Abstract
Introduction
Amphiphysin 1 (AMPH-1) is involved in endocytosis, and its expression is upregulated in osteosarcoma compared with osteofibrous dysplasia.
Methods
We investigated the role of AMPH-1 in osteosarcoma cells via both gain-of-function and loss-of-function experiments.
Results
Knockdown of AMPH-1 in osteosarcoma cells promoted cell cycle progression and cell proliferation and attenuated apoptosis. Notably, silencing AMPH-1 increased osteosarcoma progression in a mouse tumor model. The results obtained upon AMPH-1 knockdown and AMPH-1 overexpression indicates that AMPH-1 is involved in regulating MEK/ERK signaling.
Conclusion
These data suggest that AMPH-1 plays an important role in osteosarcoma and may represent a novel therapeutic target for osteosarcoma treatment.
Keywords:
Introduction
Osteosarcoma is the most common primary malignant bone tumor in children and adolescents. Approximately 70–80% of patients have an age of onset of 10–25 years, and the incidence is about 4/1,000,000 per year worldwide.Citation1 Osteosarcoma is associated with not only a high relapse rate but also a high early metastasis rate owing to its severe invasiveness and early systemic metastasis. Approximately 10–20% of newly diagnosed osteosarcoma patients have distant metastases, of which 90% are lung metastases – the main cause of death caused by osteosarcoma. The occurrence of osteosarcoma metastasis and recurrence often indicates an extremely unfavorable prognosis and the five-year overall survival rate is merely 20–30%.Citation2,Citation3
Amphiphysin 1 (AMPH-1) is enriched in the nerve terminals and plays a role in clathrin-mediated endocytosis.Citation4 Three functional domains of AMPH-1 have been identified, including the CLAP and BAR domains and a C-terminal SH3 domain.Citation5 Neurons express high levels of AMPH-1, which participates in the processes of synaptic vesicle endocytosis, neurite outgrowth, and the enrollment of dynamin to the locus of clathrin-mediated endocytosis.Citation6–Citation9 To date, previous studies have predominantly focused on the role of AMPH-1 in neuronal function.Citation7 As a human autoantigen, it was first detected in patients with stiff-person syndrome secondary to breast cancer and plays a crucial role in the process of synaptic vesicle endocytosis.Citation10–Citation12 Knockdown of AMPH-1 in neurons may cause collapse of the growth cones and decreased filopodia formation.Citation13,Citation14 Recently, several studies have explored the potential role of AMPH-1 in cancer, which demonstrated a significant anti-oncogenic function.Citation15 Moreover, the yeast homologs of AMPH, Rvs161 and Rvs167, were found to be involved in the transition from exponential cell growth to the stationary phase upon exposure to nutrient starvation, indicating a possible role in the biology of cancer.Citation16
In this study, we found that the expression level of AMPH-1 was clearly increased in osteosarcoma tissues. Moreover, the knockdown of AMPH-1 in two osteosarcoma cell lines significantly inhibited cell apoptosis and promoted proliferation and cell cycle progression. In terms of the mechanism, the knockdown of AMPH-1 activated the MEK/ERK signaling pathway in the osteosarcoma cells. Therefore, the obtained results demonstrate that AMPH-1 plays a significant role in osteosarcoma progression and may represent a novel therapeutic target for osteosarcoma treatment.
Materials And Methods
Clinical Tissue Samples
Our research was approved by the Ethics Committee of Shanghai Changzheng Hospital and written informed consent was obtained from each patient. A total of 31 osteosarcoma tissues and 20 osteofibrous dysplasia tissues were collected from osteosarcoma and osteofibrous dysplasia patients who underwent spine surgery at the Department of Orthopedic Oncology of Shanghai Changzheng Hospital between 2011 and 2016.
Cell Culture And RNA Interference
The U-2 OS and 143B cell lines were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Transient transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The U-2 OS and 143B cells were regularly cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (Gibco), 100 mg/mL streptomycin, and 100 U/mL penicillin (Gibco). The osteosarcoma cells were regularly grown in a humidified incubator (37 °C and 5% CO2).
