Abstract
Purpose
Metadherin (MTDH), as an oncogene, is associated with metastasis and poor prognosis. This study investigated MTDH expressions and development of gastric cancer (GC) cell phenotypes and the contribution of MTDH to epithelial–mesenchymal transition (EMT).
Patients and methods
MTDH expression was assayed in human GC cell lines and tumor tissue from 92 GC patients. Functional experiments were performed to characterize MTDH activity. Expressions of EMT-related proteins (vimentin and E-cadherin), phosphorylated β-catenin and β-catenin were assayed by immunohistochemistry, Western blotting, immunofluorescence, and co-immunoprecipitation, respectively.
Results
MTDH expressions were higher in GC tissue than that in gastric mucosa from the same patient. MTDH overexpression was correlated with metastasis and enhanced malignant GC phenotypes, i.e., proliferation, migration, invasiveness, and chemoresistance. MTDH overexpression was associated with expressions of vimentin, E-cadherin and cancer stem-cell biomarkers including CD44, CD133, and Oct4. MTDH complexed with β-catenin and decreased phosphorylated β-catenin levels to facilitate β-catenin translocation into the nucleus and expressions of downstream genes.
Conclusion
MTDH overexpression in GC cells is associated with EMT and development of cancer stem cell malignant phenotypes and affects the subcellular translocation of β-catenin. The results warrant investigation of the prognostic value of MTDH in GC and as a therapeutic target.
Introduction
An estimated 679,100 new cases of gastric cancer (GC) were diagnosed in China in 2015, making it the second leading cause of cancer-related death.Citation1 Despite recent advances in early diagnosis and treatment effectiveness, only 30% of GC patients survive for 5 years.Citation2 Recurrence, metastasis, and drug resistance are main obstacles to improve the response of treatment. The better understanding mechanisms that contribute to tumorigenesis are urgently needed.
Metadherin (MTDH, also known as AEG-1 and LYRIC), was first cloned in primary human fetal astrocytes infected with human immunodeficiency virus (HIV)-1 or exposed to HIV-1 envelope glycoprotein (gp)-120 in 2002.Citation3,Citation4 As an oncogene, MTDH is involved in development and progression of many cancers, including proliferation, invasion, metastasis and chemoresistance.Citation5–Citation9 MTDH can also induce epithelial–mesenchymal transition (EMT), which supports the malignant behaviors of late-stage cancers,Citation6,Citation7,Citation10 but the roles of MTDH in promoting EMT, metastasis, and tumor heterogeneity have not been fully described.
MTDH was found to influence several signaling pathways, including Wnt/β-catenin, NF- κB, PI3K/Akt and Ha-ras.Citation11–Citation14 Moreover, Wnt/β-catenin pathway is known to promote EMTCitation15 and contribute to the maintenance of cancer stem cell (CSC) populations within tumor tissues that retain the capacity of self-renewal and differentiation.Citation16,Citation17 CSCs are active in chemoresistance, immune evasion, and development of cancer cell phenotypes with increasing invasiveness.Citation18 CD44, CD133, Oct-4, and ALDH expressions may serve to distinguish GC CSCs.Citation19,Citation20
Giving the unknown roles of MTDH in GC progression, the study aims to clarify the effects of MTDH in acquisition of malignant phenotypes in GC based on the tissues from GC patients and human GC cell lines, especially the relationships between MTDH- and EMT-associated genes or CSC biomarkers that were not reported before.
Materials And Methods
Patients And Tissue Specimens
Paraffin-embedded tissues of primary GC tumors and normal gastric mucosa were obtained from each of 92 patients (70 males and 22 females) with surgical resection for GC from January 2005 to March 2006. The GC diagnosis was confirmed by two pathologists. None of patients had received preoperative chemotherapy or radiotherapy. Patient clinicopathological characteristics were retrieved from clinical records and pathology reports. mRNA expressions were assayed in cancer and normal gastric mucosa from 12 patients with surgical resection in 2017.
