Abstract
Sarcomas are a heterogeneous group of solid tumors arising from either soft tissues or bone, accounting for approximately 1% of all cancers in adults. Management of these diseases has changed little over the past 10 years, with the exception of treatment of gastrointestinal stromal tumors. Reasons for this stagnation include multiple histologies commonly grouped together in clinical trials limiting the understanding of benefit of treatment and limited investigation of molecular targeted therapies. More recently, advances in molecular pathogenesis, the advent of novel and targeted therapeutics, and increasing collaborations between sarcoma investigators has helped move the field forward in the right direction. Here, we review the recent data on novel agents tested for the management of adult soft-tissue sarcomas, excluding gastrointestinal stromal tumors.
Introduction
Sarcomas are a histologically and genetically heterogeneous group of solid tumors arising from either soft tissues or bone and are grouped together for their presumed common mesenchymal origin. Soft-tissue sarcomas (STS) constitute a little less than 1% of all cancers in adults, with around 11,000 new cases per year in the US.Citation1 Apart from the groundbreaking discovery of KIT expression and activating mutations in gastrointestinal stromal tumors (GISTs) that transformed this chemo-insensitive, routinely fatal disease to one of the most treatable sarcomas, there has been little progress in sarcoma therapy in the past 10 years. One of the major reasons for this stagnation was because all high-grade STS were being treated similarly on clinical trials. Now, due to a greater understanding of the diversity in molecular pathogenesis and the advent of novel and targeted therapeutics, the field of sarcoma has seen some exciting recent advances. Also, increasing collaboration among sarcoma oncologists nationally and internationally has made large randomized clinical trials in subtype-specific sarcomas feasible. This review focuses on promising new drugs and combination therapies that are changing the therapeutic and investigational landscape of STS, excluding GISTs.
Localized STS
Surgical resection with or without radiation therapy remains the mainstay of therapy for localized STS.Citation2,Citation3 The role of adjuvant therapy remains controversial, fraught with the challenges of performing an adequately powered randomized controlled study in a rare tumor that has tremendous heterogeneity in chemoresponsiveness of the various sarcoma subtypes. The majority of the data are with doxorubicin- containing regimens, some of which utilize doxorubicin as a single agent. To address the problem of inadequately powered adjuvant sarcoma studies showing nonsignificant benefit in survival outcomes, the Sarcoma Meta-analysis Collaboration (SMAC) performed a meta-analysis of 14 studies comprising 1568 patients, initially published in 1997.Citation4 Six of these studies evaluated doxorubicin as a single agent, and the other eight used varying combinations of doxorubicin. The 10-year disease-free survival was improved (45%–55%, P = 0.0001), but 10-year overall survival did not reach significance (50%–54%, P = 0.12).
An update of the SMAC analysis was published in 2008 and included patients from the 1997 meta-analysis and four additional randomized trials.Citation5 The pooled data from a total of 1953 patients demonstrated a statistically significant odds ratio (OR) in favor of adjuvant chemotherapy for local recurrence (OR 0.73, P = 0.02), distant recurrence (OR 0.67, P = 0.001), and overall recurrence (OR 0.67, P = 0.0001). Survival analysis showed a statistically significant benefit for doxorubicin combined with ifosfamide (OR 0.56, 95% confidence interval [CI] 0.36–0.85; P = 0.01), but not for doxorubicin alone (OR 0.84, 95% CI 0.68–1.03; P = 0.09). This study did not include the recent European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group (EORTC) studyCitation6 evaluating adjuvant doxorubicin and ifosfamide versus observation in resected grade 2 and 3 extremity tumors. A separate update of the SMAC meta-analysis including this EORTC study with a total of 2170 patients, presented by O’Connor et al, showed a benefit of adjuvant chemotherapy for disease-free and overall survival after 5 years, but only a nonsignificant trend toward improved survival after 10 years (OR 0.87, P = 0.12).Citation7 A pooled analysis of individual patient data from the two largest adjuvant trials conducted by EORTCCitation6,Citation8 using doxorubicin- and ifosfamide-based chemotherapy was also presented the same year and did not show a survival advantage for postoperative chemotherapy.Citation9
Based on some retrospective analyses,Citation10,Citation11 it has been proposed that the benefit of adjuvant chemotherapy is likely limited to patients with higher-grade and larger tumors with certain chemosensitive histologies (ie, myxoid/round-cell liposarcomas and synovial sarcomas); however, this lacks validation by a prospective clinical trial.
Metastatic STS
Chemotherapy is widely used in the treatment of nonresectable advanced disease, primarily with palliative intent. At present, initial standard chemotherapy for advanced or metastatic STS consists of single-agent anthracycline (mainly doxorubicin) or an anthracycline-based combination. Other agents with single-agent activity that are frequently combined with doxorubicin are ifosfamide and dacarbazine. The objective response rate in treatment-naive patients is somewhere between 18% and 35%.Citation12 The use of doxorubicin is limited, due to its risk of cumulative cardiac toxicity.
Gemcitabine and docetaxel is another frequently used combination as a second-line regimen in STS and occasionally used front-line for uterine leiomyosarcomas, the histology with the highest reported response rates to this combination.Citation13–Citation15 A randomized phase II study conducted by Maki et al in metastatic STS suggested a survival benefit for fixed dose-rate gemcitabine with docetaxel over fixed dose-rate gemcitabine alone.Citation16 The median progression-free survival (PFS) of gemcitabine therapy alone was 3 months compared to 6 months with the combination, and the objective response rate was 8% versus 16%, favoring the combination arm. The median overall survival (OS) was 18 months versus 12 months favoring the gemcitabine and docetaxel combination. Retrospective data from the French Sarcoma Group has demonstrated an overall objective response rate of 18.4%; when stratified by histology, leiomyosarcomas had the best objective response rate at 24.2%, compared with other sarcomas with a response rate of only 10.4%.Citation17
It is now well known that various histologic subtypes respond differently to cytotoxic therapy. As described above, leiomyosarcomas seem to have higher response with gemcitabine and docetaxel. Myxoid liposarcomas and synovial sarcomas have shown significant sensitivity to ifosfamide.Citation18 Paclitaxel appears to work well in angiosarcomas.Citation19 Some subtypes like GISTs, alveolar soft-part sarcoma (ASPS), clear-cell sarcomas, and well-differentiated liposarcomas are intrinsically resistant to chemotherapy.Citation20
As with most tumors, many chemotherapy-sensitive patients will ultimately relapse, and the prognosis for patients with metastatic sarcoma remains poor. The estimated median survival is 8–13 months from the start of first-line anthracycline-based chemotherapy, as shown in randomized studies performed over the last two decades.Citation21–Citation24 In addition, these patients are often debilitated by their sarcoma, as bulky disease can result in complications such as pain, intestinal obstruction, and other symptoms, leading to end-organ failure and death. The median survival for patients for whom conventional chemotherapy with anthracycline and ifosfamide has failed is in the range of 6 months.Citation25–Citation29
Of note, the current chemotherapy combinations in sarcoma have significant cumulative and late toxicities. The majority of patients require some form of dose adjustment due to toxicity. Newer-generation drugs with a favorable efficacy and toxicity profile are eagerly awaited and will be crucial in improving outcomes for sarcoma patients. It is also essential that we acknowledge the differences between various subtypes in designing sarcoma clinical trials. Patient numbers are the obvious challenge in a rare cancer, but collaborations across institutes and countries are making it feasible to execute such trials and focus on pathogenesis-driven therapies in some rare subtypes.
One of the frequently referenced articles in sarcoma, by Van Glabbeke et al, estimated the progression-free rate (PFR) for active and inactive agents for STS in the first-and second-line setting based on the EORTC clinical trials database.Citation30 For first-line therapy, a 6-month PFR of 30% or higher suggested drug activity, and for second-line therapy, a 3-month PFR of 40% or higher was associated with an active drug and 20% or lower meant inactivity. With more common sarcoma types, histology-specific benchmarks are also available. These provide a reference value for performing phase II studies in sarcoma with PFR as the primary end point.
