Abstract
Background
Small cell lung cancer (SCLC) is a special type of lung cancer and it is responsive to chemotherapy. Blood parameters have been proved to be associated with survival for many types of malignancies. This study aimed to investigate the prognostic significance of platelet-to-lymphocyte ratio (PLR) and mean platelet volume (MPV) for SCLC patients with etoposide-based first-line treatment.
Methods
We retrospectively identified 138 patients diagnosed as SCLC who underwent etoposide-based first-line chemotherapy. The patients’ baseline clinical characteristics and blood parameters were collected. Kaplan–Meier analysis and Cox regression methods were used to determine the factors associated with progression-free survival (PFS).
Results
The optimal cut-off value of diagnosis was depended on the ROC curve, the cut-off value of pretreatment PLR was 190 (sensitivity 39.0%, specificity 88.5%), and the cut-off value of pretreatment MPV was 10.0 (sensitivity 60.7%, specificity 61%). Kaplan–Meier analysis showed patients with high PLR levels in baseline had worse PFS than those with low PLR levels (P <0.001). Multivariate analysis revealed pretreatment MPV was an independent prognostic factor for PFS (HR: 0.815, 95% CI: 0.711–0.933, P =0.003). Further research suggested continuous high PLR indicated a poor therapy outcome (P =0.002).
Conclusion
Pretreatment MPV can be an independent predictor for first-line treatment outcome and a continuously high level of PLR suggested inferior PFS in etoposide-treated SCLC patients.
Introduction
SCLC is a major type of lung cancer with neuroendocrine tumor characteristics. Compared with non-small cell lung cancer (NSCLC), SCLC has a high degree of malignancy, a rapid doubling time and a propensity for early metastasis.Citation1 SCLC is highly responsive to chemotherapy and radiotherapy, and it has a high remission rate during the initial treatment, but it is also easy to harbor drug resistance and relapse. Therefore, searching for clinically accessible indicators such as blood parameters to predict the therapeutic effect and to determine the prognosis of patients with SCLC is particularly significant for improving the life quality and prolonging the survival of patients, which is one of the most urgent clinical problems.
Inflammation is an extremely considerable role in the development and progression of malignancies, and blood cells participate in various inflammatory processes by secreting diversified cytokines.Citation2,Citation3 The sustained inflammatory response assists in the further evolvement of the tumor by attenuating the body’s adaptive immune response.Citation4 Recently, several studies have suggested some indicators in blood were reflections of inflammatory changes in the tumor microenvironment.Citation5–Citation7
Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR) and systemic inflammation index (SII) reflected the level of systemic inflammation in the body.Citation8,Citation9 Several studies had discovered the relationship between NLR, PLR, LMR, SII and NSCLC treatment outcomes in surgery, chemotherapy, radiotherapy, targeted therapy and even immunotherapy.Citation10–Citation14 Recently, studies had attracted broad attention to the function of platelet activation to promote tumor angiogenesis, tumor cell proliferation and metastasis, thereby promoting drug resistance and disease progression, and more than that, platelet parameters could predict tumor treatment outcomes.Citation15,Citation16 Mean platelet volume (MPV) was a new indicator of platelet size and activity, which had been proposed as a possible marker of platelet function and activation.Citation17 However, there was still a rare study about the relationship between platelet parameters and SCLC treatment. Thus, the purpose of this study was to explore PLR and MPV on the prognostic effect in first-line treatment for SCLC.
Materials And Methods
Patients
All patients diagnosed as SCLC without surgery were retrospectively reviewed, from September 2015 to December 2018 in Anhui Provincial Hospital. From the group of 454 patients, 138 cases meeting the requirements as follows were included in the study ultimately: patients were pathologically diagnosed as SCLC, without other types of lung cancer containing SCLC components or combining two or more types of tumors; patients received radiological examination to define specific tumor stages; patients received first-line chemotherapy regimen with etoposide combining platinum; patients took radiological examination to assess therapeutic effect every 2–3 months, which were evaluated according to the RECIST criteria, version 1.1; patients did not have hematological diseases, immune system diseases or hepatitis virus infections; patients did not receive long-term glucocorticoid therapy; patients had first-line treatment progression.
