. Biomed Pharmacother. 2018;105:176–181. doi:10.1016/j.biopha.2018.05.12329857296
- Li Y, Lv M, Song Z, Lou Z, Wang R, Zhuang M. Long non-coding RNA NNT-AS1 affects progression of breast cancer through miR-142-3p/ZEB1 axis. Biomed Pharmacother. 2018;103:939–946. doi:10.1016/j.biopha.2018.04.08729710510
- Cai Y, Dong ZY, Wang JY. LncRNA NNT-AS1 is a major mediator of cisplatin chemoresistance in non-small cell lung cancer through MAPK/slug pathway. Eur Rev Med Pharmacol Sci. 2018;22:4879–4887. doi:10.26355/eurrev_201808_1562430070323
- Parasramka M, Yan IK, Wang X, et al. BAP1 dependent expression of long non-coding RNA NEAT-1 contributes to sensitivity to gemcitabine in cholangiocarcinoma. Mol Cancer. 2017;16:22. doi:10.1186/s12943-017-0587-x28122578
- Zhang D, Li H, Xie J, et al. Long noncoding RNA LINC01296 promotes tumor growth and progression by sponging miR-5095 in human cholangiocarcinoma. Int J Oncol. 2018;52:1777–1786. doi:10.3892/ijo.2018.436229620172
- Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the rosetta stone of a hidden RNA language? Cell. 2011;146:353–358. doi:10.1016/j.cell.2011.07.01421802130
- Kang M, Ren MP, Zhao L, Li CP, Deng MM. miR-485-5p acts as a negative regulator in gastric cancer progression by targeting flotillin-1. American Journal of Translational Research. 2015;7:2212–2222.26807169
- Jiang P, Xu C, Chen L, et al. Epigallocatechin-3-gallate inhibited cancer stem cell-like properties by targeting hsa-mir-485-5p/RXRalpha in lung cancer. J Cell Biochem. 2018;119:8623–8635. doi:10.1002/jcb.2711730058740
- Wang J, Ying Y, Bo S, Li G, Yuan F. Differentially expressed microRNA-218 modulates the viability of renal cell carcinoma by regulating BCL9. Mol Med Rep. 2016;14:1829–1834. doi:10.3892/mmr.2016.540327314976
- Beaulieu JF. Tuning WNT-beta-catenin signaling via BCL9 proteins for targeting colorectal cancer cells. EBioMedicine. 2015;2:1846–1847. doi:10.1016/j.ebiom.2015.11.03326844253
- Han W, Mu Y, Zhang Z, Su X. Expression of miR-30c and BCL-9 in gastric carcinoma tissues and their function in the development of gastric cancer. Oncol Lett. 2018;16:2416–2426. doi:10.3892/ol.2018.893430013632
- Takada K, Zhu D, Bird GH, et al. Targeted disruption of the BCL9/beta-catenin complex inhibits oncogenic Wnt signaling. Sci Transl Med. 2012;4:148ra117. doi:10.1126/scitranslmed.3003808