Stable cell lines of U-2 OS and 143B were generated by integration of retroviral shRNA vectors specific for AMPH-1 or a control vector from OriGene. The AMPH-1 shRNA (5ʹ-GCAGGAAGCUAGUGGACUATT-3ʹ) and siRNA (5ʹ- GCGAGAACUCCGAGGAUAUTT-3ʹ) were synthesized.
The antibodies used for Western blotting were those against β-actin (Sigma), AMPH-1 (Invitrogen), and MEK/ERK (Cell Signaling).
Overexpression Of AMPH-1
The PCMV-AMPH Overexpression plasmid was bought from abnova. Lipofectamine 2000 (Thermo Fisher Scientific) was employed to transfect 1ug PCMV-AMPH or PCMV-vector into 143B and U-2 OS cells.
Colony Formation
To evaluate the proliferation of the 143B and U-2 OS cells, the cells were inoculated into a six-well plate in triplicate with a density of 5×104 cells per well and incubated for seven days. Every single dish was washed twice with cold phosphate-buffered saline (PBS) and the cell colonies were fixed with paraformaldehyde and stained with 0.2% crystal violet for 30 min. Photographs of the stained osteosarcoma cell colonies were recorded using a gel imaging system (Tanon). The number of colonies was determined using a spectrophotometer (Thermo Scientific) or plate reader at 570 nm.
Apoptosis Assay And Cell Cycle Analysis
To analyze the cell apoptosis rate, flow cytometry was performed using the FITC-Annexin V Apoptosis Detection Kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. Briefly, after washing twice with cold PBS, the osteosarcoma cells (cell density 1×106 cells/mL) were resuspended in 200 μL of 1× binding buffer and then incubated with 5 μL of annexin V-FITC solution and 5 μL of propidium iodide (PI) for 15 min at 4 °C in the dark. Flow cytometry was performed using the BD FACSCalibur system (Beckman Coulter, CA, USA) with wavelength emission filters of 488–530 nm and 488–630 nm for the fluorescence signals of annexin V and PI, respectively.
For cell cycle analysis, the osteosarcoma cells with a good condition were collected as described above and stored at 4 °C overnight. After washing three times with cold 1× PBS, the cells were stained with containing PI (50 μg/mL) in the dark at 37 °C for 30 min. Finally, flow cytometry was performed using the BD FACSCalibur system to analyze the cell cycle. Each assay was independently repeated three times.
Western Blotting
Western blotting was used to analyze the total protein from each sample of cultured osteosarcoma cells following lysis using cold radioimmunoprecipitation buffer (50 mmol/L Tris–HCl pH 7.4, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 150 mmol/L NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and protease inhibitors). Similar amounts of protein were separated using 10% SDS polyacrylamide gels and transferred to nitrocellulose membranes (Millipore). Subsequently, each membrane was soaked in 5% fat-free dry milk in PBS for 1 h at room temperature and then incubated with the primary antibody at 4 °C overnight. Each membrane was then incubated with a fluorescently labeled secondary antibody (Jackson ImmunoResearch) for 1–2 h at 4 °C. The corresponding signals were then visualized using the Odyssey CLx imaging system (LI-COR). The intensity was measured using the ImageJ software.
Immunohistochemistry
Osteosarcoma and osteofibrous dysplasia tissues were obtained, fixed in 4% paraformaldehyde overnight, dehydrated using a graded ethanol series, and embedded in paraffin. The tissue specimens were cut into 4-μm sections and then deparaffinized and rehydrated. Each tissue section was subjected to antigen retrieval using ethylenediaminetetraacetic acid (EDTA), blocked with goat serum, and incubated with the anti-AMPH-1 primary antibody (1:500) at 4 °C overnight. Next, each section was sequentially incubated with secondary antibody and streptavidin–horseradish peroxidase conjugate for 20 and 30 min, respectively, at room temperature. Finally, the tissue sections were stained with 3,3ʹ-diaminobenzidine tetrahydrochloride and then counterstained with hematoxylin and dehydrated. The photographs were captured using the software (Leica Q-Win V2.0, Germany) provided with the microscope (Olympus Inc.).