Clinical Data Collection
Clinical data were collected including age, gender, tumor stage, tumor metastasis condition, first-line chemotherapy regimen, first evaluation result and radiotherapy condition. Blood parameters were recorded before every cycle of chemotherapy, including total white blood cell count (WBC), absolute neutrophil count (NEUT), absolute lymphocyte count (LYMPH), absolute number of monocytes (MONO), total number of red blood cells (RBC), hemoglobin concentration (HGB), total platelet count (PLT), platelet volume distribution width (PDW), red blood cell volume distribution width (RDW), mean red blood cell volume (MCV), mean platelet volume (MPV), and then the quantitative values of NLR, PLR, LMR, SII (SII =PLT×NEUT/LYMPH), MCV/RBC ratio and PLT/MPV ratio were calculated. Progression-free survival (PFS) was defined as the time from the randomization to the progression or death of the disease. The incidence of marrow suppression and the cause of progression during the first-line treatment were also recorded.
Laboratory Testing
Patients were at a resting state during the early morning when blood samples were collected. The analytical instrument was a Sysmex XE-5000 automatic blood analyzer. EDTA-K2 (dipotassium ethylenediaminetetraacetate) vacuum anticoagulation tube was purchased from Shanghai Kehua Biotechnology Co., Ltd. The samples were tested by the instrument, and the instrument was in the best working condition according to the instrument operation rules, the blood test routine test of Standard Operating Procedure (SOP) file and the clinical test operation rules. All samples were tested within 2 hrs.
Statistical Analysis
All analyses and graphs were performed using SPSS 19.0 (IBM Corporation, Armonk, NY, USA) and Graphpad Prism 6.0 (GraphPad Software Inc., San Diego, CA, USA) statistical software. Receiver operating characteristic (ROC) curve was constructed from the pre-treatment blood indicators, the area under the curve (AUC) was used to assess their diagnostic value, Chi-square test was used for rates comparison, Student’s t-test was used for normal distribution data comparison. Spearman test was used for correlation analysis. Potential factors were analyzed by the Kaplan–Meier method for survival analysis, log rank test for statistical difference and Cox regression analysis for multivariate analysis. The difference was statistically significant at P <0.05.
Ethical Approval And Informed Consent
This study was approved by the Ethics Committee of Anhui Provincial Hospital. All patients diagnosed with SCLC were from Anhui Provincial Hospital. The need for written informed consent was waived by the Ethics Committee because of the retrospective nature of this study. This study was conducted following the Declaration of Helsinki.
Results
Baseline Parameters
The data of 138 patients had been collected. The average age of these patients was 60.96 ±8.70 years old. There were 34 patients in the limited stage and 104 patients in the extensive stage. All patients had received etoposide-based chemotherapy for the first-line treatment. The clinical characteristics of the total patients are shown in .
Table 1 The Clinical Characteristics Of 138 Patients With SCLC
According to mean PFS value (6.59 ±3.67 months), the ROC curve of the baseline blood parameter values was evaluated (State variable: PFS =7 months). NLR0, PLR0, LMR0, SII0, MCV0, MPV0, RDW0, PDW0, PLT0/MPV0 ratio, MCV0/RBC0 ratio, as the baseline data, showed their diagnostic values of indicators (). Depending on the ROC curve results, NLR0, PLR0, LMR0, SII0, MPV0 showed favorable prognostic effects on PFS. As for the optimal cut-off point of diagnosis, PLR0 was at 190 (sensitivity =39.0%, specificity =88.5%), MPV0 was at 10.0 (sensitivity =60.7%, specificity =61%) ().
Table 2 Cut-Off Values Of ROC Curve For Pretreatment Blood Parameters
Figure 1 ROC curve based on pretreatment blood parameters.
Abbreviations: NLR, neutrophil-to-lymphocyte ratio (AUC =0.376, P =0.013); PLR, platelet-to-lymphocyte ratio (AUC =0.377, P =0.013); LMR, lymphocyte-to-monocyte ratio (AUC =0.617, P =0.019); SII, systemic inflammation index (AUC =0.402, P =0.049); MPV, mean platelet volume (AUC =0.637, P =0.006) and PLT/MPV ratio (AUC =0.410, P =0.068).
