Abstract
Gasdermin B (GSDMB) belongs to the gasdermin (GSDM) family which may adopt different mechanisms of intramolecular domain interactions to modulate their lipid-binding and pore-forming activities. The GSDM family has regulatory functions in cell proliferation and differentiation, especially in pyroptosis process. Pyroptosis is a pro-inflammatory form of regulated cell death and is designed to attract a nonspecific innate response to the site of infection. For cancer cells, the activation of pyroptosis may promote cell death and exert anticancer properties. Also, recent studies have observed the pyroptosis-like features in GSDMB and some researches have shown that GSDMB overexpression occurred in several kinds of cancers; these findings bring a contradiction with the participation of GSDMB in pyroptosis. Although people pay less attention to GSDMB, it still has some essential research value. It is a paradox that GSDMB might participate in programmed cell death, which might put forward a research direction of therapeutic targets for cancer. Here, we review the possible progress of how GSDMB participated in this inflammatory regulation mechanistically and the potential functions of GSDMB in cancer.
Introduction
High incidence and high mortality making cancer one of the leading causes of death in the world and one of the most important public health issues. Incidence rates are highest among developed countries for a wide range of types of cancer, including prostate, lung, colorectal, and breast cancers. However, the highest rates of cervical, esophageal, liver, and stomach cancers also occur in some developed countries. Epidemiological data also revealed gender- and region-specific patterns in cancer prevalence worldwide.Citation1 Cancer mortality has been associated with a wide variety of risk factors, including physical inactivity level, infection, smoking status, and obesity. Despite improvements in therapies, cancers tend to progress extremely aggressively, yielding poor survival rates, and as such, cancer research remains extremely active.Citation2–Citation6
Pyroptosis is a novel type of programmed cell death that plays a critical role in both septic shock and immune defenses.Citation7–Citation10 It was first observed in macrophages infected with the Gram-negative bacteria Shigella fexneri in 1992, and the term was named by Lawrence H. Boise in 2001.Citation11–Citation13 Pyroptosis is occurred through the action of various stimuli and inflammatory caspases and caspases trigger the cleavage of gasdermin family as well as the release of both its C-terminal inhibitory domain and N-terminal effector domain.Citation14–Citation17 The N-terminal domain oligomerizes in the cell membrane and forms pores, which leads to rapid plasma membrane rupture, thus releasing the intracellular contents and pro-inflammatory mediators such as interleukin (IL)-18 and IL-1β.Citation18 The release of damage-associated molecular patterns from lysed pyroptotic cells can recruit immune cells and further promote inflammation. For cancer cells, the activation of pyroptosis may promote cell death and exert anticancer properties.Citation7,Citation19-22
Pyroptosis is also called gasdermin-mediated programmed cell death. The gasdermin (GSDM) family has regulatory functions in normal cell proliferation and differentiationCitation23,Citation24 and include gasdermin A (GSDMA), gasdermin B (GSDMB), gasdermin C (GSDMC), gasdermin D (GSDMD), gasdermin E (GSDME), and DFNB59.Citation25–Citation27 GSDMA and GSDMB genes are located at 17q2, while GSDMC and GSDMD are located at 8q24.Citation28 With the exception of DFNB59, these gene family members share about 45% sequence homology, and all of the GSDM have two domains that are capable of binding to each other and are connected by a long flexible linker.Citation29,Citation30 Sequence homology suggests that the members of the GSDM family (except for DFNB59) share similar 3D structures.Citation29,Citation31,Citation32 The gasdermin-N domain enables most GSDM members to function as a new type of pore-forming protein. In their pore-forming protein role, different GSDM family members may adopt different mechanisms of intramolecular domain interactions that modulate their lipid-binding and pore-forming activities, which may induce pyroptosis-like features in cells. GSDMB, as a member of GSDM family, also has been observed pyroptosis-like features, and some studies have shown that GSDMB overexpression occurs in several kinds of cancers, where it might be involved in cancer progression and metastasis.Citation33,Citation34 Here, we review the progress in uncovering how GSDMB participates in this inflammatory regulation mechanistically and the potential functions of GSDMB in cancer.