Novel chemotherapeutics
Trabectedin
Trabectedin (Yondelis [ecteinascidin 743 or ET-743]; Janssen) is a tris(tetrahydroisoquinoline) alkaloid that was initially isolated from the sea squirt Ecteinascidia turbinata. Potent antitumor activity was noted in preclinical studies in vitro and in vivo, in many solid tumors, and was confirmed in several phase II trials in ovarian and breast cancer and STS. The mechanism of action of trabectedin’s cytotoxicity is not yet precisely known. It binds to the minor groove on the DNA in a sequence-specific manner and alkylates guanine at the N2 position, inducing DNA bending towards the major groove and a widening of the DNA minor groove.Citation31,Citation32 This leads to inhibition of activated transcription, and trabectedin interacts with the transcription-coupled nucleotide-excision repair, leading to irreversible breaks in DNA strands, cell-cycle arrest, and apoptosis.Citation31–Citation35
Four phase I trials testing different doses and schedules of trabectedin established 1.5 mg/m2 given as a continuous infusion over 24 hours as the recommended phase II dose. Responses were seen mainly in STS patients, and this prompted multiple sarcoma-specific trials; some of the published results are tabulated below ().
Table 1 List of published sarcoma clinical trials on ET-743
Delaloge et al published the clinical activity of trabectedin in sarcoma patients pretreated with doxorubicin and an alkylating agent in 2001, including twelve patients treated on a phase I clinical trial and 17 patients entered on a compassionate-use program.Citation36 There were four partial responses (PRs) (14%), ten disease stabilizations (34%) lasting more than 2 months, and eight patients (28%) were progression-free at 6 months. A phase II study in France enrolled 54 patients in two groups: 26 with one or two prior therapies and 28 with three or more prior therapies.Citation37 Two patients (3.7%) had a PR, and nine patients (17%) had stable disease (SD) for more than 6 months, with 39% and 24% patients progression-free at 3 and 6 months, respectively. The median PFS was 1.9 months, similar in both groups, and median OS was 12.8 months (14 months in the first group versus 8 months in the second group). A similar phase II study in the US by Garcia-Carbonero et al reported results in 36 previously treated patients with progressing STS, the majority with either leiomyosarcoma or liposarcoma, using trabectedin 1.5 mg/m2 administered as a 24-hour continuous infusion.Citation38 One complete response (CR) and two PRs (objective response rate [ORR], CR + PR = 8%) were observed, with a median PFS of 1.7 months and median OS of 12.1 months. Another trial of trabectedin 1.5 mg/m2 as a 24-hour continuous infusion was conducted by the EORTC with non-GIST STS failing prior chemotherapy.Citation39 The most common histological subtypes were leiomyosarcoma (41%), synovial sarcoma (17%), and liposarcoma (10%). There were eight (7.7%) PRs and 45 (43.3%) with SD observed. After a median follow-up of 34 months, the median PFS was 3.4 months (105 days), and the median OS was 9.2 months. Fifty-two percent, 29%, and 17% of patients were progression-free at 3, 6, and 12 months, respectively. Huygh et al reported their experience with 89 patients who received trabectedin at the Leuven Cancer Institute in Leuven, Belgium: 75 were treated on a compassionate-use program and 15 treated in a phase II trial.Citation40 In this group, where the majority (93%) of patients had previously received doxorubicin, one CR, five PRs (ORR = 7%), and 32 with SD (36%) were observed. Median PFS and OS were 2.0 and 8.2 months, respectively. A phase II trial looking at the efficacy of trabectedin in chemotherapy-naive patients accrued 36 patients with either leiomyosarcoma (42%) or liposarcoma (25%).Citation41 The median PFS was 1.6 months, and the median OS was 15.8 months. Six patients had PR (17%) and the PFRs were 24.4% and 21% at 6 and 12 months, respectively, indicating prolonged disease control in some patients. A multicenter phase II trial in patients with locally advanced or locally recurrent myxoid/round-cell liposarcoma, with three to six cycles of trabectedin in the neoadjuvant setting resulted in encouraging pathologic CR rates of 13% (95% CI, 3%–34%).Citation42
Results of a randomized phase II trial comparing trabectedin given at 580 μg/m2 over 3 hours on a weekly schedule to a schedule of 1.5 mg/m2 24-hour continuous infusion every 3 weeks in patients with leiomyosarcoma or liposarcoma failing prior therapy with doxorubicin and ifosfamide favored the 24-hour infusion every 3 weeks.Citation43 Two hundred and seventy patients were randomly assigned, and the primary end point of time to progression was 3.7 months versus 2.3 months (hazard ratio [HR] 0.734; 95% CI 0.554–0.974; P = 0.0302), and the median OS was 13.9 months versus 11.8 months (HR 0.843, 95% CI 0.653–1.090; P = 0.1920) in the 3-hour weekly versus 24-hour every-3-week schedule, respectively.
Trabectedin is generally well tolerated, and some responding myxoid liposarcoma patients stay on therapy for more than 12 months. The most common grade 3 or 4 toxicities seen in the phase II trials are neutropenia, thrombocytopenia, and biochemical hepatotoxicity. Nausea, vomiting, and fatigue are also frequently noted. Rare cases of fulminant hepatic failure and rhabdomyolysis have also been reported. An abnormal baseline alkaline phosphatase and a bilirubin > 0.6 × upper limit of normal is predictive of severe hematologic and hepatic toxicity in patients receiving trabectedin.Citation44,Citation45 Steroid premedication has been reported to reduce biochemical and hematologic toxicity,Citation46 and this in addition to more stringent hepatic function parameters and dose-reduction guidelines has decreased the incidence of severe toxicities in recent trials.
Trabectedin was approved by the European Union in 2007 for the treatment of patients with STS who progress on or are not candidates for anthracycline- or ifosfamide-based therapy. In the US, a randomized phase III registration trial of trabectedin versus dacarbazine for previously treated patients with leiomyosarcoma and liposarcoma (L-type sarcomas) is currently accruing patients. The expanded access program for non- L-type sarcomas is also open in the US to facilitate access to trabectedin in patients unlikely to benefit from other standard-of-care treatments. Some of the other ongoing trials include a phase IIB/III study comparing the efficacy of trabectedin administered as a 3-hour or 24-hour infusion to doxorubicin in patients with advanced untreated STS and a similar phase III trial specific to patients with translocation- related sarcomas looking at trabectedin over 24 hours versus doxorubicin-based chemotherapy as front-line therapy.
Palifosfamide
Palifosfamide (Zymafos [isophosphoramide mustard, ZIO 201]; Ziopharm) is a novel DNA alkylator and the active metabolite of ifosfamide, thus lacking the hemorrhagic cystitis and central nervous system toxicity associated with the nonactive ifosfamide metabolites. Isophosphoramide mustard has demonstrated broad activity against human sarcoma cell lines in vitro and in vivo, including in cyclophosphamide-resistant osteosarcoma xenograft models.Citation47 Enhanced antitumor activity was seen with the addition of doxorubicin to palifosfamide in tumor xenografts.Citation48
The initial phase I study in solid tumors and a phase I/II study in previously treated sarcoma patients used the lysine salt formulation of palifosfamide as a single agent, with the tris formulation (ZIO-201-T) introduced in the second study after an amendment.Citation49 The phase II portion of the study reported a PR in a liposarcoma patient and PFS at 3 and 6 months as 45% and 23%, respectively.Citation50 The combination of doxorubicin with palifosfamide-tris was evaluated in a phase I study, and the maximum tolerated dose (MTD) was determined to be 150 mg/m2 IV (intravenously) daily × 3 days for palifosfamide-tris and 75 mg/m2 IV on day 1 for doxorubicin repeated every 3 weeks. This led to a phase II randomized trial of palifosfamide-tris plus doxorubicin versus doxorubicin alone in first- and second-line STS (PICASSO). Patients were stratified according to age (≥ or <65) and histologic subtype (leiomyosarcoma, synovial sarcoma, or other) and received a total of six cycles at the phase I MTD dose. Nonprogressing patients after six cycles on the combination arm were eligible to continue single-agent palifosfamide, and patients progressing on single-agent doxorubicin were allowed to receive single-agent palifosfamide. A total of 62 patients were evaluable and the median PFS, which was the primary end point, was 7.8 months for the combination versus 4.4 months for doxorubicin (HR 0.427 [95% CI 0.19–0.95]; P = 0.019).Citation51 The response rate was 23% for the combination arm and 9% for doxorubicin alone. A recent press release from Ziopharm reported a positive trend in OS favoring the palifosfamide arm, with an HR of 0.79 in the intention-to-treat population. Common adverse events related to palifosfamide-tris in combination with doxorubicin include nausea, vomiting, fatigue, anemia, neutropenia, leucopenia, constipation, electrolyte abnormalities, and mucosal inflammation. No significant differences in toxicities between the two arms have been reported.