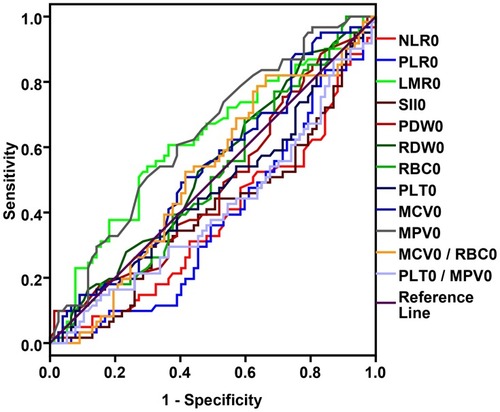
The Chi-square test demonstrated the difference between the baseline blood parameters and clinical characteristics. Gender, age, chemotherapy regimen and myelosuppression showed no difference in different blood parameter groups before the treatment. However, PFS (PFS ≥7 months vs. PFS <7 months) between all different blood parameter groups showed a significant difference. Moreover, stage and radiotherapy conditions differed in different pretreatment PLR groups, and there was still discrimination between the results of the first evaluation with different pre-treatment PLR and MPV groups ().
Table 3 Correlation Between Hematological Parameters And Clinical Features Of 138 Patients With SCLC By Chi-Square Test
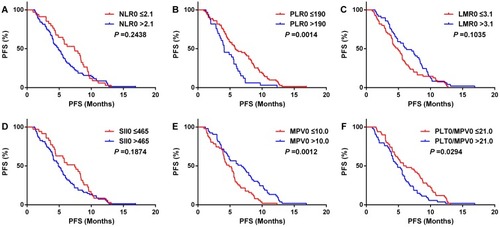
Kaplan–Meier analysis showed that PFS of the high PLR0 group was significantly shorter than the low PLR0 group in 138 patients (P <0.001) (), while other blood parameter groups were not statistically significant ( and –). Because stage conditions differed in pretreatment PLR groups, 138 patients were divided by stage condition into two groups as limited stage group (34 cases) and extensive stage group (104 cases) for further study. The outcome suggested that in patients with extensive stage, PFS of high PLR0 group was still shorter than the low PLR0 group (), as well as, the PFS of patients in low MPV0 group was shorter than high MPV0 group (). The other blood parameter groups were shown ( and –).
Figure 2 Kaplan–Meier analysis for progress-free survival (PFS) in all 138 patients with SCLC.
Notes: NLR, neutrophil-to-lymphocyte ratio (A); PLR, platelet-to-lymphocyte ratio (B); LMR, lymphocyte-to-monocyte ratio (C); SII, systemic inflammation index (D); MPV, mean platelet volume (E); PLT/MPV ratio, total platelet count/mean platelet volume (F).
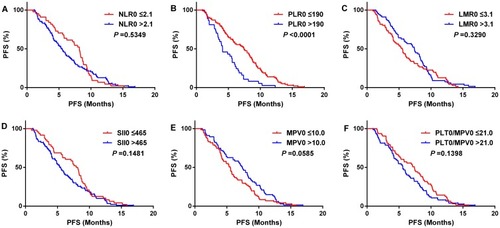
Figure 3 Kaplan–Meier analysis for progress-free survival (PFS) in 104 extensive stage SCLC patients.
Notes: NLR, neutrophil-to-lymphocyte ratio (A); PLR, platelet-to-lymphocyte ratio (B); LMR, lymphocyte-to-monocyte ratio (C); SII, systemic inflammation index (D); MPV, mean platelet volume (E); PLT/MPV ratio, total platelet count/mean platelet volume (F).
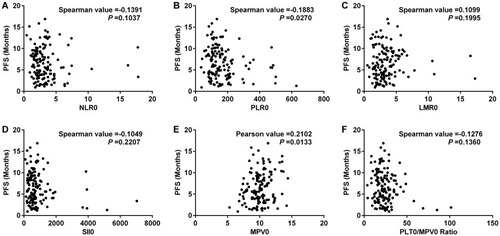
Spearman test demonstrated the correlation between pre-treatment blood parameters and PFS. The results showed that there was a pleasurable correlation between MPV0 and PFS, and it was statistically significant (P <0.05) (). The correlation between other blood parameters and PFS was shown (–).
Figure 4 Correlation analysis between progress-free survival (PFS) and blood parameters in 138 patients with SCLC.