Antibodies And Reagents
The primary antibodies were MTDH (ab45338), CD133, and LEF1 (Abcam, Cambridge, MA, USA), CD44, Oct4, CDH1/E-cadherin, and vimentin (Origene, Rockville, MD, USA), phosphorylated β-catenin and CTNNB1/β-catenin (Cell Signaling Technology; Danvers, MA 8480, USA). β-actin was obtained from ZSGB-bio (Beijing, China). IRDye 800-conjugated affinity purified anti-mouse IgG and IRDye 700DX-conjugated affinity purified anti-rabbit IgG secondary antibodies were purchased from Rockland Immunochemicals (Limerick, PA, USA) and CF666 anti-rabbit IgG secondary antibody was purchased from Biotium (Hayward, CA, USA).
Cell Culture
MKN45, MKN28, SGC7901, MGC803, and AGS human gastric cancer cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). MKN45, MKN28, SGC7901, and MGC803 cells were cultured in RPMI-1640 medium and AGS cells were cultured in F12 medium (both Gibco, Grand Island, NY, USA) at 37°C in 5% CO2 with humidity. Culture media were supplemented with 10% fetal bovine serum (FBS; PAA Laboratories, Pasching, Austria).
Plasmid And siRNA Transfection
Plasmids expressing MTDH (EX-RC207238) and siRNAs (SR314617) were obtained from Origene (Rockville, MD, USA). Lenti-Pac HIV Expression Packaging Kits and control plasmids were purchased from Genecopoeia (Rockville, MD, USA). Transfections of cells overexpressing MTDH was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). The primer sequences were listed in Supplementary Table 1. HilyMax reagent (Dojindo, Japan) was used for transient transfection as indicated instructions.
Table 1 Correlation Between MTDH Expression And Clinicopathological Features Of GC
Immunohistochemical Staining And Evaluation
Paraffin-embedded tissues were sectioned, dewaxed, hydrated, heated in EDTA (pH 7.2) for antigen retrieval, and inhibited with 3% hydrogen peroxide. The sections were incubated with primary antibodies overnight at 4°C and the antigen–antibody reactions was developed with a horseradish peroxidase–diaminobenzidine (HRP–DAB) substrate kit (ZSGB-bio, Beijing, China). Slides were counterstained with Mayer’s hematoxylin. The immunostaining index was reported as the proportion of positively stained tumor cells and staining intensity and was scored by two independent observers as previously described.Citation19 The results were scored semi-quantitatively by intensity and the proportion of positive tumor cells. The proportion of positively stained tumor cells was graded as 0 (no positively stained cells), 1 (<25%), 2 (25%–49%), 3 (50%–75%), and 4 (>75% positive cells). Staining intensity was scored as 0 (no staining), 1 (light yellow), 2 (yellow-brown), and 3 (brown-yellow). The immunostaining index was the staining intensity score multiplied by the percentage of positively stained tumor cells. Tumor with indexes of 0–2 were negative and those with indexes of 3–12 were positive.
Quantitative Real-Time PCR (qPCR)
Total RNA was extracted from harvested cultured cells or tissue samples with Trizol (Invitrogen). cDNA was synthesized with Transcriptor First Strand cDNA Synthesis Kits (Roche, Mannheim, Germany). Semi-quantitative and qPCR were performed with cDNA as the template using a LightCycler 480 SYBR Green I system (Roche).
Western Blot
Cells were lysed in RIPA buffer to extract total protein. Protein samples were separated by SDS-PAGE gel electrophoresis and transferred to PVDF membranes. After blocking with 5% blocking buffer, the membranes were incubated with primary antibodies at 4ºC overnight. HRP-conjugated anti-mouse and anti-rabbit antibodies were the secondary antibodies. The membranes were scanned using an Odyssey (Li-COR, Lincoln, NE, USA) or Fluorchem R (Santa Clara, CA, USA) imaging system.
Cell Proliferation And Viability Assays
Cells were seeded in 96-well plates at 1.5×103 cells per well and incubated for 24, 48, 72, 96, 120, or 148 hrs before pipetting MTS reagent (Promega, WI, USA). After incubating for 3 hrs at 37ºC in 5% CO2, the absorbance at 490 nm was read using a 96-well plate reader.
Colony Formation Assay
Cells were seeded with shaking into six-well plates at 400 cells per well. Colonies were stained with 0.2% crystal violet for 15 mins at 10 to 14 days, photographed, and counted after drying.
