Abstract
Objective
To investigate the therapeutic effect and survival outcome using nomogram by incorporating significant inflammatory markers in patients with thoracic esophageal squamous cell carcinoma (ESCC) who received chemoradiotherapy (CRT) or single radiotherapy (RT).
Method
A total of 266 patients diagnosed with thoracic ESCC receiving standard curative RT only or concurrent CRT were retrospectively analysed. The patients were grouped for statistical analysis depending on the median values of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and C-reactive protein/albumin (CRP/Alb) ratio. The therapeutic effect was analysed by univariate and multivariate logistic analyses. The survival prognosis was estimated by univariate and multivariate Cox analyses. At last, the nomogram was developed by incorporating the significant inflammatory markers and clinicopathological parameters, and the predictive value was verified by calibration curve, concordance index (C-index) and decision curve.
Results
The treatment responses were highly associated with clinical stage, tumor location, NLR, PLR and CRP/Alb ratio (all P<0.05) by univariate logistic analysis. However, in the multivariate logistic analysis, the results showed that only CRP/Alb ratio (P=0.000) and TNM stage (P=0.008) were independent risk parameters for tumour response. In addition, NLR, PLR, CRP/Alb ratio, age and TNM stage were significantly associated with OS by the univariate Cox analysis (all P<0.05). Furthermore, the multivariate Cox analysis showed that only CRP/Alb ratio (P=0.000), TNM stage (P=0.000) and age (P=0.001) were considered independent prognostic factors for OS. Finally, the calibration curves of nomogram were highly consistent with actual observation for the therapeutic effect and prognosis, and the decision curve analysis showed more potential of clinical benefit of the nomogram compared with TNM staging system.
Conclusion
This research found that nomogram-integrated CRP/Alb ratio was promising as a predictive model for the therapeutic effect and survival outcome in patients with thoracic ESCC receiving CRT or single RT.
Introduction
Esophageal cancer (EC) is one of the most aggressive carcinomas of the gastrointestinal tract cancers. China has the highest EC morbidity and mortality incidences in the world. Esophageal squamous cell carcinoma (ESCC) is the most common pathological type of EC in China,Citation1 which is in sharp contrast to the predominance of adenocarcinoma in the Western countries.Citation2 Because specific clinical symptoms do not emerge until the disease reaches an advanced stage, the rate of early clinical diagnosis and treatment of ESCC is small, and the average 5-year overall survival rate is only 17%.Citation3 According to the 7th edition of the American Joint Committee on Cancer (AJCC),Citation4 concurrent chemoradiotherapy was feasible for the patients with inoperable, advanced ESCC. In addition, many early-stage ESCC patients who are unwilling to undergo surgical treatment also receive curative intent concurrent chemoradiotherapy (CRT) or single radiotherapy (RT) following evidence-based medicine (EBM) guidelines. However, the recurrence rate and mortality incidence of ESCC are still high, and the therapeutic effect and prognosis remain unsatisfactory.Citation5 Although some patients with the same TNM stage receive the similar treatment strategies, the reality is that the treatment efficacy and prognosis vary widely among individuals. It is becoming more and more important to search some biomarkers for predicting tumor response to treatment and prognosis before CRT or RT only.