Notes: NLR, neutrophil-to-lymphocyte ratio (A); PLR, platelet-to-lymphocyte ratio (B); LMR, lymphocyte-to-monocyte ratio (C); SII, systemic inflammation index (D); MPV, mean platelet volume (E); PLT/MPV ratio, total platelet count/mean platelet volume (F).
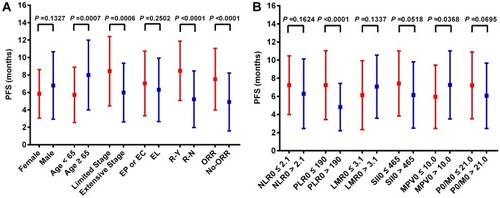
Afterward, Student’s t-test revealed the differentiation of PFS between different clinical and blood parameter groups. PFS showed no statistical difference in gender, chemotherapy regimen (), NLR0, LMR0, SII0 and PLT0/MPV0 groups. But in low PLR0 group (7.24 ±3.80 months), PFS was longer than high PLR0 group (4.82 ±2.61 months) (P <0.001); meanwhile, in high MPV0 group (7.26 ±3.76 months), PFS was longer than low MPV0 group (5.96 ±3.50 months) (P =0.037) ().
Figure 5 Student’s t-test for progress-free survival (PFS) between different clinical features and blood parameters. (A) Comparison of mean progress-free survival (PFS) between the different clinical feature groups; (B) comparison of mean progress-free survival (PFS) between the different blood parameter groups.
Abbreviations: EP or EC group, chemotherapy regimen was etoposide + cisplatin or etoposide + cisplatin; RL group, chemotherapy regimen was etoposide + lobaplatin; RY group, patients received radiotherapy during first-line treatment; RN group, patients never received radiotherapy during first-line treatment; ORR group, the first-time evaluation results was CR or PR; No-ORR group, the first-time evaluation results was SD or PD; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; SII, systemic inflammation index; MPV, mean platelet volume, PLT/MPV ratio, total platelet count/mean platelet volume.
The univariate analysis identified that gender, age, tumor stage, radiotherapy, results of the first evaluations and pretreatment PLR were significantly associated with PFS (). Multivariate analysis distinctly revealed that age (HR: 0.973, 95% CI: 0.954–0.992, P =0.006), stage (HR: 0.600, 95% CI: 0.385–0.937, P =0.025), radiotherapy (HR: 2.548, 95% CI: 1.721–3.772, P <0.0001), results of the first evaluations (HR: 2.155, 95% CI: 1.475–3.146, P =0.0001) and MPV0 (HR: 0.815, 95% CI: 0.711–0.933, P =0.003) were independent prognostic factors for PFS ().
Table 4 Univariate Analysis Of PFS In 138 Patients With SCLC
Table 5 Multivariate Analysis Of PFS In 138 Patients With SCLC
Post-Chemotherapy Parameters
There were 4 patients having been received recombinant human granulocyte colony-stimulating factor (G-CSF) in hospitalization because of myelosuppression. In order to prevent these patients from interfering with the experiment result, the following analysis excluded them. In the remaining 134 patients, NLR1, PLR1, LMR1, SII1, as the post-chemotherapy data, were gathered for further research. The quartile of NLR1 was 2.155 (1.490–3.175), the quartile of PLR1 was 170.69 (122.77–232.13), the quartile of LMR1 was 2.425 (1.578–3.283), the quartile of SII1 was 467.55 (294.25–734.47). 134 patients were divided into three groups on the basis of pre-chemotherapy and post-chemotherapy data. We classified the patients into three subsets as follows: High NLR group (NLR0 >2.1 and NLR1 >2.1), Low NLR group (NLR0 ≤2.1 and NLR1 ≤2.1), Medium NLR group (NLR0 >2.1 and NLR1 ≤2.1 or NLR0 ≤2.1 and NLR1>2.1). Like NLR, patients were divided into three groups according to the changes of PLR, LMR and SII similarly.
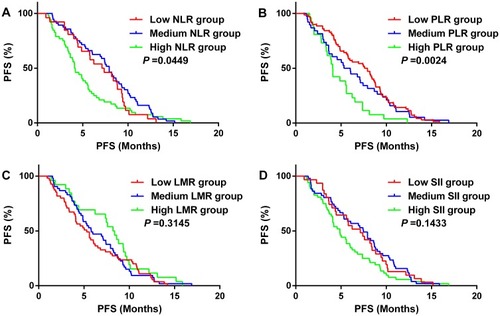
Kaplan–Meier analysis demonstrated that PFS of the High PLR group was shorter than other groups (P =0.002) (), while the NLR change groups had a similar result () but other blood parameter groups were not statistically significant ( and ).