Xenograft Experiments
The xenograft animal model was established using 6–8-week-old male BALB/c nude mice provided by the Shanghai Laboratory Animal Center (Shanghai, China). The mice were bred in the animal experimental center following procedures approved by East China Normal University and cared for in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Osteosarcoma cells of sh AMPH-1 and sh NC were suspended in cold PBS (cell density 5×106 cells/100 μL) and subsequently subcutaneously injected into the flanks of the mice.
Statistical Measurements
Quantitative data are presented as the mean ± SEM for independent experiments analyzed using the Prism V5.0 software (GraphPad Software). The statistical significance of means was determined using ANOVA, and p values of <0.05 were considered significantly significant.
Results
AMPH-1 Is Downregulated In Osteosarcoma Samples And Knockdown Of AMPH-1 Promotes Osteosarcoma Cell Growth
AMPH-1 was expressed at lower levels in osteosarcoma tissues (), and we therefore supposed that AMPH-1 may possess tumor-suppressing activity. In an effort to elucidate the function of AMPH-1 in osteosarcoma cells, we investigated the knockdown of AMPH-1 in 143B and U-2 OS cells. Western blotting results revealed that the AMPH-1 level clearly decreased after siRNA transfection of the 143B and U-2 OS cells ( and C). Furthermore, knockdown of AMPH-1 in the osteosarcoma cells dramatically enhanced the cell growth compared with the control group, according to the results of colony formation assays ( and ).
Figure 1 Influence of AMPH-1 on osteosarcoma progression. (A) Immunohistochemical results showing AMPH-1 protein expression in osteosarcoma and osteofibrous dysplasia tissue samples. (B, C) Western blotting analysis of AMPH-1 protein expression in 143B cells and U-2 OS cells transfected with scrambled siRNA (si N and si AMPH-1). (D, E) Cell colony formation and statistical analysis for 143B cells and U-2 OS cells transfected with scrambled siRNA (si N and si AMPH-1). (F) Flow cytometry analysis of cell apoptosis for 143B and U-2 OS osteosarcoma cells; FL1-H is annexin V-FITC and FL2-H is PI. (G) Cell cycle distribution and proportion analysis for the AMPH-1 knockdown (sh AMPH-1) and control (sh NC) groups of 143B and U-2 OS cells.
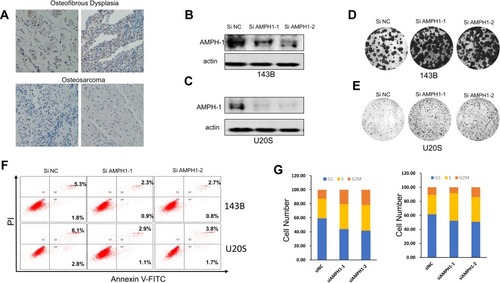
In addition, the role of AMPH-1 in cell apoptosis was examined. The results preliminarily demonstrated that knockdown of AMPH-1 noticeably inhibited the apoptosis of the osteosarcoma cells. Flow cytometry analysis revealed clear decreases in the numbers of early and late apoptotic cells for both 143B and U-2 OS cells after the knockdown of AMPH-1 (). Furthermore, cell cycle analysis results demonstrated that the proportions of osteosarcoma cells in the G2 and S stages increased whereas the proportion in the G1 phase decreased in both cell lines after the knockdown of AMPH-1 (). Consequently, these results suggest that the knockdown of AMPH-1 attenuates cell apoptosis and enhances cell cycle progression in vitro.