In 1863, Virchow was the first to note a significant effect of chronic inflammation on tumors by discovering leukocytes in malignant tissue specimens.Citation6 Since then, the relationship between tumourigenesis and inflammatory response has been demonstrated step by step. To date, many investigators have clarified several potential blood biomarkers, such as the neutrophil-to-lymphocyte ratio (NLR),Citation7 the platelet lymphocyte ratio (PLR),Citation8 plasma fibrinogen and serum albumin levels,Citation9 and the Glasgow prognostic score (GPS)Citation10 or the modified GPS (mGPS)Citation11,Citation12 for predicting disease recurrence, tumor response to treatment and prognosis in several malignancies, including ESCC. Recently, a new prognostic parameter, C-reactive protein/albumin(CRP/Alb) ratio, has also been found as an independent prognostic variable in different malignancies. Liu et alCitation13 suggested that the preoperative CRP/Alb ratio was a superior index for predicting OS in patients undergoing curative resection for gastric cancer. Tao et alCitation14 reported that pretreatment CRP/Alb ratio was a useful parameter with significant prognostic value for OS in nasopharyngeal carcinoma. However, the role of the CRP/Alb ratio has not yet been evaluated in patients with ESCC receiving CRT or RT. Therefore, the purpose of this research was to clarify whether pretreatment inflammatory markers, including NLR, PLR and CRP/Alb ratio, are useful for assessing the therapeutic effects and prognosis of thoracic ESCC patients receiving concurrent CRT or RT only, and finally develop a nomogram incorporating the significant independent factors to provide individualized risk assess.
Patients And Methods
Patient Groups And Demographic Characteristics
In this retrospective study, we enrolled patients with a confirmed pathological diagnosis of thoracic ESCC who were treated with RT only or concurrent CRT in the affiliated Taixing People’s Hospital of Yangzhou University from January 2012 to December 2014. The inclusion criteria in this research were as follows: (1) patients with histologically verified thoracic ESCC; (2) patients who were undergoing curative intent concurrent CRT or RT only; (3) patients with a performance status of 0 or 1; and (4) patients with a complete record of the necessary pretreatment haematological variables. The exclusion criteria for this study were as follows: (1) the presence of distant metastasis (n=11), (2) patients with additional primary cancers before diagnosis (n=5); (3) patients with incomplete follow-up data of <1 year (n=15); (4) the presence of an infection or inflammatory conditions (n=21). Finally, 318 patients met the inclusion criteria, among which 52 patients were later excluded from this study; therefore, the data from 266 patients were obtained and analysed in this study. All patients were evaluated by blood examinations, oesophagogastroduodenoscopy, fluoroscopy, and computed tomography (CT) before starting treatment. According to the results of the medical examinations, patients were tumor-node-metastasis (TNM) staged based on the 7th International Union Against Cancer (UICC) criteria for oesophageal carcinoma.Citation15 This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Ethics Committee of the affiliated Taixing People’s Hospital of Yangzhou University in March 2017. The written informed consents were acquired from all patients for the use and publication of their information.
Treatment Modalities And Assessment Of Therapeutic Effect
All patients received curative RT only or concurrent CRT. The total dose of 60.0–66.0Gy was delivered by standard fractionated radiotherapy in 30–33 fractions. Concurrent chemotherapy consisted of a daily dose of cisplatin (25 mg/m2, over days 1–4) with paclitaxel (135–175 mg/m2, on day 1) for 28 days per cycle for a total of 2 cycles.
Clinical responses were assessed by pneumobarium double contrast examination and a comprehensive assessment of computed tomography (CT) scans at 1 month after RT. Tumor response was evaluated by the Response Evaluation Criteria in Solid Tumours (RECIST) guideline (version 1.1).Citation16 Accordingly, patients were divided into four groups depending on tumor response as follows: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Patients who demonstrated CR or PR after treatment were defined as responders, whereas those who demonstrated SD or PD were listed as non-responders.
Hematological Parameters Calculation And Follow-Up
The pretreatment hematological parameters, including C-reactive protein, albumin count, lymphocyte count, neutrophil count, platelet count and monocyte count, were gathered within 1 week before treatment. Then, the NLR, PLR, and CRP/Alb were calculated by division of the absolute values of the corresponding hematological parameters.
After treatment, the patients were required to return to the hospital every 3 months in the first year for examination, and every 6 months in the next two years, and then once a year. Follow-up time was calculated from the day of treatment to the day of death or May 2017.