The phase III randomized, double-blind study of doxorubicin plus palifosfamide versus doxorubicin plus placebo has just completed enrollment of the target 424 patients. The study is powered for PFS as the primary end point for accelerated approval and OS for full approval. If positive, this combination is likely going to change the treatment paradigm for sarcomas, given its advantages over the combination of doxorubicin and ifosfamide: safe for outpatient administration and an improved safety profile.
TH-302
TH-302 (Threshold Pharmaceuticals) is a nitroimidazole prodrug of the cytotoxin bromo-isophosphoramide mustard (Br-IPM). When exposed to hypoxic conditions, TH-302 is reduced at the nitroimidazole site by intracellular reductases, leading to the release of the alkylating agent Br-IPM.Citation52 Br- IPM is similar in action to the active metabolite of ifosfamide, IPM, but because of the preferential activation in hypoxic tissues and the absence of acrolein as a by-product, TH-302 is anticipated to have less renal, central nervous system, and bladder toxicity than ifosfamide. In vitro cytotoxicity and clonogenic assays indicate that TH-302 has little activity under normoxic conditions, but is highly cytotoxic under hypoxic conditions. In addition, studies in xenograft models of sarcoma and non-small cell lung cancer (NSCLC) show that TH-302 adds to the activity of maximally tolerated doses of doxorubicin, leading to tumor regressions and growth delay.
Two phase I studies were conducted to determine the safety, efficacy, and pharmacokinetics of TH-302; one was with single-agent TH-302 in advanced solid tumors,Citation53 and the other was in combination with full-dose doxorubicin in subjects with STS.Citation54 In the combination study, the MTD of TH-302 was determined to be 300 mg/m2 IV on days 1 and 8 in combination with 75 mg/m2 IV of doxorubicin on day 1, with prophylactic growth-factor support added due to grade 4 neutropenia. Five out of 15 evaluable patients had a PR. Enrollment was expanded at the MTD for subjects with advanced STS previously untreated with chemotherapy (neoadjuvant and adjuvant chemotherapy permitted), and updated results from this phase II portion of the study were reported at the 2011 Connective Tissue Oncology Society (CTOS) meeting.Citation55 Patients received up to six cycles of the combination, and nonprogressing patients with acceptable toxicity were allowed to continue single-agent TH-302. Tumor response rates for the 89 evaluable patients out of the 91 enrolled were as follows: CR (2) 2%, PR (30) 34%, SD (43) 48%, and PD (14) 16%. The median PFS was 6.7 months (95% CI, 6.2–8.1 months). The 6-month PFR was 63%, and the 3-month PFR was 83%. The median OS was 17.5 months (95% CI, 16.1 months to not reached). The tumor response, PFS, and OS are all higher than those reported historically with single-agent doxorubicin. The combination regimen was well tolerated with acceptable grade 3 and 4 hematologic toxicities and some grade 3 fatigue, diarrhea, and pyrexia. Skin and mucosal toxicities related to activation of the TH-302 in these tissues were noted, and supportive measures such as application of ice to these areas during infusion is recommended.
The phase III randomized study of doxorubicin versus doxorubicin and TH-302 as front-line therapy in patients with advanced STS has recently started accruing patients and will help us ultimately determine the value of this combination in the sarcoma armamentarium.
Eribulin
Eribulin mesylate (Halaven; Eisai) is a synthetic analogue of halichondrin B, a novel inhibitor of microtubule dynamics, extracted from the marine sponge Halichondria okadia. Eribulin is FDA-approved for the treatment of metastatic breast cancer in patients who have received at least two prior lines of therapy for late-stage disease. Based on preclinical activity seen in fibrosarcoma and leiomyosarcoma xenograft models, EORTC conducted a phase II nonrandomized multicenter trial for patients with high-grade sarcoma, who had received no more than two prior chemotherapeutic agents for advanced disease.Citation56 In this EORTC trial 62052, eribulin was given intravenously at a concentration of 1.4 mg/m2 over 2–5 minutes on days 1 and 8 every 3 weeks with the primary end point of PFS at 12 weeks. Patients were prestratified based on tumor subtype into four categories; leiomyosarcoma, adipocytic sarcoma, synovial sarcoma, and other defined soft-tissue sarcomas. Twelve of the 38 (32%) leiomyosarcoma patients and 15 of the 32 (47%) adipocytic sarcoma patients were progression-free at 12 weeks, whereas only four of the 19 (21%), and five of the 26 (19%) patients with synovial sarcoma and other sarcomas, respectively, were progression-free at 12 weeks. The most common grade 3–4 adverse events were neutropenia (52%), leucopenia (35%), anemia (7%), fatigue (7%), febrile neutropenia (6%), abnormal alanine aminotransferase concentrations (5%), mucositis (3%), and sensory neuropathy (3%).
A phase III trial comparing eribulin to dacarbazine in patients with intermediate to high-grade advanced liposarcoma or leiomyosarcoma who have progressed after standard therapy is ongoing. This is similar to the trabectedin trial, and OS is the primary end point.
Targeted therapies
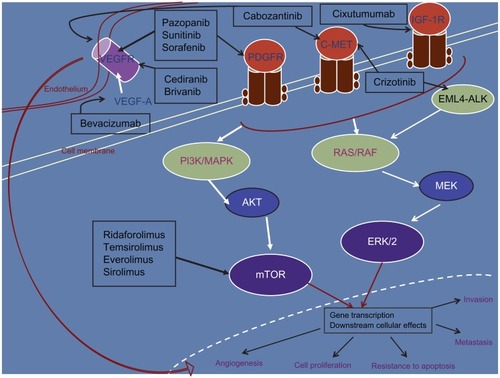
A variety of drugs targeting the molecular pathways involved in carcinogenesis are also being investigated in sarcomas, as more data are emerging regarding the molecular aberrations in this heterogeneous group of diseases. Detailed below are some agents that have shown encouraging activity, starting with pazopanib, an antiangiogenic agent that recently became the first molecular-targeted therapy to be FDA-approved in STS. highlights the targets for the agents discussed in this section.
Figure 1 Molecular-targeted therapy with activity in soft-tissue sarcoma. Only the major target(s) for each of the inhibitors have been depicted.
Abbreviations: VEGFR, vascular endothelial growth-factor receptor; STS, soft-tissue sarcoma; PDGFR, platelet-derived growth-factor receptor; IGF-1R, insulin-like growth factor-1 receptor; PI3K, phosphatidylinositol 3-kinase; MAPK, mitogen-activated protein kinase; EML4, echinoderm microtubule-associated protein-like 4; ALK, anaplastic lymphoma kinase; MEK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinases; AKT, protein kinase B; mTOR, mammalian target of rapamycin.
Angiogenesis inhibitors
Pazopanib
Pazopanib (Votrient [GW786034]; GlaxoSmithKline) is an orally bioavailable, adenosine triphosphate-competitive tyrosine kinase inhibitor of vascular endothelial growth-factor receptor (VEGFR)-1, -2, and -3, platelet-derived growth-factor receptor (PDGFR)-α and –β, and v-kit Hardy– Zuckerman 4 feline sarcoma viral oncogene homolog (c-kit) that has activity in multiple cancers, including renal cell cancer, sarcoma, NSCLC, and cervical and ovarian cancer. It is FDA-approved for the treatment of advanced renal-cell carcinoma and was recently approved for the treatment of patients with advanced STS who have received prior chemotherapy, excluding GIST and adipocytic STS patients. Sarcomas appear to be a good tumor type for antiangiogenic inhibitors, particularly those preventing VEGF action.Citation57 Circulating angiogenic factor levels were shown to correlate with extent of disease and risk of recurrence in patients with STS.Citation58 Pazopanib was evaluated as a single agent in STS in an EORTC phase II trial, and showed single-agent activity in STS, including leiomyosarcoma and synovial sarcomas, but not in adipocytic tumors.Citation59 This was followed by a randomized phase III placebo-controlled study of pazopanib in previously treated STS patients that showed a statistically significant improvement in PFS, leading to the recent approval. The 800 mg daily dosing regimen is the established active monotherapy dose used in these trials, providing optimal biologic and clinical effects associated with VEGFR inhibition and generally well tolerated.