Characterization of GSDMB
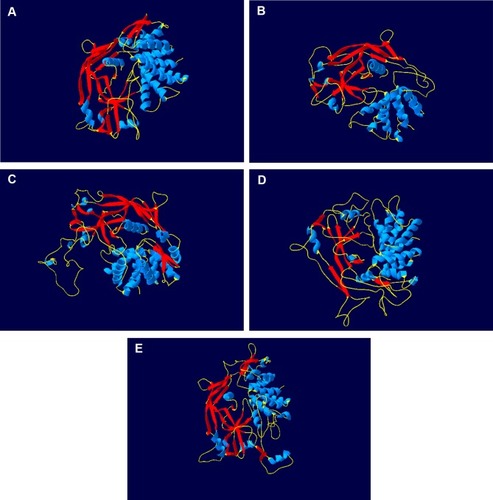
GSDMB used to be known as GSDML (gasdermin-like protein) and is located at 17q21, which may also harbor genes that influence diseases associated with aberrant immune responses, and 17q21 also includes ORMDL3, which also regulates the expression of GSDMB. It is hypothesized that GSDMB might be generated due to a duplication of GSDMA gene which is also a member of GSDM family and is located at 17q21 too. The GSDMB protein is comprised of 411 amino acids.Citation28 As shown in , GSDMB is so different from other GSDM in its structure and domain architecture. However, there are two different promoters in the cis-regulatory elements of GSDMB, an Alu-derived promoter which directs expression solely in normal stomach tissues and a long-terminal-repeat (LTR) derived promoter which directs GSDMB expression in various types of cancer and normal tissues. These two promoters drive all GSDMB transcriptions except for those starting from exon1a and exon1b.Citation35,Citation36 However, the molecular mechanisms by which the cellular promoter regulates GSDMB expression have not yet been elucidated.Citation37 Human GSDMB has six splicing variants, and each of them can encode a protein with a molecular weight ranging from 35 to 50 kDa. These isoforms have different expression profiles and subcellular localization patterns in different cell types and are also different in the sequence of their linkers between the N- and C-terminal domains which is weaker and instability than those of any other gasdermins, except for isoform 5, which comprises only the C-terminal domain of the protein.Citation30,Citation38 The C-terminal domain does not inhibit lipid binding because of the weaker N- and C-domain interactions in GSDMB.
Figure 1 GSDMB is different from other gasdermins in its structure and domain architecture. The protein of GSDMA (A), GSDMB (B), GSDMC (C), GSDMD (D) and GSDME (E) is shown as cartoon. Blue cartoon represents the helices of protein. Red cartoon represents the sheets of protein. Yellow cartoon represents the coils of protein.
Some studies discovered that the N-terminal domains of GSDMA3, GSDMA and GSDMD could bind phospholipids and cardiolipin and the C-terminal domains inhibit this ability to the full-length proteins.Citation39 Therefore, these GSDMs could function only upon cleavage between their N- and C-terminal domains because phospholipids were components of the cell membrane’s inner leaflets. The lipid-binding activity of GSDMB is also located in its N-terminal domain, both the full-length GSDMB protein as well as the N-terminal domain of GSDMB uniquely bind phosphoinositides and sulfatides (sulfated galactocerebrosides and 3-O-sulfogalactosylceramides).Citation40 Strikingly, unlike other GSDMs, the presence of the GSDMB C-terminal domain in the full-length protein does not prevent phospholipid binding. The reason for this strange phenomenon is that the N-terminal domain bound liposomes containing sulfatide whereas the C-terminal domain does not. Sulfatides, which are esters of sulfuric acid with galactosylceramides, are synthesized from galactosylceramide in the Golgi apparatus and degraded in the lysosome. Sulfatides are multifunctional molecules, which have several important biological functions in the immune system, nervous system, microbial infections, cancer, and so on. Elevated levels of sulfatides are documented on the surface membrane of epithelial cells in many cancers, which suggests that GSDMB may have an important function in cellular transport of sulfatides. Abundant sulfatides on the surface of cancer cells are native ligands for P-selectin expressed by activated endothelial cells, which could facilitate the formation of aggregates and promote cancer cell migration and metastasis. Overall, the N-terminal domain of GSDMB can bind with sulfatide uniquely, while sulfatide over-expression is frequently related to cancer progression, suggesting that GSDMB might play a significant role in cancer cell migration and metastasis.Citation28,Citation30,Citation41-44
Therefore, the difference in the inherent stability of these isoforms and susceptibility to cellular protein degradation machinery may lead to different cellular concentrations, which may thus increase some disease risk.Citation30 A recent research has revealed an association between single nucleotide polymorphisms in GSDMB and type 1 diabetes and ankylosing spondylitis.Citation35 In particular, polymorphisms in GSDMB were also associated with an increased risk of developing complex-trait inflammatory diseases, such as asthma, inflammatory bowel disease, Crohn’s disease, and ulcerative colitis.Citation30,Citation45 Humans may also be more susceptible to inflammatory disease than mice because humans carry a gene encoding GSDMB but rodents do not. Recent research has observed that GSDMB has the ability to induce pyroptosis-like features, but it remains unclear whether GSDMB can induce pyroptosis or how GSDMB participates in this inflammatory regulation mechanistically.Citation35 In the process of exploring whether GSDM family members can induce programmed cell death, Shi et al found that GSDM family proteins were expressed normally in cells and that the N-terminal domains of GSDM family members, with exception of DFNB59, have the ability to induce cell death.Citation29 However, many scholars pay more attention to the role of GSDMB in pyroptosis.