Knockdown Of AMPH-1 Activates The MEK/ERK Signaling Pathway And Promotes Osteosarcoma Growth In A Xenograft Mouse Model
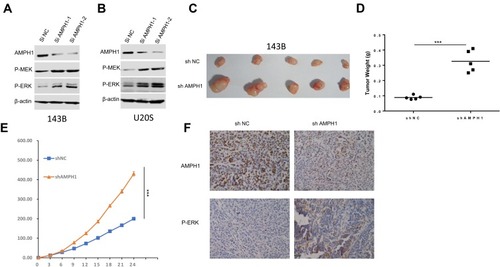
The MEK/ERK signaling pathway is considered to be mainly related to the process of mitogenesis in metazoans, and mutational activation of this signaling pathway is relatively common in human cancer. To dissect the significant role of AMPH-1 in regulating the activity of the MEK/ERK signaling pathway, we evaluated the influence of AMPH-1 knockdown in osteosarcoma cells. Western blotting results indicated that the knockdown of AMPH-1 increased the levels of both MEK and ERK in the 143B and U-2 OS cell lines ( and ). To verify the significant role of AMPH-1 in osteosarcoma tumorigenesis in vivo, a xenograft mouse model was established in which nude mice were subcutaneously inoculated with 143B stable osteosarcoma cells (sh NC or sh AMPH-1). The knockdown of AMPH-1 was found to enhance the proliferation of the osteosarcoma cells in the xenograft model. The AMPH-1-knockdown 143B cells (sh AMPH-1) exhibited considerably higher tumorigenicity compared with the control group (sh NC). The tumors were removed after three weeks, and the tumor volume and weight in the AMPH-1 knockdown group were found to be significantly increased compared with the control group (–). In addition, the immunostaining of p-ERK1/2 was significantly increased in the knockdown group compared with the control group ().
Figure 2 Influence of AMPH-1 knockdown on osteosarcoma cells. (A, B) 143B and U-2 OS cells were treated with scrambled siRNA (si N and si AMPH-1) for 72 h and then collected for Western blotting analysis. The total protein from (A) 143B and (B) U-2 OS cells was loaded and analyzed by immunoblotting with anti-p-MEK and anti-p-ERK antibodies. (C) Comparison of tumor size three weeks after inoculation of nude mice with shAMPH-1 and shNC 143B osteosarcoma cells. (D, E) Knockdown of AMPH-1 significantly increased the final tumor weights and every group involved five tumors. (F). Immunostaining of AMPH-1 and P-ERK in tumor samples.
Overexpression Of AMPH-1 Inhibits Osteosarcoma Growth And Cell Cycle Arrest And Promotes Cell Apoptosis
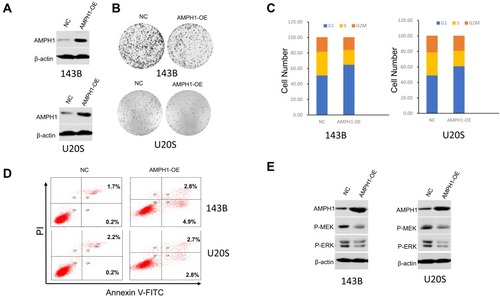
To further confirm that AMPH-1 is a suppressor of osteosarcoma cell proliferation, we developed a gain-of-function assay by transfecting AMPH-1 into 143B and U-2 OS cell lines (). In addition, overexpression of AMPH-1 significantly decreased cell proliferation compared with the controls () by colony formation assay. The results also demonstrated that overexpression of AMPH-1 promoted cell apoptosis and inhibited cell cycle progression, indicating an anti-cancer role ( and ). Western blotting analyses demonstrated that overexpression of AMPH-1 decreased the levels of both MEK and ERK in the 143B and U-2 OS cell lines (). These data indicate that AMPH-1 is an essential factor that acts to protect against human osteosarcoma formation, whose effects are mediated by the MEK/ERK signaling pathway.
Figure 3 Inhibition of osteosarcoma growth and cell cycle arrest and promotion of cell apoptosis by overexpression of AMPH-1. (A) Western blotting analysis of AMPH-1 protein expression in 143B and U-2 OS cells transfected with AMPH-1 expression vector (AMPH-1 OE). (B) Cell colony formation by the transfected 143B and U-2 OS cells. (C) Cell cycle distribution and proportion analysis for the transfected 143B and U-2 OS cells. (D) Flow cytometry analysis of cell apoptosis for the transfected 143B and U-2 OS cells; FL1-H is annexin V-FITC and FL2-H is PI. (E) 143B and U-2 OS cells were treated with AMPH-1 expression vector (AMPH-1 OE) for 24 h and then collected for Western blotting analysis. The total protein was analyzed by immunoblotting with anti-p-MEK and anti-p-ERK antibodies.