The phase II study of pazopanib in patients with relapsed or refractory STS used a Simon two-stage design to evaluate the primary end point of PFR, using RECIST at 12 weeks after start of treatment, in four different STS categories.Citation59 Patients with leiomyosarcoma, synovial sarcoma, and “the other types of sarcoma” experienced a 12-week PFR of ≥40%, the predefined threshold indicating antitumor activity. The liposarcoma group did not meet its prespecified end point at the end of stage 1 and did not progress to stage 2. A total of 142 patients were enrolled and there were nine documented PRs, with at least 14 patients receiving more than 1 year of protocol therapy. The phase III randomized, double-blind study of pazopanib versus placebo in subjects with STS, excluding patients with GIST or adipocytic sarcomas (PALLETE), was initiated in 2008. A total of 369 STS patients, who had received at least one prior anthracycline-containing regimen were randomized in a 2:1 ratio to pazopanib or placebo.Citation60 Results published this year showed patients on the pazopanib arm had a statistically significant improvement in PFS, with a median increase of 13 weeks (median PFS 4.6 months versus 1.6 months; HR = 0.31 (95% CI 0.24–0.40), P < 0.0001). OS showed a trend toward improvement, but was not statistically significant (median 12.5 versus 10.7 months; HR = 0.86 [95% CI 0.67–1.11]). The median relative-dose intensity of pazopanib was 768 mg daily.
The most common adverse events reported for pazopanib monotherapy to date are diarrhea, fatigue, nausea, hypertension, hair color changes (hair depigmentation), anorexia, vomiting, dysgeusia, headache, abdominal pain, rash, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) increases, constipation, cough, and arthralgia. The majority of these events are low-grade. The most frequent grade 3 or 4 toxicities include hypertension, fatigue, diarrhea, hyperbilirubinemia, and AST and ALT increases. Less common adverse events of note include hand-foot syndrome, mucositis/stomatitis, proteinuria, venous thrombotic events, and bleeding.
Future studies in sarcoma are focusing on pazopanib’s role in earlier lines of therapy, in specific histologic subtypes, and in combination with chemotherapeutic agents.
Sorafenib
Sorafenib (BAY 43-9006; Onyx Pharmaceuticals) is an oral multikinase inhibitor with antiangiogenic and proapoptotic properties, targeting wild-type and mutant B-raf and C-raf kinases, as well as VEGFR-2, PDGFR-β, fms-like tyrosine kinase 3 (Flt-3), c-KIT and p38a, a member of the MAP kinase family. PDGFR expression has been demonstrated in sarcomas, and hence is a target of interest in addition to VEGFR.Citation61,Citation62 Sorafenib is currently FDA-approved for unresectable hepatocellular carcinoma and renal-cell carcinoma.
Based on an encouraging 12-week nonprogression rate seen in metastatic sarcoma patients in a phase I study,Citation63 Maki and his colleagues conducted a phase II multiarm study with single-agent sorafenib at a dose of 400 mg twice daily in patients with advanced STS.Citation64 Each of the six arms, representing different subtypes, including leiomyosarcoma, malignant peripheral nerve-sheath tumor, synovial sarcoma, vascular sarcomas, high-grade undifferentiated pleomorphic sarcoma, and “other” sarcomas, had its own Simon two-stage design. Out of the 145 patients treated on the study, 144 were evaluable for toxicity, and 122 were evaluable for response. Sixty- three percent of patients had received prior chemotherapy. Clinical response was only seen in the leiomyosarcoma and angiosarcoma subtypes, and the angiosarcoma arm was the only arm to meet the RECIST response-rate primary end point with five out of 37 patients responding (one CR and four PRs). The PFS (Kaplan–Meier estimates) at 3 and 6 months was 53% and 22%, respectively, and the median OS was 14.3 months (95% CI, 12.2–19.2 months).
A similar phase II trial conducted through the Southwest Oncology Group (SWOG) evaluated the role of sorafenib in advanced vascular sarcomas (angiosarcomas, malignant hemangiosarcomas, and solitary fibrous tumors), grade 3 and 4 liposarcomas and leiomyosarcomas, based on known expression of VEGFR and PDGFR in these histologies.Citation65 Of the 37 patients evaluable for response and toxicity, no confirmed responses were documented. The median PFS was 3 months (95% CI, 2–5 months), and OS was 17 months (95% CI, 13–25 months). The vascular sarcoma group had the longest median PFS of 5 months (95% CI, 3–6 months). A third phase II trial in advanced angiosarcomas by the French Sarcoma Group showed minimal activity, with a 40% tumor control rate and a 23% response rate only in patients who had previously received conventional chemotherapy.Citation66
One of the most common toxicities reported with sorafenib is dermatologic, with the most severe symptoms observed within the first 2 weeks of treatment. A majority of patients required a dose interruption and or reduction in these studies. Other antiangiogenic class effects, including hemorrhage, deep venous thrombosis, bowel perforation, and congestive heart failure, though rare, have also been reported.
Sorafenib also appears to have activity in desmoid fibromatosis, a tumor that lacks metastatic potential but can be locally aggressive, leading to significant morbidity. A retrospective review of 26 patients treated at a single institution, out of which 23 had documented progression prior to sorafenib, revealed a 25% PR rate and 70% SD rate.Citation67 Imatinib (Gleevec [ST1571]; Novartis) was the first tyrosine kinase inhibitor to demonstrate clinical benefit in this disease with validation in a prospective clinical trial.Citation68
In the hopes of improving antitumor activity, sorafenib is being combined with conventional chemotherapeutic agents in ongoing phase I/II clinical trials.
Sunitinib
Sunitinib (Sutent [SU11248]; Pfizer) is another multitargeted, small-molecule inhibitor of the receptor tyrosine kinases involved in tumor proliferation and angiogenesis, including VEGFR-1, -2, and -3, PDGFR-α and -β, KIT, the tyrosine kinase receptor encoded by the ret proto-oncogene, and Flt3. It is approved for use in GIST, renal cell, and pancreatic neuroendocrine tumors. The standard dosing is considered to be 50 mg daily for 4 weeks followed by 2 weeks off, but continuous dosing with 37.5 mg daily might be equally efficacious and hence has been incorporated in more recent trials.Citation69
A phase II trial in non-GIST sarcoma patients reported by George et al looked at continuous dosing with 37.5 mg daily, with RECIST response as the primary end point.Citation70 One patient with desmoplastic small-round cell tumor achieved a confirmed PR that was durable, and prolonged SD beyond 16 weeks was seen in two patients with solitary fibrous tumor, one each with ASPS, giant-cell tumor of bone, and synovial sarcoma, as well as four patients with chordoma. A retrospective series comprising 35 patients with solitary fibrous tumors also showed clinical activity for sunitinib in this population.Citation71 A phase II trial of sunitinib in previously treated metastatic uterine leiomyosarcoma patients, however, revealed minimal activity in this subtype.Citation72
Ongoing studies with sunitinib in sarcoma are focusing on its role in certain subtypes like ASPS and vascular sarcomas.
Brivanib
Brivanib (BMS-540215; Bristol-Myers Squibb) is an oral, selective dual inhibitor of VEGF and fibroblast growth factor (FGF) signaling. Results from a randomized discontinuation phase II trial, with the primary end point being PFS of brivanib versus placebo in sarcoma patients with FGF2 overexpression by immunohistochemistry, were recently reported.Citation73 Brivanib 800 mg once daily was initially given to 251 previously treated sarcoma patients for 12 weeks, after which the patients with PR (n = 7, including three angiosarcomas) were continued on brivanib open-label, and patients with progressive disease were taken off therapy. The patients with SD (n = 76) were then randomized 1:1 to either brivanib or placebo and stratified by FGF2 expression (53 positive/23 negative). Patients who received placebo were eligible to receive brivanib as open-label at the time of progression. PFS from week 12 in FGF2-positive patients demonstrated a doubling of PFS from 1.4 to 2.8 months (HR 0.58, 90% CI 0.34–0.97; P = 0.08). In the smaller FGF2- negative subset, brivanib demonstrated an improvement in PFS from 1.4 to 2.6 (HR 0.80, 90% CI 0.36–1.79; P = 0.69). This trial was not powered to determine a difference in efficacy between FGF2-positive and -negative patients. The most common grade 3 or 4 adverse effects included fatigue and hypertension.