Statistical Analysis
NLR, PLR, as well as CRP/Alb ratio were divided into high and low groups based on the respective median values. The therapeutic effect of these markers was analysed by univariate and multivariate logistic analyses. The Kaplan–Meier method was used for calculating the overall survival (OS) rates and the Cox proportional hazards models were adopted for the univariate and multivariate analyses. ROC curves were also plotted to verify the accuracy of NLR, PLR and CRP/Alb ratio for therapeutic effect and OS prediction. A 2-tailed P-value ≤0.05 was considered statistically significant. The above statistical analyses were performed using the Statistical Package for Social Sciences version 17.0 (SPSS, Chicago, IL, USA).
Finally, the nomogram was developed according to the results of multivariate analysis and by using the rms package in R version 2.14.1 (http://www.r-project.org/). The performance of the nomogram was assessed by calibration curve, concordance index (C-index) and decision curve.
Results
Patient Characteristics
The basic features of the enrolled patients are provided in . Among the 266 cases, 172 (65%) were male and 94 (35%) were female, with a sex ratio of 1.83:1. The median age was 67 years (range from 48 to 87). There were 35, 145 and 86 patients with stage I, II and III, respectively. A total of 128 patients received RT alone, and concurrent CRT was administered to 138 patients. With a median survival time of 24 months (95% CI 21.003–26.997), the 1-, 2-, 3-year OS rates were 85.7%, 49.6%, 35.7%, respectively.
Table 1 Clinicopathological Characteristics Of 266 Patients With Thoracic Esophageal Squamous Cell Carcinoma
Correlations Between Tumour Response And NLR, PLR, CRP/Alb Ratio And Clinicopathological Parameters
A total of 266 ESCC patients were classified depending on the median values of NLR, PLR, and CRP/Alb ratio, as provided in . Based on NLR median values, patients were divided into two groups, with 131 and 135 patients in the low and high groups, respectively. 133 and 133 patients were divided into the low and high PLR groups, respectively. Similarly, patients were classified into low and high groups based on CRP/Alb ratio median values, consisting of 152 and 114 patients, respectively. The responsiveness to treatment of these markers was analyzed by univariate logistic analyses. The results found that the treatment responses were highly associated with clinical TNM stage (P=0.001), tumor location (P=0.047), NLR (P=0.000), PLR (P=0.008) and CRP/Alb ratio (P=0.000). Furthermore, the multivariate logistic analysis discovered that only the CRP/Alb ratio (HR=9.606, 95% CI 4.572–20.182, P=0.000) and the TNM stage (HR=0.397, 95% CI 0.201–0.785, P=0.008) were independent risk factors for tumor response.
Table 2 Univariate And Multivariate Logistic Regression Analysis Between Tumor Response And Inflammation-Based Markers And Clinicopathological Characteristics In Patients With Thoracic ESCC (n=266)
Prognostic Analysis Based On NLR, PLR, Or CRP/Alb Ratio
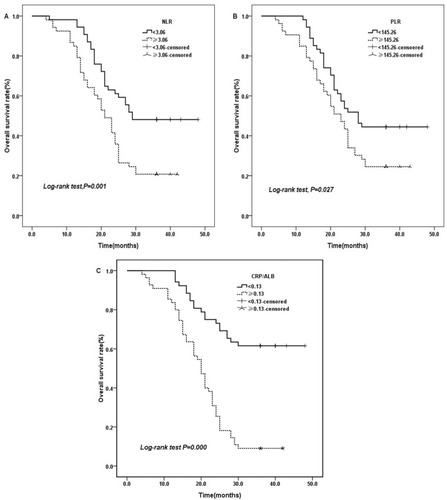
The 3-year OS rates for NLR≥3.06 and NLR<3.06 were 20.8% and 48.1%, respectively (. χ2=10.211, P=0.001). Similarly, the 3-year OS rates for PLR≥145.26 and PLR<145.26 were 24.5% and 44.4%, respectively (. χ2=4.921, P=0.027). Additionally, the 3-year OS rates for CRP/Alb ratio≥0.13, compared with CRP/Alb ratio<0.13, were significantly associated with poor overall survival (χ2=34.558, P=0.000, ).