Discussion And Conclusions
AMPH-1 is a protein that is extremely abundant in nerve terminals.Citation14 Previous studies have revealed that AMPH-1 is a highly dominant autoantigen that is also associated with breast cancer and paraneoplastic sensory neuropathy and present in normal human mammary tissue.Citation17 In recent years, several studies have indicated that AMPH-1 also plays a significant role in suppressing cancer by inhibiting signaling pathways.Citation15 However, to date there has been no report concerning the association between AMPH-1 and bone tumors. This study has demonstrated for the first time the significant role of AMPH-1 in the mechanism of osteosarcoma formation and progression.
In the present study, we have demonstrated for the first time that the knockdown of AMPH-1 dramatically enhances human osteosarcoma cell proliferation both in vitro and in vivo, attenuates apoptosis, and enhances cell cycle progression. To investigate the cellular function of AMPH-1 in osteosarcoma in vivo, we established a xenograft nude mouse model to explore the influence of AMPH-1 on osteosarcoma growth. The in vivo and in vitro results demonstrated that cell growth, tumor volume, and tumor weight dramatically increased after the knockdown of AMPH-1, indicating that AMPH-1 plays a protective role to prevent osteosarcoma. Indeed, gain-of-function experiments proved that high expression of AMPH-1 protected against human osteosarcoma formation. Similar results were also found for other cancers, such as breast cancer and non-small cell lung cancer. Taken together, these findings indicate that AMPH-1 acts as a tumor suppressor that inhibits cancer progression.
The MEK/ERK pathway (also known as the Ras/Raf/MEK/ERK pathway) is an important signaling pathway that plays a critical role in various cell functions involving the cell cycle, proliferation, and differentiation, and is also a crucial downstream signaling pathway of angiogenesis. Previous studies have revealed that the MEK/ERK signaling pathway is often aberrantly activated in various cancers and it has therefore become an promising target in the development of novel antineoplastic targeted drugs.Citation15 In the present study, we found that the knockdown of AMPH-1 in osteosarcoma cells dramatically activated the MEK/ERK signaling pathway, as determined via Western blotting. Therefore, AMPH-1 may serve as a tumor suppressor during human osteosarcoma development by inhibiting the MEK/ERK signaling pathway. A previous study reported the detection of AMPH-1 antibodies in the serum of patients with small-cell lung carcinoma, which may be linked to the clinical presentation.Citation18 Furthermore, Otsuka et al reported that AMPH-1 has the ability to suppress vitronectin-mediated cell adhesion, movement, and migration in vitro.Citation19 Consequently, it would be interesting to investigate the influence of AMPH-1 on several other signaling pathways associated with cancer formation and metastasis to further explore its role in the mechanism of osteosarcoma. Previous studies have indicated that mutations of the kinases of the MEK/ERK pathway occur frequently in various types of human cancers.Citation20 The results obtained in this study demonstrate that AMPH-1 downstream might serve as a significant effector in the MEK/ERK signaling pathway according to knockdown or overexpression of AMPH-1, although the detailed mechanism should be elucidated in future studies.
Overall, the results obtained in this study demonstrate that AMPH-1 plays an important role in osteosarcoma development via the MEK/ERK signaling pathway. The knockdown of AMPH-1 contributes to cancer cell proliferation and enhanced cell growth both in vivo and in vitro. These findings may assist in the identification and development of novel therapeutic approaches for treating osteosarcoma.
Ethics Statement
All experimental protocols and methods were approved by the Sixth People’s Hospital of Nantong. Mice were bred in the Animal Core Facility following procedures approved by the Institutional Animal Care and Use Committee of the Sixth People’ s Hospital of Nantong.