Figure 6 Kaplan–Meier analysis for progress-free survival (PFS) in 134 patients with SCLC. (A) High NLR group (NLR0 >2.1 and NLR1 >2.1), Low NLR group (NLR0 ≤2.1 and NLR1 ≤2.1), Medium NLR group (NLR0 >2.1 and NLR1 ≤2.1, or NLR0 ≤2.1 and NLR1 >2.1); (B) High PLR group (PLR0 >190 and PLR1 >190), Low PLR group (PLR0 ≤190 and NLR1 ≤190), Medium PLR group (PLR0 >190 and PLR1 ≤190, or PLR0 ≤190 and PLR1 >190); (C) High LMR group (LMR0 >3.1 and LMR1 >3.1), Low LMR group (LMR0 ≤3.1 and LMR1 ≤3.1), Medium LMR group (LMR0 >3.1 and LMR1 ≤3.1, or LMR0 ≤3.1 and LMR1 >3.1). (D) High SII group (SII0 >465 and SII1 >465), Low SII group (SII0 ≤465 and SII1 ≤465), Medium SII group (SII0 >465 and SII1 ≤465, or SII0 ≤465 and SII1 >465).
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; SII, systemic inflammation index.
Discussion
There were some studies about the relationship between inflammatory indexes in hematology and SCLC.Citation18–Citation21 However, most studies revealed a relationship between parameters and overall survival (OS),Citation21,Citation22 the relationship between PFS of first-line treatment and blood parameters had not been fully uncovered.
The study demonstrated that the baseline PLR had a preliminary prognostic value of PFS in the light of univariate analysis, and high PLR group (PLR0 >190) had an inferior PFS, the same results were obtained in all patients and extensive stage patients. However, multivariate analysis showed PLR was not the independent factor of PFS. Taking it into account that the Chi-square test suggested PLR0 was related to radiotherapy condition, more importantly, radiotherapy condition was an independent factor of PFS; therefore, it conjectured that radiotherapy condition had a deep influence on PLR to predict the PFS. A former study in 187 Korean patients had got a similar conclusion that baseline PLR was not associated with OS or PFS in patients treated with platinum-based chemotherapy.Citation23 So, further research showed that continuous high PLR (high PLR0 and high PLR1) group patients had shorter PFS than the others. The combination of pretreatment and post-treatment PLR partly reduced the impact of radiotherapy factor, which made the results more reliable.
MPV was considered as a hallmark of platelet activation. Shi et alCitation24 retrospectively reviewed advanced NSCLC patients and the study showed MPV and plateletcrit (PCT) were negative predictors of drug resistance and both were independent factors associated with OS. Another studyCitation25 in advanced NSCLC patients showed OS was significantly shorter in the group with a low MPV/PLT ratio than in the other group, and a low MPV/PLT ratio was an unfavorable independent prognostic factor for OS. In our study, the Chi-square test, Spearman test and Student’s t-test all revealed that MPV of baseline had a relationship with PFS, and Kaplan–Meier analysis showed PFS of patients in low MPV group was shorter than the other group in extensive stage patients. Strangely, MPV was not statistically significant in univariate analysis but multivariate analysis. It suggested MPV was an independent prognostic factor for PFS in SCLC patients. We considered univariate analysis had its shortness of test efficacy, and the result of multivariate analysis was more reliable. There had been no reports related to MPV and SCLC treatment so far. Only one study showed increased MPV was an important prognostic factor and an increased MPV level might be used as a prognostic biomarker to estimate for poor OS in patients with NSCLC.Citation26
Notably, this study had not found the important significance of NLR in SCLC. Suzuki R et alCitation27 found the link between high NLR and inferior OS, but his conclusion was based on 122 patients with limited-stage SCLC without defined chemotherapy regimens. On the contrary, Bernhardt et alCitation18 did not find NLR was an independent prognostic factor for OS in 350 SCLC in limited-stage. Suzuki R et alCitation20 found high NLR predicted inferior survival in extensive-stage SCLC patients received platinum-based chemotherapy. Liu et alCitation21 found NLR was an independent prognostic factor and could be used to predict the mortality risk of SCLC patients but they did not clearly describe the tumor stage or chemotherapy regimens. There still remained confusion about the role of NLR in SCLC. The cut-off value, therapeutic regimen and tumor stage of this study were partly different. Put it another way, patients with SCLC were prone to platelet-related complications such as superior vena cava obstruction; it hypothesized that platelet parameters had a better prognostic effect in SCLC than traditional white blood cell parameters; furthermore, platelet parameters were not easily affected by drugs like G-CSF.