GSDMB Participates in Pyroptosis
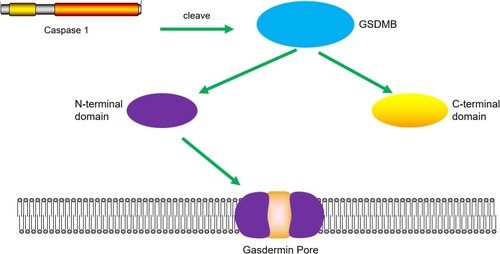
Cleavage of GSDMB Protein by Caspase-1 Induces Pyroptosis
Ding et al found that the N-terminal domain of GSDMB indeed induces pyroptosis in HEK293T cells.Citation39 Accordingly, Panganiban et al found that expression of GSDMB proteins alone in the absence of caspase-1 did not induce pyroptosis.Citation46 Moreover, transfection of plasmids expressing GSDMB and caspase-1 into 293T cells induced GSDMB to be cleaved into two short forms. As shown in , subsequent experiments have indicated that GSDMB is cleaved by caspase-1 at site 236. One of the cleaved forms is the N-terminus of the GSDMB protein, which is about 20 kDa. The release of the N-terminal domain can induce cell pyroptosis. In contrast, the full-length N-terminal domain or the C-terminal fragment could not induce any increase in pyroptosis. Importantly, they investigated whether the splicing variant rs11078928, which corresponds to one of the single nucleotide polymorphisms in GSDMB, abolishes the biochemical ability of GSDMB to induce pyroptosis cell death, because genotypes of rs11078928 result in a change of proline to serine (P311S) at amino acid residue 311 of the GSDMB protein. This substitution destroys a splicing acceptor site and prevents the splicing of exon 643, leading to the deletion of 13 amino acids from the N-terminus of GSDMB.Citation46,Citation47
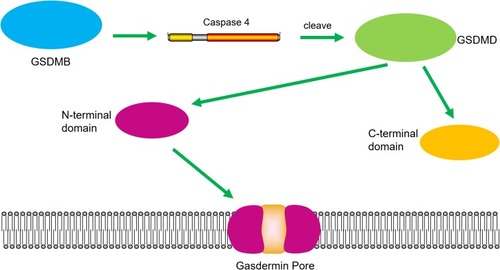
GSDMB Promotes Non-Canonical Pyroptosis by Enhancing Caspase-4 Activity
Chen et al found that the N-terminus of GSDMB by itself was unlike other GSDM family proteins in its association with sulfatide, but without inducing pyroptosis.Citation48 The binding of full-length GSDMB with the CARD domain of caspase-4 may result in the oligomerization of caspase-4 proteins, which in turn causes conformational changes to caspase-4 proteins, thus increasing enzymatic activity and promoting GSDMD cleavage, thereby inducing non-canonical pyroptosis just as seen in . In addition, Chen et al also posited that the positive effect of GSDMB on caspase-4 in non-canonical pyroptosis can be terminated by a negative feedback mechanism, which may be an important protective function when excessive or prolonged pyroptosis in response to infectious pathogens is detrimental. Taken together, the authors concluded that the GSDMB N-terminus cannot form pores and that the enhanced cell death caused by GSDMB actually occurs through enhancing the enzymatic activity of caspase-4.Citation49
Figure 3 GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. The binding of GSDMB with the CARD domain of caspase-4 result in the oligomerization of the caspase-4 proteins, and caspase-4 promotes the cleavage of GSDMD and the releasing of the N-terminal effector domain and the C-terminal inhibitory domain. The N-terminal domain oligomerizes in the cell membrane and forms pores, which leads to pyroptosis.