Author Contributions
All authors contributed toward data analysis and drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13.20213383
- Franke M, Hardes J, Helmke K, et al. Solitary skeletal osteosarcoma recurrence. Findings from the cooperative osteosarcoma study group. Pediatr Blood Cancer. 2011;56:771–776.21370409
- de Azambuja E, Ameye L, Paesmans M, Zielinski CC, Piccart-Gebhart M, Preusser M. The landscape of medical oncology in Europe by 2020. Ann Oncol. 2014;25:525–528. doi:10.1093/annonc/mdt55924425791
- Pittock SJ, Lucchinetti CF, Parisi JE, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol. 2005;58:96–107. doi:10.1002/ana.v58:115984030
- Wigge P, Kohler K, Vallis Y, et al. Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis. Mol Biol Cell. 1997;8:2003–2015. doi:10.1091/mbc.8.10.20039348539
- De Jesus-Cortes HJ, Nogueras-Ortiz CJ, Gearing M, Arnold SE, Vega IE. Amphiphysin-1 protein level changes associated with tau-mediated neurodegeneration. Neuroreport. 2012;23:942–946. doi:10.1097/WNR.0b013e32835982ce22975846
- Sekiguchi M, Katayama S, Hatano N, Shigeri Y, Sueyoshi N, Kameshita I. Identification of amphiphysin 1 as an endogenous substrate for CDKL5, a protein kinase associated with X-linked neurodevelopmental disorder. Arch Biochem Biophys. 2013;535:257–267. doi:10.1016/j.abb.2013.04.01223651931
- Wu Y, Matsui H, Tomizawa K. Amphiphysin I and regulation of synaptic vesicle endocytosis. Acta Med Okayama. 2009;63:305–323. doi:10.18926/AMO/3182220035287
- Takei K, Slepnev VI, Haucke V, De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol. 1999;1:33–39. doi:10.1038/900410559861
- Decamilli P, Thomas A, Cofiell R, et al. The synaptic vesicle-associated protein amphiphysin is the 128-kD autoantigen of stiff-man syndrome with breast cancer. J Exp Med. 1993;178:2219–2223. doi:10.1084/jem.178.6.22198245793
- David C, McPherson PS, Mundigl O, de Camilli P. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc Natl Acad Sci U S A. 1996;93:331–335. doi:10.1073/pnas.93.1.3318552632
- Folli F, Solimena M, Cofiell R, et al. Autoantibodies to a 128-kd synaptic protein in three women with the stiff-man syndrome and breast cancer. N Engl J Med. 1993;328:546–551. doi:10.1056/NEJM1993022532808058381208
- Mundigl O, Ochoa GC, David C, Slepnev VI, Kabanov A, De Camilli P. Amphiphysin I antisense oligonucleotides inhibit neurite outgrowth in cultured hippocampal neurons. J Neurosci. 1998;18:93–103. doi:10.1523/JNEUROSCI.18-01-00093.19989412489
- Yoo J, Jeong MJ, Kwon BM, Hur MW, Park YM, Han MY. Activation of dynamin I gene expression by Sp1 and Sp3 is required for neuronal differentiation of N1E-115 cells. J Biol Chem. 2002;277:11904–11909. doi:10.1074/jbc.M11178820011809758
- Yang H, Wan Z, Huang C, Yin H, Song D. AMPH-1 is a tumor suppressor of lung cancer by inhibiting Ras-Raf-MEK-ERK signal pathway. Lasers Med Sci. 2019;34:473–478. doi:10.1007/s10103-018-2616-430143925
- Crouzet M, Urdaci M, Dulau L, Aigle M. Yeast mutant affected for viability upon nutrient starvation: characterization and cloning of the RVS161 gene. Yeast. 1991;7:727–743. doi:10.1002/(ISSN)1097-00611776363
- Floyd S, Butler MH, Cremona O, et al. Expression of amphiphysin I, an autoantigen of paraneoplastic neurological syndromes, in breast cancer. Mol Med. 1998;4:29–39. doi:10.1007/BF034017279513187
- Dropcho EJ. Antiamphiphysin antibodies with small-cell lung carcinoma and paraneoplastic encephalomyelitis. Ann Neurol. 1996;39:659–667. doi:10.1002/(ISSN)1531-82498619552
- Otsuka A, Hirose K, Kilimann MW, Kamata T. Amphiphysin1 inhibits vitronectin-mediated cell adhesion, spreading, and migration in vitro. Biochem Biophys Res Commun. 2003;301:769–775. doi:10.1016/S0006-291X(03)00040-812565847
- Faghfuri E, Nikfar S, Niaz K, Faramarzi MA, Abdollahi M. Mitogen-activated protein kinase (MEK) inhibitors to treat melanoma alone or in combination with other kinase inhibitors. Expert Opin Drug Metab Toxicol. 2018;14:317–330. doi:10.1080/17425255.2018.143259329363351