Figure 1 Kaplan–Meier curves for OS stratified according to NLR, PLR, and CRP/ALB ratio median values.
Notes: (A) OS curves grouped by NLR median value (the 3-year OS for low NLR 48.1%, high NLR 20.8%, P=0.001). (B) OS curves stratified according to PLR median value (the 3-year OS for low PLR 44.4%, high PLR 24.5%, p=0.027). (C) OS curves stratified by CRP/ALB ratio median value (the 3-year OS for low CRP/ALB ratio 61.5%, high CRP/ALB ratio 9.1%, P=0.000).
Abbreviations: OS, overall survival; NLR, neutrophil to lymphocyte ratio; PLR, platelet lymphocyte ratio; CRP/Alb, C-reactive protein/albumin.
Our results indicated that NLR, PLR, CRP/Alb ratio, age, and TNM stage were significantly associated with OS using the univariate analysis. Furthermore, the results of multivariate Cox model analysis showed that only CRP/Alb ratio, TNM stage and age were considered independent prognostic factors for OS ().
Table 3 Univariate And Multivariate Analysis Of Clinicopathological Parameters And Hematological Markers For The Prediction Of Overall Survival In Patients With Thoracic ESCC (N =266)
ROC Curve For Therapeutic Efficacy And Overall Survival Prediction
ROC curves for therapeutic responsiveness were plotted to verify the median values of NLR, PLR, and CRP/Alb ratio. As provided in , the areas under the curve (AUCs) for NLR, PLR, and CRP/Alb ratio were 0.668 (95% CI 0.558–0.778, P=0.006), 0.602 (95% CI 0.486–0.718, P=0.093), and 0.742 (95% CI 0.643–0.840, P=0.000), respectively. The results indicated that CRP/Alb ratio was superior to NLR and PLR as a predictive parameter for therapeutic efficacy in ESCC patients.
Figure 2 The ROC curves grouped by NLR, PLR and CRP/ALB ratio.
Notes: (A) ROC curves based on therapeutic effect. (B) ROC curves for OS.
Abbreviations: AUC, area under the curve; NLR, neutrophil lymphocyte ratio; PLR, platelet lymphocyte ratio; CRP/Alb, C-reactive protein/albumin; ROC, receiver operating characteristic.
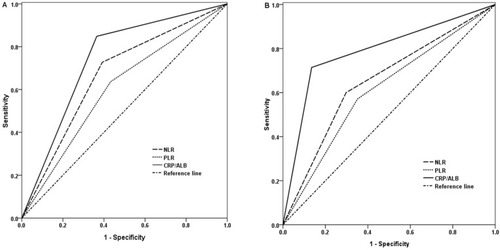
ROC curves for OS were also plotted, and the AUC was 0.651 (95% CI 0.542–0.760, P=0.010) for NLR, 0.610 (95% CI 0.498–0.722, P=0.062) for PLR and 0.790 (95% CI 0.699–0.880, P=0.000) for CRP/Alb ratio, indicating that CRP/Alb ratio was superior to NLR and PLR as a predictive factor for OS in patients with ESCC ().
Prediction Nomogram For Therapeutic Efficacy And Overall Survival
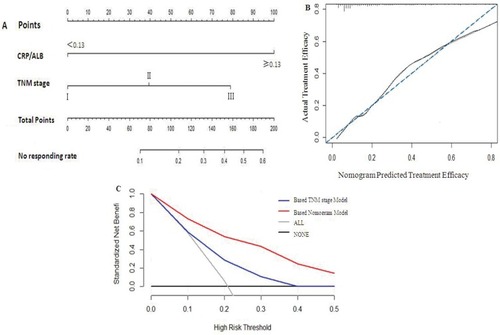
The multivariate logistic regression analysis identified CRP/Alb ratio and TNM stage as independent predictors for therapeutic efficacy (see ). The model that incorporated the above independent parameters was developed and presented as the nomogram (). The calibration curve of the nomogram for the tumor response demonstrated good agreement between nomogram prediction and actual observation (). The C-index for the prediction nomogram was 0.756 (95% CI 0.737–0.772) by internal bootstrapping validation. In the decision curve analysis, the nomogram model showed high potential of clinical application, because it ensured better net benefits throughout the entire range of threshold probabilities for therapeutic efficacy compared with the TNM staging systems ().