Cediranib
Cediranib (Recentin [AZD2171]; AstraZeneca) is a potent oral pan-VEGFR signaling inhibitor that has shown activity in patients with ASPS, a rare tumor accounting for <1% of STS. ASPS is typically indolent but has a high incidence of metastasis to the lung, and can also spread to the brain. It is a highly vascular tumor and does not respond to conventional chemotherapy. A preliminary report of seven patients with ASPS treated with cediranib at a starting dose of 45 mg once daily on two separate phase II trials showed an encouraging response: four patients experienced a PR, two patients had a confirmed reduction in maximum tumor diameter of ≥10% and <30%, and one patient experienced SD.Citation74
Thereafter, a phase II trial of cediranib in patients with ASPS was initiated to determine the response rate, effect on tumor angiogenesis, and proliferation via imaging and to evaluate changes in the gene-expression profile on pre- and posttreatment tumor biopsies.Citation75 Patients received 30 mg cediranib orally once a day in 28-day cycles. At the time of this report, 28 patients were evaluable for response, with a disease control rate (PR + SD) of 78% at 6 months; 12 patients had PR (43%), 4 patients had > 20% reduction in target lesions, and six were stable at 6 months. Common toxicities reported were transaminitis, hypertension, diarrhea, tumor pain, and proteinuria. Preliminary data from gene-expression profiling of five patients revealed an early effect of treatment, with downregulation of certain proangiogenic or endothelial-specific genes and upregulation of genes involved in the inflammatory response. This and other studies are ongoing to confirm the role of cediranib in ASPS.
Bevacizumab
Bevacizumab (Avastin; Genentech/Roche) is a monoclonal antibody to circulating VEGF and has been evaluated as a single agent or in combination with various chemotherapy agents in the management of metastatic STS. It is FDA-approved for the treatment of metastatic colorectal cancer, non–small cell lung cancer, renal cell cancer and glioblastoma multiforme.
An open-label, single-agent phase II study of bevacizumab was conducted in relapsed or refractory angiosarcoma and epithelioid hemangioendothelioma patients.Citation76 Bevacizumab at 15 mg/kg IV every 3 weeks resulted in a response rate of 12% and tumor stabilization rate of 62% among the 26 patients evaluable for response. Two case reports have also reported dramatic responses in cutaneous angiosarcoma, one with bevacizumab monotherapy and one in combination with paclitaxel.Citation77,Citation78
Bevacizumab at 15 mg/kg IV was combined with doxorubicin 75 mg/m2 IV repeated every 3 weeks in a phase II study for metastatic STS.Citation79 However, the response rate of 12% (two PRs out of 17 patients) did not compare favorably to the historical response rate of single-agent doxorubicin. In addition, 35% of patients had a grade 2 or worse decline in left ventricular ejection fraction, despite administering dexrazoxane once doxorubicin doses exceeded 300 mg/m2. A phase IB study combining docetaxel, gemcitabine, and bevacizumab in patients with chemotherapy-naive STS was also completed.Citation80 Gemcitabine was given at three dose levels (1000, 1250, and 1500 mg/m2) every 2 weeks in the dose-finding portion of the study, followed by the expansion cohort with gemcitabine at 1500 mg/m2, docetaxel at 50 mg/m2, and bevacizumab at 5 mg/kg every 2 weeks. The overall response rate observed in the 35 assessable patients was 31.4%, with five CRs and six PRs, and 51.4% with SD lasting for a median of 6 months. There was no significant hematologic toxicity. Unfortunately, the observed grade 3 and 4 toxicities attributed to bevacizumab included pneumothorax, bowel perforation, fatigue, skin ulceration, and hemorrhage. Serum and tissue VEGF-A biomarkers studied did not show correlation with outcome. The best responses were seen in the angiosarcoma patients. A retrospective case series of 14 patients reported a 79% response rate for the combination of temazolomide 150 mg/m2 orally on days 1–7 and 15–21 along with bevacizumab 5 mg/kg on days 8 and 22, in patients with solitary fibrous tumors/hemangiopericytomas.Citation81 Eleven patients demonstrated a PR and two patients had SD based on Choi criteria.Citation82
Bevacizumab is actively being investigated in combination with a variety of standard chemotherapy regimens in STS subtypes. One would need to consider the added toxicity while determining the benefit of increased efficacy with combination therapy.
Insulin growth factor-receptor inhibitors
Cixutumumab
Cixutumumab (IMC-A12; Imclone Systems) is a fully human immunoglobulin G1 monoclonal antibody that selectively inhibits the insulin-like growth factor-1 receptor (IGF-1R), sparing the insulin receptor. Insulin growth factor (IGF)-1 and IGF-2 bind to IGF-1R, activating the receptor, and stimulate intracellular signaling, primarily through the downstream Ras/Raf/mitogen-activated protein kinase (MAPK) and the phosphatidylinositol 3-kinase (PI3-K)/AKT/mammalian target of rapamycin (mTOR) pathways. The IGF pathway plays a major role in cancer-cell proliferation, survival, and resistance to therapy and has been implicated in the pathogenesis of various types of bone and soft-tissue sarcoma.Citation83,Citation84
Phase I studies of IGF-1R monoclonal antibodies reported objective responses in several Ewing’s sarcoma patients and disease stabilization in one patient each with fibrosarcoma and ASPS.Citation85,Citation86 This prompted a few phase II studies, including a study of cixutumumab in five subtypes of sarcoma, with PFS as the primary end point.Citation87 One hundred and thirteen patients with previously treated metastatic soft-tissue sarcoma and the Ewing family of tumors were enrolled. Cixutumumab was given intravenously at 10 mg/kg over 1 hour every other week until disease progression or discontinuation for other reasons. The only subtype to meet the response criteria to proceed to stage 2 of the enrolment based on a Simon two-stage design was the adipocytic tumor. One patient experienced PR, and 57% of patients experienced SD in this subgroup. Other evidence of activity included one PR in the Ewing sarcoma subtype and SD in 41% of leiomyosarcoma, 35% of synovial sarcoma, 28% of Ewing’s, and 24% of rhabdomyosarcoma patients. Grade 3 or 4 toxicities with single-agent therapy were rare, and included hyperglycemia, asthenia, pain, and thrombocytopenia.
Since mTOR is involved in signal transduction downstream of IGF-1R, combination therapy with an mTOR inhibitor can potentially enhance IGF-1R inhibition. In turn, IGF-1R inhibition could mitigate the activation of upstream signaling molecules that occurs with mTOR inhibition. Preclinical data support the additive antitumor effects of combining these two targeted agents.Citation88 Cixutumumab at 6 mg/kg IV weekly was combined with temsirolimus, an mTOR inhibitor at 25–37.5 mg IV weekly in 4-week cycles, in heavily pretreated patients with the Ewing family of tumors.Citation89 This was well tolerated, with preliminary evidence of durable antitumor activity. Of the 20 patients (17 Ewing’s 3 DSRCT) enrolled, seven (35%) achieved a CR/PR or SD for more than 5 months. The same combination has also been evaluated in a phase II study for STS and bone tumors, stratifying for the expression of IGF-1R on tumor tissue.Citation90 These recently reported data showed each of the three arms: IGF-1R-positive STS (A), IGF-1R-positive bone sarcoma (B), and IGF-1R-negative bone and STS (C) met the primary end point of improved PFS, and the effect was independent of IGF-1R expression by immunohistochemistry. By intention-to- treat analysis, the median PFS in weeks was 6.3 (95% CI 5.9–12.0) for arm A, 11.0 (95% CI 8.0–18.0) for arm B, and 11.6 (95% CI 9.0–17.9) for arm C. Adverse events reported are higher with combination therapy, though grade 3 or 4 toxicities were rare (lymphopenia and mucositis).