Drug Resistance Assay
Cells were seeded in 96-well plates (5 × 103 cells per well) and cultured under standard conditions for 48 hrs with 0, 1, 10, 100, 1000 μmol/L 5-fluoruracil (5-FU) or 0, 3.125, 6.25, 12.5, 25, or 50 μg/mL oxaliplatin for 48 hrs. The medium was then replaced with 100 μL fresh medium plus 20 μL MTS and the absorbance was read at 490 nm as described above. Cell viability was calculated as the percentage of surviving cells = (ODdrug-treated − ODblank control)/(ODvector control − ODempty control) ×100.
Transwell Migration And Invasion Assays
Cell migration and invasion were assays in 24-well plates with 0.4-μm pore size hanging inserts (Costar, Kennebunk, ME, USA) and 24-well Matrigel-coated invasion chambers (BD Biosciences, Bedford, USA). Cells (5 × 104) resuspended in serum-free medium were added to the upper chambers. Medium with 10% FBS was added to the lower chambers. The cells remaining in the upper chamber were removed after 30-hr culture, and the lower surface of membrane was fixed, stained with 0.2% crystal violet. The cells in five randomly selected fields from each membrane were counted with a Nikon D3-L3 system.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X (Sigma-Aldrich), blocked with 4% bovine serum albumen and incubated with primary antibody overnight at 4ºC before incubating with secondary antibody for 1 hr at room temperature. Cells were counterstained with anti‐fade DAPI (Invitrogen) and observed by fluorescent confocal microscopy (DM-RXA2, Leica). Images were processed with a MetaMorph Imaging System (Universal Imaging Corp).
Co-Immunoprecipitation Assay
MKN28 cells transfected with plasmids were harvested with NP-40 lysis buffer (Wanlei Bio, Shenyang, China). An 80 µL volume of the supernatant was used as input, and the remaining was as IP. The samples were incubated with 5 µg anti-MTDH antibody (Abcam, Cambridge, MA, USA) and anti-IgG antibody (Cell Signaling Technology; Danvers, MA, USA) and then rotated for 4 hrs at 4ºC. After centrifugation, the beads were washed with lysis buffer and protein complex was eluted, resolved by SDS-PAGE and boiled. The entire supernatant was assayed by Western blot.
Statistical Analysis
Statistical analysis was performed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad 5.0 (https://www.graphpad.com). Differences in MTDH expression and clinicopathological characteristics were evaluated using Pearson’s χ2 test. Independent Student's t-tests and analysis of variance were used for analysis of enumeration data obtained in the in vitro assays. Statistical significance was defined as *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001.
Results
MTDH Was Upregulated In Human GC Cells And Associated With Metastasis And Patient Prognosis
The expression of MTDH mRNA was upregulated in GC compared with the paired gastric mucosal tissues obtained from 12 patients during gastric resection surgery (). Immunohistochemical staining of 92 postoperative specimens ( and ) found that MTDH expression was localized in the cytoplasm and cell membrane. Expression was higher in GC (51%) than that in mucosal tissues (15%, p < 0.001). As shown in , MTDH overexpression was significantly correlated with T (p < 0.001), N (p = 0.001), and recurrence (p = 0.019). Western blot found that MTDH protein expressions () were higher in MKN45, MGC803, and SGC7901 cells than those in MKN28 and AGS cells. Therefore, two cell lines with relatively high MTDH expressions (MKN45 and MGC803) or with relatively low expressions (MKN28 and AGS) were used for the subsequent investigation.