Figure 3 Prediction Nomogram for therapeutic efficacy.
Notes: (A) Therapeutic evaluation of nomogram-integrated CRP/Alb ratio in the patients with esophageal squamous cell cancer receiving CRT RT only. (B) The calibration curve of the nomogram for the tumor response. (C) The decision curve analysis of the nomogram for the tumor response.
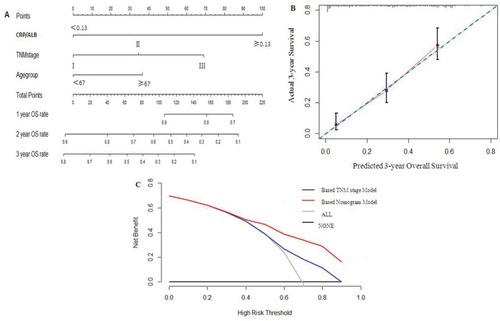
The prognostic nomogram that integrated all significant independent factors for OS is shown in . The C-index for OS prediction was 0.728 (95% CI 0.690–0.762). The calibration plot for the probability of survival at 3 years after treatment demonstrated an optimal agreement between the prediction by nomogram and actual observation (see ). The decision curve analysis for the nomogram is presented in . The decision curve showed that if the threshold probability>30%, using the nomogram to show net benefit for predicting overall survival compared to the TNM staging-based model.
Figure 4 Prediction nomogram for overall survival.
Notes: (A) Survival evaluation of nomogram-integrated CRP/Alb ratio in the patients with esophageal squamous cell cancer receiving CRT RT only. (B) The calibration plot for the probability of survival at 3-year after treatment. (C) The decision curve analysis of the nomogram for the 3-year survival rate.
Discussion
The inflammatory response (IR) is common in many malignancies and is correlated with tumour progression or recurrence. However, it is difficult to predict the tumour response and survival prognosis prior to treatments. In this research, we investigated the significance of pretreatment PLR, NLR and CRP/Alb ratio levels for tumor treatment response and survival prognosis in ESCC patients receiving CRT or RT alone. This study indicated that CRP/Alb ratio and TNM stage were independent risk factors for tumor response. While CRP/Alb ratio, TNM stage and age were considered independent prognostic factors for OS. Finally, the nomogram was developed based on the results of multivariate analysis; the calibration curves of nomogram were highly consistent with actual observation for the therapeutic effect and survival prognosis, and the decision curve analysis showed more potential of clinical benefit of the nomogram compared with TNM staging system.
In clinical practice, it is hard to distinguish a responder from a non-responder before CRT or RT. Furthermore, there were few variables for predicting tumor response in patients with ESCC. It is critical to dispose of these urgent issues in clinical management. The systemic inflammatory response has been known to closely correlate with tumor progression and metastasis in various malignancies, including ESCC.Citation17–Citation20 Interestingly, several research have discovered that hyperfibrinogenemia and elevated NLR or the combination of NLR with PLR (CNP) were predictors of a poor therapeutic efficacy before initial treatment.Citation21–Citation23 In the present study, we calculated and compared the predictive value to the treatment responses of the pretreatment NLR, PLR, and CRP/Alb ratio in patients with ESCC who were treated with CRT or single RT. Our univariate logistic regression analyses have validated the NLR, PLR and CRP/Alb ratio as predictors for treatment responses; However, the multivariate logistic regression analysis showed that only the CRP/Alb ratio was independent risk factor for tumor response. In ROC analysis, our findings also indicated that the CRP/Alb ratio was superior to NLR and PLR as a predictive factor for the therapeutic responsiveness in patients with ESCC. At last, the nomogram model that incorporated the CRP/Alb ratio and TNM stage was developed. The nomogram performed well in predicting tumor response, and its prediction was supported by the C-index, the calibration curve and decision curve analysis.