Cixutumumab is also being evaluated in a phase I study in combination with doxorubicin in advanced STS patients.Citation91 Dose-limiting toxicities reported so far have been hyperglycemia and mucositis, with two patients experiencing grade 3 decrease in cardiac ejection fraction after repeated cycles.
mTOR inhibitors
Ridaforolimus
The mTOR is a member of the phosphatidylinositol kinase-related kinase family and functions to regulate protein translation, cell-cycle progression, and cellular proliferation.Citation92,Citation93 Activating mutations in growth-factor receptors lead to activation of the downstream PI3K/AKT/mTOR pathway. Ridaforolimus (AP23573; Ariad Pharmaceuticals/Merck) is derived from rapamycin, an immunosuppressive agent, and functions as an mTOR inhibitor, leading to G1 arrest and inactivation of protein synthesis. Based on early evidence of some activity in sarcoma, a phase II study in advanced bone sarcoma and STS was conducted, with ridaforolimus given at 12.5 mg/day IV for 5 days every 2 weeks.Citation94 Of the total 212 patients, 61 (28.8%) achieved clinical benefit, though the response rate was only 1.9% in this heavily pretreated population. Median PFS was 15.3 weeks, and median OS was 40 weeks. No potential tumor markers for response were identified out of those analyzed. Toxicities commonly associated with ridaforolimus include mild to moderate stomatitis, mucosal inflammation, rash, and fatigue.
Data from the phase III Sarcoma Multi-Center Clinical Evaluation of the Efficacy of Ridaforolimus (SUCCEED) study were first reported in 2011.Citation94 Efficacy of oral ridaforolimus was evaluated in the maintenance setting, with 711 patients with SD or better after first-line chemotherapy, randomized to either maintenance ridaforolimus or placebo. The study met its primary end point, with a statistically significant improvement in PFS of 3.1 weeks (17.7 versus 14.6 weeks) favoring ridaforolimus, but there was no significant improvement in OS. Due to this marginal benefit with added side effects in the maintenance setting, the FDA did not approve ridaforolimus for this indication.
Temsirolimus
Temsirolimus (Toricel [CCI-779]; Wyeth) is another mTOR inhibitor that has been studied in STS patients. A phase II study in 41 patients with advanced STS showed limited activity and moderate toxicity as a single agent, with two patients experiencing a PR and a median time to progression of 2 months.Citation95 Temsirolimus in combination with IGF-1R inhibitor cixutumumab showed activity in refractory Ewing sarcoma patients, and this combination is also being evaluated for soft-tissue and bone sarcomas, as described above under IGF-1R receptor inhibitors.
Everolimus
Everolimus (Afinitor [RAD001]; Novartis) was evaluated in a phase I study with figitumumab (CP-751871; Pfizer), an IGF-1R inhibitor, in which the majority of patients had advanced sarcoma, and the best response of PR was seen in a patient with malignant solitary fibrous tumor.Citation96 There are ongoing phase II studies looking at everolimus as a single agent and in combination with imatinib (Gleevec; Novartis) for STS.
Sirolimus
Sirolimus (rapamycin) is an allosteric mTOR inhibitor that was initially developed as an immunosuppressant and later evaluated for its anticancer properties. Clinical activity for sirolimus alone or in combination with oral cyclophosphamide was reported in four heavily pretreated advanced-sarcoma patients.Citation97 This was followed by a phase II study of oral cyclophosphamide and sirolimus in patients with advanced previously treated STS and bone sarcomas.Citation98 Ten out of the 47 patients were progression-free for 6 or more months, and the 6-month PFS rate was 21%. However, mTOR levels did not correlate with activity, and sarcomas with higher levels of mTOR activation progressed faster on sirolimus. Further studies with second-generation mTOR inhibitors described above might help us better understand the biologic correlatives for response and resistance with mTOR pathway inhibitors in STS.
Sirolimus has also been described in the management of malignant perivascular epithelioid tumors (PEComas). These sarcomas are part of a family of diseases that includes angiomyolipomas and lymphangioleiomyomatosis and are seen in patients with tuberous sclerosis, which is associated with mutations in TSC1 and TSC2, which lead to activation of the mTOR pathway. One report in the literature described three patients with malignant PEComas treated with sirolimus, all of whom had evidence of response.Citation99 Later the same year, another report described two patients with malignant PEComa who were successfully treated with temsirolimus.Citation100
Other novel inhibitors
Mesenchymal–epithelial transition factor (MET) is a protooncogene encoding the MET receptor tyrosine kinase, that upon stimulation from hepatocyte growth factor/scatter factor induces downstream signaling leading to invasive cellular growth, morphogenic differentiation, and angiogenesis. MET can be detected by immunohistochemistry in 87% of sarcomas, and higher levels of expression correlate with higher tumor grade.Citation101,Citation102 Cabozantinib (XL-184; Exelixis) is a small-molecule inhibitor, primarily targeting MET and VEGFR-2 and leading to inhibition of tumor growth, metastasis, and angiogenesis. Additional targets include RET, AXL, KIT, and TIE-2. Antitumor activity was seen in a phase I study in advanced solid-tumor patients, and SD was noted in one patient with clear-cell sarcoma and another with ASPS.Citation103 Further studies will be needed to clarify a potential role in these rare subtypes.
Crizotinib (PF-02341066; Pfizer) is a selective anaplastic lymphoma kinase (ALK) kinase inhibitor with additional activity against c-MET tyrosine kinase. Crizotinib was recently granted accelerated FDA approval for the treatment of patients with metastatic NSCLC harboring ALK translocations, which comprise around 5% of the NSCLC population. Inflammatory myofibroblastic tumor, a rare aggressive sarcoma in young adults and children, is associated with rearrangements in the ALK locus in around 50% of patients. One such patient treated with crizotinib experienced a sustained response, which has led to the inclusion of this tumor type in ongoing studies looking at ALK inhibitors in tumors that test positive for ALK translocations.Citation104
The murine double minute 2 (MDM2) oncogene has also been found to be amplified in well-differentiated and dedifferentiated liposarcoma, making it a promising target for therapy. Citation105 MDM2 antagonists have demonstrated preclinical activity and are now entering early clinical development, including sarcoma-specific trials in combination with chemotherapy.
There had been a prolonged hiatus in therapeutic advancement for STS other than GIST. It is now heartening to see an increased awareness and interest in STS, with an increasing number of sarcoma trials incorporating novel cytotoxics and targeted therapies. Positive results from two phase III trials were reported last year (SUCCEED and PALETTE), one of which led to the approval of pazopanib for previously treated nonadipocytic STS patients this year. There are a number of promising drugs for advanced STS patients currently in phase III testing (). This is a relatively exciting time for sarcoma therapy, with new targets and drugs being identified, and national and international collaborations are making large randomized and subtype-specific trials executable.