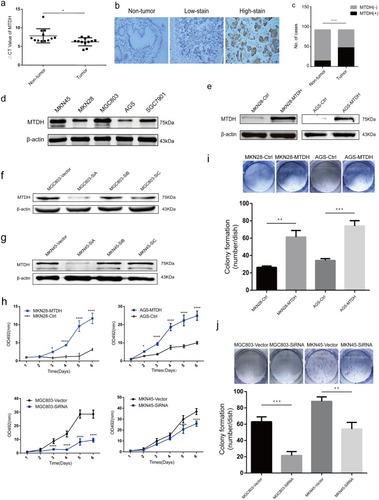
Figure 1 MTDH is significantly elevated in GC and promotes proliferation and clonogenicity in different GC cell lines. (A) mRNA expression of MTDH in GC and paired non-tumor mucosa (n=12). y-Axis indicates the ΔCt value. (B) Images of MTDH immunochemistry stain in non-tumor mucosa and primary cancer specimens were negative, low and high expressions as followed (magnification fold was 400×). (C) Bar chart summary of MTDH expressions in paired non-tumor mucosa and cancer tissues in b. + or - stand for high or low stain, respectively, and y-axis represents the number of cases in each group. (D) The MTDH levels in GC cell lines. (E) Western blot showed MTDH expression of transfection groups elevated versus control in MKN28 and AGS. (F) and (G) SiA sequence could significantly inhibit expressions of MTDH protein levels in MGC803 and MKN45. (H) MTS assays were performed in MTDH-upregulated MKN28 and AGS cells and MTDH-downregulated MGC803 and MKN45 cells. (I) and (J) The colony formation assay was carried out to detect colony formation ability. All experiments were repeated three times, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
MTDH Enhanced GC Cell Proliferation And Clonogenicity
Western blot confirmed successful establishment of stable MTDH expressions in MKN28 and AGS cells (). The MTS showed that the proliferation rates were higher in MKN28 and AGS cells transfected with MTDH plasmids than those transfected with empty plasmids (). MTDH-transfected cells also formed more and bigger colonies than control cells (). MTDH expressions were successfully knocked down in MKN45 and MGC803 cells that were transiently transfected with three anti-MTDH SiRNAs ( and ). And downregulation of MTDH expressions was accompanied with decreasing colony formation activity and cell proliferation ().
MTDH Regulated GC Cells Migration, Invasion, And Drug Resistance
Transwell migration and invasion assays were used to clarify association of MTDH expressions with the behavior of GC cells. Upregulation of MTDH expressions increased the numbers of MKN28 and AGS cells that passed through the transwell chamber membranes compared with control cells (). Downregulation of MTDH in MKN45 and MGC803 cells decreased cell invasiveness compared with control groups (). The numbers of fixed and stained cells passing through the membranes in migration assay were consistent with those observed in invasion assay ().
Figure 2 MTDH increases the migration and invasion capacity of GC cells and enhances 5-FU and Oxaliplatin chemoresistance. (A) Migration, (B) invasion assay showed that MTDH overexpression increased the cells numbers passing through membranes compared with control (p<0.05). Simultaneously, MTDH downregulation decreased the capability of migration and invasion versus vector (p<0.05). (C) And (D) to ascertain the effect of MTDH on drug resistance of GC cell lines, we tested drug sensitivity in MTDH-overexpression and downregulation cells at different concentration of oxaliplatin and 5-FU using an MTS assay. The results were shown as mean±SD. SD, standard deviation. All experiments were repeated three times, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
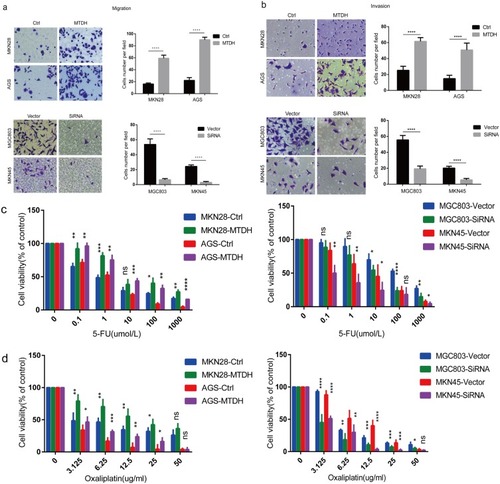
Drug resistance leads to poor prognosis. The influence of MTDH expression on GC resistance was assayed after 48-hr exposure to increasing concentrations of 5-FU and oxaliplatin, both of which are widely used first-line drugs. The MTS assay indicated MTDH overexpression in MKN28 and AGS cells increased 5-FU and oxaliplatin resistance, but downregulation increased drug sensitivity in MKN45 and MGC803 cells ( and ).
Expression Of MTDH In GC Induces EMT And Was Correlated With β-Catenin Nuclear Translocation
EMT is a key step in GC cell metastasis. The association of MTDH expressions and appearance of malignant phenotypes of GC cells was investigated by assaying the expressions of EMT markers with immunohistochemical (IHC) staining of GC patient tissue specimens. The expression of vimentin, which is a mesenchymal marker, was positively correlated with MTDH expressions (). The expressions of E-cadherin, an epithelial marker, were negatively correlated with MTDH expressions (). The results are consistent with promotion of EMT by overexpression of MTDH in GC.