The prognosis of ESCC patients who have undergone CRT or RT mainly depends on the clinical stage of the disease; however, there are many differences in outcomes between patients even when they are within the same clinical stage. To date, oncological studies have already concentrated on determining tumor biomarkers with important influences on clinical outcomes. Relatively specific biomarkers for patient survival prognosis and tumor relapse have been found for some cancers, including AFP for hepatoma, PSA for prostate cancer, and CA-199 for pancreatic cancer.Citation23 However, there are currently no specific hallmarkers that can be used to predict survival prognosis in ESCC patients.
Many studies showed that there were statistically significant differences in the survival rates classified by NLR, PLR and CRP/Alb ratio levels for several types of malignancies.Citation24–Citation26 Feng reported that the preoperative NLR and PLR were significant predictors of overall survival in patients with ESCC.Citation8 Liu discovered that the CRP/Alb was a novel independent marker for prognosis in ovarian cancer patients.Citation27 Currently, we first investigate the prognostic significance of NLR, PLR and CRP/Alb ratio in ESCC patients receiving CRT or RT only. The multivariate Cox analysis showed that only the CRP/Alb ratio was significantly associated with OS and had prognostic value independent of TNM stage. Then, the prognostic nomogram model that integrated all significant independent factors for OS was constructed. This nomogram predicted OS with an accuracy of C-index 0.728. The calibration plot revealed good correlation between the predicted survival probability and the actual survival rate. The decision curve analysis also showed more potential of clinical benefit of the predictive nomogram model compared with current TNM staging system.
Although the nomogram integrated CRP/Alb ratio demonstrated accuracy for the prediction of therapeutic efficacy and survival prognosis for ESCC receiving RT alone or CRT, there are some limitations in this research. Firstly, not all inflammatory parameters were used for analysis, because some biomarkers were not routinely examined, such as tumor necrosis factor alpha, Interleukin-1and Interleukin-6. Secondly, it was a single-institution, retrospective study. Thirdly, 266 ESCC patients were enrolled in this study, and the sample size is relatively small. In view of these limitations, more randomized trials are needed to clarify these results in the future.
In conclusion, this study discovered that nomogram-integrated CRP/Alb ratio was a promising prediction model for the therapeutic effect and survival outcome in patients with ESCC receiving CRT or single RT. However, considering the retrospective nature of this study, large-scaled prospective trials are still warranted to verify our results.
Disclosure
The authors declare no competing interests in this work.
Acknowledgment
This work was supported by the grant from Jiangsu Provincial Medical Youth Talent (QNRC2016234).