Table 2 Ongoing phase II trials for advanced STS (excluding GIST)
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- StojadinovicALeungDHHoosAJaquesDPLewisJJBrennanMFAnalysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomasAnn Surg2002235342443411882765
- O’SullivanBDavisAMTurcotteRPreoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trialLancet200235993252235224112103287
- [No authors listed]Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis CollaborationLancet19973509092164716549400508
- PervaizNColterjohnNFarrokhyarFTozerRFigueredoAGhertMA systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcomaCancer2008113357358118521899
- WollPJVan GlabbekeMHohenbergerPAdjuvant chemotherapy (CT) with doxorubicin and ifosfamide in resected soft tissue sarcoma (STS): interim analysis of a randomised phase III trialJ Clin Oncol200725Suppl 1810008
- O’ConnorJMChacónMPetracciFEChacónRDAdjuvant chemotherapy in soft tissue sarcoma (STS): a meta-analysis of published dataJ Clin Oncol200826 Suppl10526
- BramwellVRouesseJStewardWAdjuvant CYVADIC chemotherapy for adult soft tissue sarcoma – reduced local recurrence but no improvement in survival: a study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma GroupJ Clin Oncol1994126113711498201375
- Le CesneAVan GlabbekeMWollPJThe end of adjuvant chemotherapy (adCT) era with doxorubicin-based regimen in resected high-grade soft tissue sarcoma (STS): pooled analysis of the two STBSG-EORTC phase III clinical trialsJ Clin Oncol200826 Suppl10525
- FerrariAGronchiACasanovaMSynovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institutionCancer2004101362763415274077
- EilberFCEilberFREckardtJThe impact of chemotherapy on the survival of patients with high-grade primary extremity liposarcomaAnn Surg20042404686695 discussion 695–68715383796
- VerweijJMouridsenHTNielssenOSThe present state of the art in chemotherapy for soft tissue sarcomas in adults: the EORTC point of viewCrit Rev Oncol Hematol19952031932018748009
- HensleyMLMakiRVenkatramanEGemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trialJ Clin Oncol200220122824283112065559
- HensleyMLBlessingJADegeestKAbulafiaORosePGHomesleyHDFixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II studyGynecol Oncol2008109332332818394689
- HensleyMLBlessingJAMannelRRosePGFixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trialGynecol Oncol2008109332933418534250
- MakiRGWathenJKPatelSRRandomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]J Clin Oncol200725192755276317602081
- BayJORay-CoquardIFayetteJDocetaxel and gemcitabine combination in 133 advanced soft-tissue sarcomas: a retrospective analysisInt J Cancer2006119370671116496406
- SleijferSOualiMvan GlabbekeMPrognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG)Eur J Cancer2010461728319853437
- FuryMGAntonescuCRVan ZeeKJBrennanMFMakiRGA 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapyCancer J200511324124716053668
- ScurrMHistology-driven chemotherapy in soft tissue sarcomasCurr Treat Options Oncol2011121324521359911
- AntmanKCrowleyJBalcerzakSPAn intergroup phase III randomized study of doxorubicin and dacarbazine with or without ifosfamide and mesna in advanced soft tissue and bone sarcomasJ Clin Oncol1993117127612858315425
- BramwellVHAndersonDCharetteMLDoxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft-tissue sarcoma: a meta-analysis and clinical practice guidelineSarcoma20004310311218521288
- JelicSKovcinVMilanovicNRandomised study of high-dose epirubicin versus high-dose epirubicin-cisplatin chemotherapy for advanced soft tissue sarcomaEur J Cancer19973322202259135492
- Le CesneAJudsonICrowtherDRandomized phase III study comparing conventional-dose doxorubicin plus ifosfamide versus high-dose doxorubicin plus ifosfamide plus recombinant human granulocyte-macrophage colony-stimulating factor in advanced soft tissue sarcomas: a trial of the European Organization for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma GroupJ Clin Oncol200018142676268410894866
- HartmannJTOechsleKHuoberJAn open label, non-comparative phase II study of gemcitabine as salvage treatment for patients with pretreated adult type soft tissue sarcomaInvest New Drugs200624324925316133789
- PazdurRSamsonMKBakerLHFludarabine phosphate. Phase II evaluation in advanced soft-tissue sarcomasAm J Clin Oncol19871043413432441592
- ReichardtPOechsleKPinkDAn open label, non-comparative phase II study of topotecan as salvage treatment for patients with soft tissue sarcomaInvest New Drugs200321448148614586217
- RosePGBlessingJASoperJTBarterJFProlonged oral etoposide in recurrent or advanced leiomyosarcoma of the uterus: a gynecologic oncology group studyGynecol Oncol19987022672719740703
- WollPJJudsonILeeSMTemozolomide in adult patients with advanced soft tissue sarcoma: a phase II study of the EORTC Soft Tissue and Bone Sarcoma GroupEur J Cancer199935341041210448291
- Van GlabbekeMVerweijJJudsonINielsenOSProgression-free rate as the principal end-point for phase II trials in soft-tissue sarcomasEur J Cancer200238454354911872347
- David-CordonnierMHGajateCOlmeaODNA and non-DNA targets in the mechanism of action of the antitumor drug trabectedinChem Biol200512111201121016298299
- TakebayashiYPourquierPZimonjicDBAntiproliferative activity of ecteinascidin 743 is dependent upon transcription-coupled nucleotide-excision repairNat Med20017896196611479630
- FriedmanDHuZKolbEAGorfajnBScottoKWEcteinascidin- 743 inhibits activated but not constitutive transcriptionCancer Res200262123377338112067978
- TakebayashiYGoldwasserFUrasakiYKohlhagenGPommierYEcteinascidin 743 induces protein-linked DNA breaks in human colon carcinoma HCT116 cells and is cytotoxic independently of topoisomerase I expressionClin Cancer Res20017118519111205907
- HerreroABMartin-CastellanosCMarcoEGagoFMorenoSCross- talk between nucleotide excision and homologous recombination DNA repair pathways in the mechanism of action of antitumor trabectedinCancer Res200666168155816216912194
- DelalogeSYovineATaammaAEcteinascidin-743: a marine-derived compound in advanced, pretreated sarcoma patients – preliminary evidence of activityJ Clin Oncol20011951248125511230465
- YovineARiofrioMBlayJYPhase II study of ecteinascidin- 743 in advanced pretreated soft tissue sarcoma patientsJ Clin Oncol200422589089914990645
- Garcia-CarboneroRSupkoJGManolaJPhase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapyJ Clin Oncol20042281480149015084621
- Le CesneABlayJYJudsonIPhase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trialJ Clin Oncol200523357658415659504
- HuyghGClementPMDumezHEcteinascidin-743: evidence of activity in advanced, pretreated soft tissue and bone sarcoma patientsSarcoma200620065628217496996
- Garcia-CarboneroRSupkoJGMakiRGEcteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic studyJ Clin Oncol200523245484549216110008
- GronchiABuiBNBonvalotSPhase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcomaAnn Oncol201223377177621642514
- DemetriGDChawlaSPvon MehrenMEfficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedulesJ Clin Oncol200927254188419619652065
- GomezJLázaroLLGuzmanCGonzalezAMissetJTwelvesCIdentification of biochemical parameters that predict the onset of severe toxicities in patients treated with ET-743Proc Am Soc Clin Oncol200019727
- TaammaAMissetJLRiofrioMPhase I and pharmacokinetic study of ecteinascidin-743, a new marine compound, administered as a 24-hour continuous infusion in patients with solid tumorsJ Clin Oncol20011951256126511230466
- GrossoFDileoPSanfilippoRSteroid premedication markedly reduces liver and bone marrow toxicity of trabectedin in advanced sarcomaEur J Cancer200642101484149016737808
- HingoraniPZhangWPiperdiSPreclinical activity of palifosfamide lysine (ZIO-201) in pediatric sarcomas including oxazaphosphorine- resistant osteosarcomaCancer Chemother Pharmacol200964473374019224214
- JonesBKomarnitskyPMillerGTAmedioJWallnerBPAnticancer activity of stabilized palifosfamide in vivo: schedule effects, oral bioavailability, and enhanced activity with docetaxel and doxorubicinAnticancer Drugs201223217318422027537
- ChughRChawlaSBenjaminRHeatonJStevensJSchwartzBPhase I/II study of ZIO-201 in advanced sarcomaESMO 32nd CongressJuly 5–8, 2007Lugano, Switzerland
- ChawlaSChuaVAbbadessaGLoBueLLewisJA study of palifosfamide in combination with doxorubicin: safety and preliminary efficacyConnective Tissue Oncology Society 14th Annual MeetingNovember 13–15, 2008London, UK
- VerschraegenCFMitaMMRyanCWA phase II, randomized, controlled trial of palifosfamide plus doxorubicin versus doxorubicin in patients with soft tissue sarcoma (PICASSO)J Clin Oncol201028Suppl 1510004