Figure 3 MTDH induces EMT and affects the subcellular translocation of β-catenin. (A) and (B) Immunohistochemical staining of vimentin and E-cadherin in GC samples (magnification, 400×). Statistical analysis showed that MTDH expressions were negatively correlated with E-cadherin expressions, while, positively correlated with vimentin expressions (P<0.05). (C) Immunohistochemical expression MTDH and β-catenin in human GC samples (magnification, 400×). (D) Western blot analysis of MTDH, E-cadherin, vimentin, phosphorylated β-catenin (p-β-catenin), β-catenin and LEF-1 in GC cell lines. (E) Immunofluorescence displayed that MTDH overexpression increased β-catenin nucleus ectopic expression. (F) Correspondingly, knockdown of MTDH decreased β-catenin nucleus expression (scale bar, 20 μm). (G) Co-immunoprecipitation (Co-ip) was carried out in MTDH-overexpressed cell line MKN28.
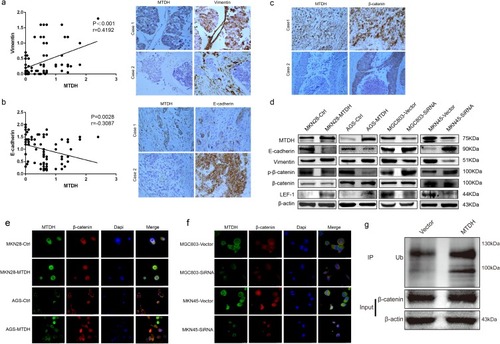
Aberrant activation of the WNT/β-catenin pathway plays important roles in EMT and metastasis in cancer.Citation21 β-Catenin is degraded by the proteasome after ubiquitylation when acting as a target of GSK3β that maintains the phosphorylation state of β-catenin. Thereby, blocking the phosphorylation event allows β-catenin to accumulate.Citation22 In canonical Wnt/β-catenin pathway, activation of signaling follows nuclear translocation of β-catenin. MTDH overexpression was associated with ectopic β-catenin expression in the nucleus ( and ). We further determined the protein expression levels of E-cadherin, vimentin, phosphorylated β-catenin, β-catenin, and LEF1 by Western blot in GC cell lines and showed that E-cadherin expression and phosphorylated β-catenin decreased, and vimentin expression increased in MTDH-overexpressed MKN28 and AGS cells and decreased when MTDH expression was knocked down (). β-Catenin protein expression was not significantly changed by knockdown of MTDH expression nor was that of LEF1, another Wnt/β-catenin signaling protein (). The translocation of β-catenin in GC cells was shown by immunofluorescence staining, which showed that overexpression of MTDH in MKN28 and AGS cells was accompanied by β-catenin expression in nucleus (), but downregulation led to the absence of β-catenin nuclear expression in MKN45 and MGC803 cells (). Overall, the results showed that MTDH mediated EMT and affected the subcellular translocation of β-catenin. Co-immunoprecipitation in MKN28 cells transfected with MTDH () provided direct evidence of physical interaction between MTDH and β-catenin, meanwhile MTDH expression resulted in decrease of phosphorylated β-catenin that contributes to accumulate β-catenin (), which could account for the prevention of β-catenin degradation and facilitation of β-catenin translocation in cell nucleus.
Table 2 Relationship Between MTDH And β-Catenin Expressions
MTDH Overexpression In GC Cells Was Associated With The Expressions Of CSC Markers
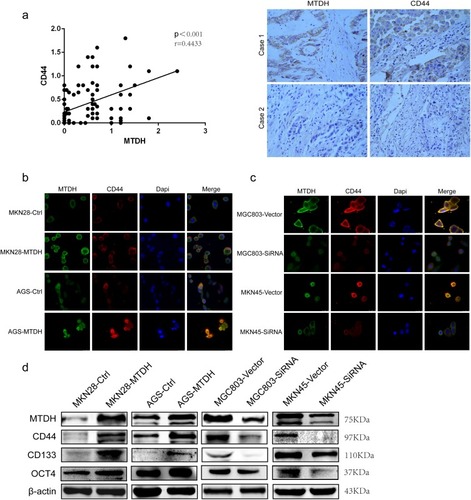
Wnt/β-catenin signaling may promote development and maintenance of CSCs. MTDH overexpression was positively correlated with expression of CD44, which is a CSC marker (). Immunofluorescent staining confirmed that MTDH overexpression or knockdown could increase or decrease CD44 expression, respectively ( and ). Western blot showed that MTDH overexpression or downregulation could increase or decrease the expressions of CD44, CD133, and Oct4, respectively, which are CSC markers (). The results supported MTDH could lead to expansion of CSC population and be a participant in molecular mechanisms of metastasis and drug resistance in GC.