There were still some limitations in this study. First, the cut-off value was individually determined by the ROC curve from the baseline blood parameters of 138 patients involved in this study. Second, as for the blood parameters of the first evaluation time, the impact from myelosuppression due to chemotherapy drugs could not be ignored; myelosuppression also was the reaction of the human body for treatment. Though 4 patients were excluded on account of having been received G-CSF in hospitalization, the situation of myelosuppression and support treatment in the discharge period were unknown, and the influence of G-CSF injection to NLR was unspecified. Lastly, the data of this study were from a single center and the research was a retrospective study. The outcomes still need larger and randomized clinical trials for validation.
In conclusion, baseline MPV was a significant predictor of outcome and a continuously high level of PLR suggested inferior PFS in etoposide-treated SCLC patients.
Disclosure
The authors declare no conflicts of interest in this work.
Acknowledgments
All blood tests were one of the normal treatment procedures and did not cause additional harm to the patients. The authors thank all patients participated in this research.
References
- Kalemkerian GP, Loo BW, Akerley W, et al. NCCN guidelines insights: small cell lung cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16(10):1171–1182. doi:10.6004/jnccn.2018.007930323087
- Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev. 2018;281(1):57–61. doi:10.1111/imr.1261429247996
- Loffek S. Transforming of the tumor microenvironment: implications for TGF-β inhibition in the context of immune-checkpoint therapy. J Oncol. 2018;2018:9732939. doi:10.1155/2018/973293930631358
- Varga G, Foell D. Anti-inflammatory monocytes-interplay of innate and adaptive immunity. Mol Cell Pediatr. 2018;5(1):5. doi:10.1186/s40348-018-0083-429616417
- Magdy M, Hussein T, Ezzat A, Gaballah A. Pre-treatment peripheral neutrophil-lymphocyte ratio as a prognostic marker in gastric cancer. J Gastrointest Cancer. 2018;1–6. doi:10.1007/s12029-018-0144-x29110227
- Vallard A, Garcia MA, Diao P, et al. Outcomes prediction in pre-operative radiotherapy locally advanced rectal cancer: leucocyte assessment as immune biomarker. Oncotarget. 2018;9(32):22368–22382. doi:10.18632/oncotarget.2502329854285
- Hong YF, Chen ZH, Wei L, et al. Identification of the prognostic value of lymphocyte-to-monocyte ratio in patients with HBV-associated advanced hepatocellular carcinoma. Oncol Lett. 2017;14(2):2089–2096. doi:10.3892/ol.2017.642028789436
- Yang J, Xu H, Guo X, et al. Pretreatment inflammatory indexes as prognostic predictors for survival in colorectal cancer patients receiving neoadjuvant chemoradiotherapy. Sci Rep. 2018;8(1):3044. doi:10.1038/s41598-018-21093-729445100
- Yang J, Guo X, Wang M, Ma X, Ye X, Lin P. Pre-treatment inflammatory indexes as predictors of survival and cetuximab efficacy in metastatic colorectal cancer patients with wild-type RAS. Sci Rep. 2017;7(1):17166. doi:10.1038/s41598-017-17130-629215037
- Aguiar-Bujanda D, Dueñas-Comino A, Saura-Grau S, et al. Neutrophil to lymphocyte ratio as a prognostic factor in European patients with epidermal growth factor receptor-mutant non-small cell lung cancer treated with tyrosine kinase inhibitors. Oncol Res Ther. 2018;41(12):755–761. doi:10.1159/000492344
- Suh KJ, Kim SH, Kim YJ, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2018;67(3):459–470. doi:10.1007/s00262-017-2092-x29204702