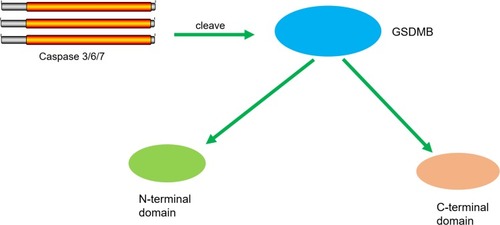
GSDMB Could Be Cleaved by Caspase-3/-6/-7
When Chao et al investigated whether GSDMB is a substrate of human apoptotic caspases, they found that GSDMB is not a substrate for the inflammatory caspases 1 and 4/5/11 because it lacks the specific interdomain linker region site, like the GSDMD cleavage sequence.Citation30 Accordingly, they characterized the phospholipid-binding activities of GSDMB and the cleavage profile of caspase and was also demonstrated that GSDMB proteins are substrates of the apoptotic executioner apoptotic caspases-3, -6, and -7 proteins, not inflammatory caspases, which implies potential cross-talk between the apoptosis pathway and the non-canonical pyroptosis pathway. The molecular weights of the proteolytic fragments indicate that the caspase recognition site is in the N-terminal domain of GSDMB and not in the interdomain linker region. Additionally, just as shown in , the N-terminal sequence of the C-terminal fragment from a caspase-3 cleavage reaction revealed that the cleavage site is after 88DNVD91 and 304EDPD307, with a probability score of 0.803. Thus, human caspases did not generate an entire GSDMB N-terminal domain, analogous to the pyroptosis N-terminal domain of GSDMD, which binds signaling lipids in contrast to other GSDM, whose C-terminal domains inhibit lipid binding, and only their cleaved forms can bind lipids, form pores, and kill cells. However, there has been no evidence that a stable N-terminal domain is generated from a proteolytic cleavage of GSDMB, and the pyroptosis activity of the N-terminal domain may be decoupled from its phospholipid-binding activity because the full-length GSDMB is noncytotoxic.Citation30,Citation35,Citation39 In general, the cleavage by caspase-3/-6/-7 does not form a complete N domain. And whether the cleaved product can form pores and induce pyroptosis is currently exclusive. Thus, the functional significance of caspase-3/-6/-7 cleavage at an internal GSDMB’s N-terminal domain and its impact on protein activity is worthy of in-depth study.
Figure 4 GSDMB could be cleaved by caspase-3/-6/-7. Caspase-3/-6/-7 promotes the cleavage of GSDMB and the releasing of the N-terminal effector domain and the C-terminal inhibitory domain.
In conclusion, how GSDMB participates in pyroptosis is still debatable. Experiments have different ideas in the role of caspases in the cleavage of GSDMB in pyroptosis. As shown in , there are many differences in the experiments that were performed. Taken together, different cell lines and conditions lead to different cleavage sites for caspases. However, only GSDMB cleaved by special caspase at the particularly site can produce the N-terminus that have a pore-forming ability, which might be the possible reason resulted indifferent conclusions. Thus, further research should be undertaken to investigate whether GSDMB can induce cell pyroptosis by cleaving caspases and how GSDMB participates in pyroptosis mechanistically.
Table 1 Differences in the Experiments About the Mechanism of GSDMB in Pyroptosis
GSDMB and Cancer
Recently, GSDMB expression has been detected in lymphocytes as well as esophagus, stomach, liver, colon, skin epithelium, gastrointestinal tract, and immune cells. Based on previous studies of human cancers, people have found that GSDMB is highly expressed not only in healthy tissues but also in cancer tissues, such as those of gastric, uterine, cervical, and breast cancers.Citation35 Researchers have demonstrated that GSDMB located in amplicons, genomic regions that are often amplified during cancer development. Therefore, GSDMB may be involved in cancer progression and metastasis.Citation34 However, the specific functions of GSDMB in carcinogenesis, cancer metastasis, and progression are still not well understood.
GSDMB in Breast Cancer
The expression of fusion genes created by genomic rearrangements, such as GSDMB in the TATDN1–GSDMB fusion, was recently observed to be especially common in association with high-level DNA amplification, which may be associated with the selective advantage provided by DNA amplification and deletion. In human epidermal growth factor receptor 2-positive breast cancer, GSDMB overexpression has been shown to occur in approximately 60% of the cases, where it appears to promote cancer cell invasion, progression, and metastasis. Thus, GSDMB may be used as a novel marker in breast cancer screening. As demonstrated by Molina-Crespo et al intracellular delivery of an antibody targeting GSDMB can reduce human epidermal growth factor receptor 2 breast cancer metastasis, migration, and resistance to therapy efficiently and specifically. Thus, there is potential for aggressive human epidermal growth factor receptor 2 breast cancers to be effectively treated by inducing cytoplasmic overexpression of GSDMB. However, other researchers have found that GSDMB expression is also linked to poor prognosis, poor therapeutic responses in terms of relapse-free survival, and the development of distant metastasis in human epidermal growth factor receptor 2-positive breast cancer. As demonstrated across a range of human epidermal growth factor receptor 2-positive breast carcinoma cells, GSDMB expression promotes survival under treatment with trastuzumab. The same researchers also found that in vivo GSDMB expression is closely related to trastuzumab resistance in patient-derived xenografts.Citation33,Citation50-52 Overall, GSDMB is a critical determinant of poor prognosis and therapeutic response in human epidermal growth factor receptor 2-positive breast cancer, and the mechanisms underlying resistance remain somewhat unknown.