Figure 4 MTDH promotes CSCs-related markers expressions. (A) Immunohistochemical staining of stem cell marker CD44 in human GC samples, Spearman correlation analysis indicated that MTDH expressions were positively correlated with CD44 in 92 GC specimens. (B) and (C) Overexpression of MTDH increases expression of CD44, whereas downregulation of MTDH decreased protein expression of CD44 (scale bar, 20 μm). (D) Western blot indicated the correlation of MTDH and CSCs-related markers CD44, CD133 and OCT4 expressions in MKN28, AGS, MGC803 and MKN45 cell lines.
Discussion
MTDH is an oncogene known to be active in human malignancies.Citation7,Citation10,Citation23 This study investigated the roles of MTDH in promoting malignant GC phenotypes. The expressions of MTDH increased in GC tumor tissues, which was significantly correlated with multiple clinicopathological characteristics (). MTDH expression is thus associated with tumorigenesis and predicts a worse prognosis in GC. It is reported that MTDH induced inflammation, promoted gastric tumorigenesis, and indicated a poor prognosis.Citation8 Moreover, MTDH induced GC metastasis with upregulation of eIF4E expression.Citation24 Our findings are in line with others and confirm the roles of MTDH in tumorigenesis and EMT, but an explanation of how prognosis is affected remains incomplete.
Metastasis, recurrence, and drug resistance contribute to the progression of GC and are obstacles to successful treatment. MTDH overexpression significantly increased cell proliferation, colony formation, migration, invasiveness and enhanced drug resistance. Furthermore, MTDH downregulation inhibited aforementioned biological properties and increased drug sensitivity. The results found that MTDH was a key promotor of GC progression, which facilitated tumor metastasis and drug resistance.
EMT is a key step in the development of cancer metastasis that allows tumor cells to escape from the primary tissue to surrounding tissues.Citation25 EMT also involves the acquisition of invasive potential by primary cancer cells via loss of epithelial cell polarity to gain a mesenchymal cell phenotype.Citation26 EMT is accompanied by an increase in the expressions of mesenchymal markers like vimentin and loss of expressions of epithelial markers like E-cadherin.Citation27 MTDH is active during EMT in lung and breast cancer.Citation7,Citation28 In our study, overexpression of MTDH was correlated with decreased expression of E-cadherin and increased expression of vimentin. MTDH was thus associated with the expressions of EMT markers and metastasis.
Previous studies described the roles of MTDH in various signaling pathways,Citation12–Citation15 including the Wnt/β-catenin pathway, which has been associated with EMT.Citation29 In the canonical Wnt/β-catenin signaling pathway, activated β-catenin is translocated into the nucleus where it forms a transcriptional complex with LEF/TCF to induce transcription of downstream genes implicated in carcinogenesis.Citation15 And MTDH interacts with β-catenin to increase tumor cell migration and invasion in colorectal carcinoma.Citation30 In this study, MTDH overexpression was colocalized with β-catenin in the nucleus, decreased the phosphorylated β-catenin expression levels but not β-catenin, which indicated MTDH allowed or prevented β-catenin protein translocation from the cytoplasm into the nucleus. What is more, LEF1 expression corresponded with changes of MTDH expression. The results showed that MTDH was an activator to regulate the subcellular translocation of β-catenin, which may hint the roles of Wnt/β-catenin signaling pathway in the abovementioned process. Therefore, a further study of Wnt/β-catenin signaling pathway in this study is required in the future. Co-immunoprecipitation confirmed that MTDH directly interacted with β-catenin, consistent with the accumulation of β-catenin in cell nucleus by MTDH.