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2 ):87–108. doi:10.3322/caac.2126225651787
- Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the cancer incidence in five continents database. Int J Epidemiol. 2001;30(6 ):1415–1425. doi:10.1093/ije/30.6.141511821356
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19(34 ):5598–5606. doi:10.3748/wjg.v19.i34.559824039351
- Rice TW, Rusch VW, Ishwaran H, Blackstone EH, Worldwide Esophageal Cancer Collaboration. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116(16 ):3763–3773. doi:10.1002/cncr.2514620564099
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (jcog9907). Ann Surg Oncol. 2012;19(1 ):68–74. doi:10.1245/s10434-011-2049-921879261
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255 ):539–545. doi:10.1016/S0140-6736(00)04046-011229684
- Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg. 2012;36:617–622. doi:10.1007/s00268-011-1411-122223293
- Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12:58. doi:10.1186/1477-7819-12-5824641770
- Matsuda S, Takeuchi H, Kawakubo H, et al. Cumulative prognostic scores based on plasma fibrinogen and serum albumin levels in esophageal cancer patients treated with transthoracic esophagectomy: comparison with the Glasgow prognostic score. Ann Surg Oncol. 2015;22(1 ):302–310. doi:10.1245/s10434-014-3857-524952029
- Kimura J, Kunisaki C, Makino H, et al. Evaluation of the Glasgow prognostic score in patients receiving chemoradiotherapy for stage III and IV esophageal cancer. Dis Esophagus. 2016;29(8 ):1071–1080. doi:10.1111/dote.1242026471766
- Hirashima K, Watanabe M, Shigaki H, et al. Prognostic significance of the modified Glasgow prognostic score in elderly patients with gastric cancer. J Gastroenterol. 2014;49(6 ):1040–1046. doi:10.1007/s00535-013-0855-523821018
- Nakagawa K, Tanaka K, Nojiri K, et al. The modified Glasgow prognostic score as a predictor of survival after hepatectomy for colorectal liver metastases. Ann Surg Oncol. 2014;21(5 ):1711–1718. doi:10.1245/s10434-013-3342-624452408
- Liu X, Sun X, Liu J, et al. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol. 2015;8(4 ):339–345. doi:10.1016/j.tranon.2015.06.00626310380
- Tao CJ, Chen YY, Jiang F, et al. The C-reactive protein/albumin ratio is an independent prognostic factor for overall survival in patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy. J Cancer. 2016;7(14 ):2005–2011. doi:10.7150/jca.1621027877215
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6 ):1471–1474. doi:10.1245/s10434-010-0985-420180029
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2 ):228–247. doi:10.1016/j.ejca.2008.10.02619097774
- McNamara MG, Templeton AJ, Maganti M, et al. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur J Cancer. 2014;50(9 ):1581–1589. doi:10.1016/j.ejca.2014.02.01524630393
- Mohammed ZM, Going JJ, Edwards J, et al. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. 2012;107(5 ):864–873. doi:10.1038/bjc.2012.34722878371
- Thurner EM, Krenn–Pilko S, Langsenlehner U, et al. The elevated C-reactive protein level is associated with poor prognosis in prostate cancer patients treated with radiotherapy. Eur J Cancer. 2015;51(5 ):610–619. doi:10.1016/j.ejca.2015.01.00225618827
- Szkandera J, Stotz M, Absenger G, et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer. 2014;110(1 ):183–188. doi:10.1038/bjc.2013.70124201751
- Kijima T, Arigami T, Uchikado Y, et al. Combined fibrinogen and neutrophil-lymphocyte ratio as a prognostic marker of advanced esophageal squamous cell carcinoma. Cancer Sci. 2017;108(2 ):193–199. doi:10.1111/cas.1312727889946
- Cho IR, Park JC, Park CH, et al. Pre-treatment neutrophil to lymphocyte ratio as a prognostic marker to predict chemotherapeutic response and survival outcomes in metastatic advanced gastric cancer. Gastric Cancer. 2014;17(4 ):703–710. doi:10.1007/s10120-013-0330-224442663
- Zhu S, Miao CW, Wang ZT, Peng L, Li B. Sensitivity value of hematological markers in patients receiving chemoradiotherapy for esophageal squamous cell carcinoma. Onco Targets Ther. 2016;9:6187–6193. doi:10.2147/OTT.S11501127789959
- Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104:1288–1295. doi:10.1038/bjc.2011.10021448173
- Sun W, Zhang L, Luo M, et al. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma: neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Head Neck. 2016;38(Suppl 1 ):E1332–E1340. doi:10.1002/hed.2422426362911
- Zhang W, Liu K, Ye B, Liang W, Ren Y. Pretreatment C-reactive protein/albumin ratio is associated with poor survival in patients with stage IB-IIA cervical cancer. Cancer Med. 2018;7:105–113. doi:10.1002/cam4.127029193777
- Liu Y, Chen S, Zheng C, et al. The prognostic value of the preoperative c-reactive protein/albumin ratio in ovarian cancer. BMC Cancer. 2017;17:285. doi:10.1186/s12885-017-3220-x28431566