- DuanJXJiaoHKaizermanJPotent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugsJ Med Chem20085182412242018257544
- WeissGJInfanteJRChioreanEGPhase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignanciesClin Cancer Res20111792997300421415214
- GanjooKNCranmerLDButrynskiJEA phase I study of the safety and pharmacokinetics of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcomaOncology2011801–2505621625179
- ChawlaSPAdkinsDReedDTH-302 in combination with doxorubicin in patients with first line advanced soft tissue sarcoma previously untreated with chemotherapy2011 Combined Meeting of the Connective Tissue Oncology Society and the Musculoskeletal Tumor SocietyOctober 26–29, 2011Chicago, US
- SchoffskiPRay-CoquardILCioffiAActivity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histological subtypesLancet Oncol201112111045105221937277
- HeymachJVAngiogenesis and antiangiogenic approaches to sarcomasCurr Opin Oncol200113426126911429484
- YoonSSSegalNHOlshenABBrennanMFSingerSCirculating angiogenic factor levels correlate with extent of disease and risk of recurrence in patients with soft tissue sarcomaAnn Oncol20041581261126615277268
- SleijferSRay-CoquardIPapaiZPazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043)J Clin Oncol200927193126313219451427
- van der GraafWTBlayJYChawlaSPPazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trialLancet201237998291879188622595799
- WeinerTMLiuETCravenRJCanceWGExpression of growth factor receptors, the focal adhesion kinase, and other tyrosine kinases in human soft tissue tumorsAnn Surg Oncol19941118277834423
- FranklinWAChristisonWHColleyMMontagAGStephensJKHartCEIn situ distribution of the beta-subunit of platelet-derived growth factor receptor in nonneoplastic tissue and in soft tissue tumorsCancer Res19905019634463482169345
- StrumbergDRichlyHHilgerRAPhase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumorsJ Clin Oncol200523596597215613696
- MakiRGD’AdamoDRKeohanMLPhase II study of sorafenib in patients with metastatic or recurrent sarcomasJ Clin Oncol200927193133314019451436
- von MehrenMRankinCGoldblumJRPhase 2 Southwest Oncology Group-directed intergroup trial (S0505) of sorafenib in advanced soft tissue sarcomasCancer2012118377077621751200
- Ray-CoquardIItalianoABompasESorafenib for patients with advanced angiosarcoma: a phase II trial from the French Sarcoma Group (GSF/GETO)Oncologist201217226026622285963
- GounderMMLefkowitzRAKeohanMLActivity of sorafenib against desmoid tumor/deep fibromatosisClin Cancer Res201117124082409021447727
- ChughRWathenJKPatelSREfficacy of imatinib in aggressive fibromatosis: Results of a phase II multicenter Sarcoma Alliance for Research through Collaboration (SARC) trialClin Cancer Res201016194884489120724445
- GeorgeSBlayJYCasaliPGClinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failureEur J Cancer200945111959196819282169
- GeorgeSMerriamPMakiRGMulticenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomasJ Clin Oncol200927193154316019451429
- StacchiottiSNegriTLibertiniMSunitinib malate in solitary fibrous tumor (SFT)Ann Oncol Epub June 17, 2012
- HensleyMLSillMWScribnerDRJrSunitinib malate in the treatment of recurrent or persistent uterine leiomyosarcoma: a Gynecologic Oncology Group phase II studyGynecol Oncol2009115346046519811811
- SchwartzGKRatainMJUndeviaSDBrivanib (BMS-582664) in advanced soft-tissue sarcoma (STS): biomarker and subset results of a phase II randomized discontinuation triaJ Clin Oncol201129 Suppl10000
- GardnerKLeahyMBarquinEActivity of cediranib, a highly potent and selective VEGF signaling inhibitor, in alveolar soft part sarcomaJ Clin Oncol200927 Suppl 1510523
- KummarSMonksAIvySPAn evaluation of cediranib as a new agent for alveolar soft part sarcoma (ASPS)J Clin Oncol201129 Suppl10001
- AgulnikMVon MehrenMJovanovicBBrocksteinBBenjaminRSEvensAMAn open-label multicenter phase II study of bevacizumab for the treatment of angiosarcomaJ Clin Oncol200927 Suppl 1510522
- FullerCKCharlsonJADankleSKRussellTJDramatic improvement of inoperable angiosarcoma with combination paclitaxel and bevacizumab chemotherapyJ Am Acad Dermatol2010634e83e8420846560
- RosenAThimonSTernantDMachetMCPaintaudGMachetLPartial response to bevacizumab of an extensive cutaneous angiosarcoma of the faceBr J Dermatol2010163122522720394623
- D’AdamoDRAndersonSEAlbrittonKPhase II study of doxorubicin and bevacizumab for patients with metastatic soft-tissue sarcomasJ Clin Oncol200523287135714216192597
- VerschraegenCFArias-PulidoHLeeSJPhase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimenAnn Oncol201223378579021746804
- ParkMSPatelSRLudwigJAActivity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumorCancer2011117214939494721480200
- ChoiHCharnsangavejCFariaSCCorrelation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteriaJ Clin Oncol200725131753175917470865
- RikhofBde JongSSuurmeijerAJMeijerCvan der GraafWTThe insulin-like growth factor system and sarcomasJ Pathol2009217446948219148905
- PrieurATirodeFCohenPDelattreOEWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3Mol Cell Biol200424167275728315282325
- MalempatiSWeigelBIngleAMPhase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children’s Oncology GroupJ Clin Oncol201230325626222184397
- TolcherAWSarantopoulosJPatnaikAPhase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1J Clin Oncol200927345800580719786654
- SchoffskiPAdkinsDBlayJPhase II trial of anti-IGF-IR antibody cixutumumab in patients with advanced or metastatic soft-tissue sarcoma and Ewing family of tumorsJ Clin Oncol201129 Suppl 1510004
- KurmashevaRTDudkinLBillupsCDebelenkoLVMortonCLHoughtonPJThe insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcomaCancer Res200969197662767119789339
- NaingALoRussoPFuSInsulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumorsClin Cancer Res20121892625263122465830
- SchwartzGK TWQinLA phase II multicenter study of the IGF-1 receptor antibody cixutumumab (A12) and the mTOR inhibitor temsirolimus (TEM) in patients (pts) with refractory IGF-1R positive (+) and negative (−) bone and soft tissue sarcomas (STS)J Clin Oncol201230 Suppl10003
- ChughRGriffithKABrocksteinBUndeviaSDStadlerWMSchuetzeSPhase I study of doxorubicin (D) plus anti-insulin-like growth factor 1 receptor (IGF-1R) antibody cixutumumab (IMC-A12) in advanced soft tissue sarcoma (STS)J Clin Oncol201230 Suppl10028
- SchmelzleTHallMNTOR, a central controller of cell growthCell2000103225326211057898
- BrownEJSchreiberSLA signaling pathway to translational controlCell19968645175208752206
- ChawlaSPStaddonAPBakerLHPhase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomasJ Clin Oncol2012301788422067397
- OkunoSBaileyHMahoneyMRA phase 2 study of temsirolimus (CCI-779) in patients with soft tissue sarcomas: a study of the Mayo phase 2 consortium (P2C)Cancer2011117153468347521287536
- QuekRWangQMorganJACombination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumorsClin Cancer Res201117487187921177764
- SchuetzeSMBakerLHMakiRGSirolimus reduced tumor-related morbidity and resulted in biochemical and radiographic response in patients with progressive sarcomaJ Clin Oncol200624Suppl 18 Abstr 9503
- SchuetzeSZhaoLChughRResults of a phase II trial of sirolimus (S) and cyclophosphamide (C) in advanced sarcomaJ Clin Oncol201129 Suppl10003
- WagnerAJMalinowska-KolodziejIMorganJAClinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumorsJ Clin Oncol201028583584020048174
- ItalianoADelcambreCHosteinITreatment with the mTOR inhibitor temsirolimus in patients with malignant PEComaAnn Oncol20102151135113720215136
- KuhnenCMuehlbergerTHonselMTolnayESteinauHUMullerKMImpact of c-Met expression on angiogenesis in soft tissue sarcomas: correlation to microvessel-densityJ Cancer Res Clin Oncol2003129741542214605882
- OdaYSakamotoASaitoTKinukawaNIwamotoYTsuneyoshiMExpression of hepatocyte growth factor (HGF)/scatter factor and its receptor c-MET correlates with poor prognosis in synovial sarcomaHum Pathol200031218519210685632
- KurzrockRShermanSIBallDWActivity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancerJ Clin Oncol201129192660266621606412
- ButrynskiJED’AdamoDRHornickJLCrizotinib in ALK-rearranged inflammatory myofibroblastic tumorN Engl J Med2010363181727173320979472
- SingerSSocciNDAmbrosiniGGene expression profiling of liposarcoma identifies distinct biological types/subtypes and potential therapeutic targets in well-differentiated and dedifferentiated liposarcomaCancer Res200767146626663617638873