Wnt/β-catenin signaling pathway was active in the maintenance of CSC phenotypes and their undifferentiated state.Citation31–Citation33 They express surface proteins including CD44, CD133, and Oct4, which may serve as markers to identify gastric CSCs.Citation19,Citation34 It has been reported that MTDH overexpression in CD133+ glioma cells maintains stemness, differentiation, and drug resistance via Wnt/β-catenin signaling.Citation35 In this study, MTDH expression affected expression of at least one CSC marker. IHC of GC patient specimens found that MTDH and CD44 expressions were positively correlated. Importantly, the relationship of MTDH expression with that of CSCs has seldom been reported.
Conclusion
We conclude that MTDH overexpression is closely related to increased aggressiveness and predicted poor prognosis in GC. MTDH induces EMT and expressions of CSC characteristics and affects the subcellular translocation of β-catenin. The findings support future study of MTDH as a prognostic marker and therapeutic target of GC.
Abbreviations
MTDH, metadherin; GC, gastric cancer; EMT, epithelial–mesenchymal transition; CSCs, cancer stem cells; 5-FU, 5-fluoruracil; CO-IP, co-immunoprecipitation.
Ethics Approval And Informed Consent
Our study was granted ethical approval by Ethical Committee of Harbin Medical University Cancer Hospital, and all the patients provided written informed consent.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We thank Dr. Meng and all our colleagues for helpful discussions. We also thank International Science Editing for editing this manuscript.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.2133826808342
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi:10.3322/caac.2125425559415
- Su ZZ, Kang DC, Chen Y, et al. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21(22):3592–3602. doi:10.1038/sj.onc.120544512032861
- Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353(1):8–15. doi:10.1016/j.gene.2005.04.00615927426
- Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5(4):365–374.15093543
- Liu X, Wang D, Liu H, et al. Knockdown of astrocyte elevated gene-1 (AEG-1) in cervical cancer cells decreases their invasiveness, epithelial to mesenchymal transition, and chemoresistance. Cell Cycle. 2014;13(11):1702–1707. doi:10.1016/j.gene.2005.04.00624675891
- He W, He S, Wang Z, et al. Astrocyte elevated gene-1(AEG-1) induces epithelial-mesenchymal transition in lung cancer through activating Wnt/beta-catenin signaling. BMC Cancer. 2015;15:107. doi:10.1186/s12885-015-1124-125880337
- Li G, Wang Z, Ye J, et al. Uncontrolled inflammation induced by AEG-1 promotes gastric cancer and poor prognosis. Cancer Res. 2014;74(19):5541–5552. doi:10.1158/0008-5472.CAN-14-096825092897
- Tang J, Shen L, Yang Q, Zhang C. Overexpression of metadherin mediates metastasis of osteosarcoma by regulating epithelial-mesenchymal transition. Cell Prolif. 2014;47(5):427–434. doi:10.1111/cpr.1212925174891
- Zhu K, Dai Z, Pan Q, et al. Metadherin promotes hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2011;17(23):7294–7302. doi:10.1158/1078-0432.CCR-11-132721976539
- Mao J, Fan S, Ma W, et al. Roles of Wnt/beta-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014;5:e1039. doi:10.1158/1078-0432.CCR-11-132724481453
- Emdad L, Sarkar D, Su ZZ, et al. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: implications for tumor progression and metastasis. Cancer Res. 2006;66(3):1509–1516. doi:10.1158/0008-5472.CAN-05-302916452207
- Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27(8):1114–1121. doi:10.1038/sj.onc.121071317704808
- Hu G, Wei Y, Kang Y. The multifaceted role of MTDH/AEG-1 in cancer progression. Clin Cancer Res. 2009;15(18):5615–5620. doi:10.1158/1078-0432.CCR-09-004919723648
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi:10.1016/j.cell.2012.05.01222682243