- Gao Y, Zhang H, Li Y, Wang D, Ma Y, Chen Q. Preoperative increased systemic immune-inflammation index predicts poor prognosis in patients with operable non-small cell lung cancer. Clin Chim Acta. 2018;484:272–277. doi:10.1016/j.cca.2018.05.05929860033
- Luo H, Ge H, Cui Y, et al. Systemic inflammation biomarkers predict survival in patients of early stage non-small cell lung cancer treated with stereotactic ablative radiotherapy – a single center experience. J Cancer. 2018;9(1):182–188. doi:10.7150/jca.2170329290784
- Song X, Chen D, Yuan M, Wang H, Wang Z. Total lymphocyte count, neutrophil-lymphocyte ratio, and platelet-lymphocyte ratio as prognostic factors in advanced non-small cell lung cancer with chemoradiotherapy. Cancer Manag Res. 2018;10:6677–6683. doi:10.2147/CMAR.S18857830584362
- Guo F, Zhu X, Qin X. Platelet distribution width in hepatocellular carcinoma. Med Sci Monit. 2018;24:2518–2523.29689031
- Cui MM, Li N, Liu X, et al. Platelet distribution width correlates with prognosis of non-small cell lung cancer. Sci Rep. 2017;7(1):3456. doi:10.1038/s41598-017-03772-z28615714
- Lee JH, Park M, Han S, et al. An increase in mean platelet volume during admission can predict the prognoses of patients with pneumonia in the intensive care unit: a retrospective study. PLoS One. 2018;13(12):e0208715. doi:10.1371/journal.pone.020871530533065
- Bernhardt D, Aufderstrasse S, König L, et al. Impact of inflammatory markers on survival in patients with limited disease small-cell lung cancer undergoing chemoradiotherapy. Cancer Manag Res. 2018;10:6563–6569. doi:10.2147/CMAR.S18099030555261
- Zheng Y, Wang L, Zhao W, et al. Risk factors for brain metastasis in patients with small cell lung cancer without prophylactic cranial irradiation. Strahlenther Onkol. 2018;194(12):1152–1162. doi:10.1007/s00066-018-1362-730218136
- Suzuki R, Lin SH, Wei X, et al. Prognostic significance of pretreatment total lymphocyte count and neutrophil-to-lymphocyte ratio in extensive-stage small-cell lung cancer. Radiother Oncol. 2018;126(3):499–505. doi:10.1016/j.radonc.2017.12.03029398150
- Liu D, Huang Y, Li L, Song J, Zhang L, Li W. High neutrophil-to-lymphocyte ratios confer poor prognoses in patients with small cell lung cancer. BMC Cancer. 2017;17(1):882. doi:10.1186/s12885-017-3893-129268698
- Kasmann L, Bolm L, Schild SE, Janssen S, Rades D. Neutrophil-to-lymphocyte ratio predicts outcome in limited disease small-cell lung cancer. Lung. 2017;195(2):217–224. doi:10.1007/s00408-017-9976-628154994
- Kang MH, Go SI, Song HN, et al. The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br J Cancer. 2014;111(3):452–460. doi:10.1038/bjc.2014.31724921916
- Shi L, Li Y, Yu T, et al. Predictable resistance and overall survival of gemcitabine/cisplatin by platelet activation index in non-small cell lung cancer. Med Sci Monit. 2018;24:8655–8668. doi:10.12659/MSM.91112530498189
- Inagaki N, Kibata K, Tamaki T, Shimizu T, Nomura S. Prognostic impact of the mean platelet volume/platelet count ratio in terms of survival in advanced non-small cell lung cancer. Lung Cancer. 2014;83(1):97–101. doi:10.1016/j.lungcan.2013.08.02024189108
- Omar M, Tanriverdi O, Cokmert S, et al. Role of increased mean platelet volume (MPV) and decreased MPV/platelet count ratio as poor prognostic factors in lung cancer. Clin Respir J. 2018;12(3):922–929. doi:10.1111/crj.1260528026133
- Suzuki R, Wei X, Allen PK, Cox JD, Komaki R, Lin SH. Prognostic significance of total lymphocyte count, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio in limited-stage small-cell lung cancer. Clin Lung Cancer. 2019;20(2):117–123. doi:10.1016/j.cllc.2018.11.01330611672