- Deshpande AJ, Ahmed F, Buske C. Identification of murine and human acute myeloid leukemia stem cells. Methods Mol Biol. 2009;568:21–35. doi:10.1007/978-1-59745-280-9_319582419
- Rocco A, Compare D, Nardone G. Cancer stem cell hypothesis and gastric carcinogenesis: experimental evidence and unsolved questions. World J Gastrointest Oncol. 2012;4(3):54–59. doi:10.4251/wjgo.v4.i3.5422468184
- Iv Santaliz-Ruiz LE, Xie X, Old M, Teknos TN, Pan Q. Emerging role of nanog in tumorigenesis and cancer stem cells. Int J Cancer. 2014;135(12):2741–2748. doi:10.1002/ijc.2869024375318
- Brungs D, Aghmesheh M, Vine KL, Becker TM, Carolan MG, Ranson M. Gastric cancer stem cells: evidence, potential markers, and clinical implications. J Gastroenterol. 2016;51(4):313–326. doi:10.1007/s00535-015-1125-526428661
- Takaishi S, Okumura T, Tu S, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27(5):1006–1020. doi:10.1002/stem.3019415765
- Li Q, Lai Q, He C, et al. RUNX1 promotes tumour metastasis by activating the Wnt/beta-catenin signalling pathway and EMT in colorectal cancer. J Exp Clin Cancer Res. 2019;38(1):334. doi:10.1186/s13046-019-1330-931370857
- Metcalfe C, Bienz M. Inhibition of GSK3 by Wnt signalling–two contrasting models. J Cell Sci. 2011;124(Pt21):3537–3544. doi:10.1242/jcs.09199122083140
- Gnosa S, Zhang H, Brodin VP, Carstensen J, Adell G, Sun XF. AEG-1 expression is an independent prognostic factor in rectal cancer patients with preoperative radiotherapy: a study in a Swedish clinical trial. Br J Cancer. 2014;111(1):166–173. doi:10.1038/bjc.2014.25024874474
- Wu S, Yang L, Wu D, et al. AEG-1 induces gastric cancer metastasis by upregulation of eIF4E expression. J Cell Mol Med. 2017;21(12):3481–3493. doi:10.1111/jcmm.1325828661037
- Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(Suppl 7):vii89–vii92. doi:10.1093/annonc/mdq29220943648
- Yoon C, Cho SJ, Chang KK, Park DJ, Ryeom SW, Yoon SS. Role of Rac1 pathway in epithelial-to-mesenchymal transition and cancer stem-like cell phenotypes in gastric adenocarcinoma. Mol Cancer Res. 2017;15(8):1106–1116. doi:10.1158/1541-7786.MCR-17-005328461325
- Yao L, Zhang D, Zhao X, et al. Dickkopf-1-promoted vasculogenic mimicry in non-small cell lung cancer is associated with EMT and development of a cancer stem-like cell phenotype. J Cell Mol Med. 2016;20(9):1673–1685. doi:10.1111/jcmm.1286227240974
- Li X, Kong X, Huo Q, et al. Metadherin enhances the invasiveness of breast cancer cells by inducing epithelial to mesenchymal transition. Cancer Sci. 2011;102(6):1151–1157. doi:10.1111/j.1349-7006.201121371176
- Shi X, Wang X. The role of MTDH/AEG-1 in the progression of cancer. Int J Clin Exp Med. 2015;8(4):4795–4807.26131054
- Zhang F, Yang Q, Meng F, et al. Astrocyte elevated gene-1 interacts with beta-catenin and increases migration and invasion of colorectal carcinoma. Mol Carcinog. 2013;52(8):603–610. doi:10.1002/mc.2189422431469
- Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–1134. doi:10.1038/nm.440928985214
- Malanchi I, Peinado H, Kassen D, et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452(7187):650–653. doi:10.1038/nature0683518385740
- Pandit H, Li Y, Li X, Zhang W, Li S, Martin RCG. Enrichment of cancer stem cells via beta-catenin contributing to the tumorigenesis of hepatocellular carcinoma. BMC Cancer. 2018;18(1):783. doi:10.1186/s12885-018-4683-030075764
- Sun J, Sun B, Zhu D, et al. HMGA2 regulates CD44 expression to promote gastric cancer cell motility and sphere formation. Am J Cancer Res. 2017;7(2):260–274.28337375
- Hu B, Emdad L, Kegelman TP, et al. Astrocyte elevated gene-1 regulates beta-catenin signaling to maintain glioma stem-like stemness and self-renewal. Mol Cancer Res. 2017;15(2):225–233. doi:10.1158/1541-7786.MCR-16-023927903708