GSDMB in Gastric Cancer
Gastric cancer is a major malignant disease and the second most common cause of cancer death worldwide.Citation53 Recently, Komiyama et al examined GSDMB expression in healthy and cancerous stomach tissue samples.Citation34 They found that GSDMB had extremely low expression levels in most normal gastric tissues, and the expression level was low or effectively absent in the few healthy samples exhibiting GSDMB expression. Unlike the healthy tissues, GSDMB was amplified and overexpressed in gastric cancers, showing that GSDMB might play an important role in the regulation of cancer cell proliferation and have no cell-growth inhibition activity in gastric cancer cells. These findings indicated that GSDMB might be an oncogene.Citation34 Accordingly, Carl-McGrath et al found that GSDMB could also be overexpressed in hepatic carcinoma and colon cancer tissues.Citation54
Komiyama et al found an Alu element, approximately 300-bp-long and belonging to the SINE family of retrotransposons, residing in the 5′ region upstream of GSDMB. The Alu element was found to regulate GSDMB expression in cancer cells, and a putative IKZF binding motif in this element played an important role in upregulating GSDMB expression.Citation34 Another study, by Saeki et al found that GSDMB expression is also driven by two promoters, including a cellular promoter and LTR-derived promoter.Citation55 Additionally, the LTR promoter drives GSDMB expression in gastric cancer specimens. These two studies concluded that GSDMB expression levels and Alu versus LTR-derived promoter usage may be useful markers for the evaluation of gastric cancer development and progression. In addition, Saeki et al also identified a region downstream of the LTR promoter that showed transcriptional activity in gastric cancer cell lines.Citation55 They used this region to construct a herpes simplex virus thymidine kinase-expression viral vector, which could be used as a therapeutic vector for expression in cancer cells. Accordingly, the GSDMB-driven herpes simplex virus thymidine kinase expression vector can alter a targeted regulatory region through cancer cell-specific expression of cytotoxic genes, which could be used to treat gastric cancer with peritoneal dissemination, a condition that has a critical impact on patient survival.Citation11,Citation34,Citation54-56
GSDMB in Cervical Squamous Cell Carcinomas
Cervical cancer is both the third most common form of cancer and a major cause of mortality among women worldwide. Cervical cancer is caused by persistent infection with an oncogenic strain of high-risk human papillomavirus.Citation57,Citation58 Tumor stages, grade of differentiation, smoking status, use of oral contraceptives, and postmenopausal age may each increase cervical carcinogenesis risk. In squamous cell carcinomas, Lutkowska et al showed that polymorphisms within two non-major histocompatibility loci are associated with invasive Cervical cancer.Citation59 These polymorphisms include the single nucleotide polymorphism NC_000017.10: g.38051348A>G (rs8067378), which is located 9.5 kb downstream of GSDMB. This corresponds to the cellular promoter and the Long Terminal region, which can drive the expression of GSDMB. The same researchers also assessed the effect of rs8067378 genotype on GSDMB levels in both non-cancerous and squamous cell carcinomas tissues, finding a clear increase in the GSDMB levels observed in squamous cell carcinoma tissues. Accordingly, rs8067378 single nucleotide polymorphism variants appear to increase GSDMB expression, as associated with the development and progression of cervical squamous cell carcinoma tissues. As such, GSDMB expression could be a risk factor of cervical squamous cell carcinoma tissues, in which it may promote cancer cell growth and accelerate metastasis beyond that of lower-grade cancerous cells. Thus, there is a potential link between GSDMB protein expression and the development and progression of uterine cervical cancer.Citation59,Citation60
In conclusion, researchers found that GSDMB is highly expressed in human cancer tissues, including gastric, hepatic, colon, uterine, esophagus, stomach and cervical cancers, and other cancer-derived cell lines. But as shown in , there are some similarities and differences among these cancers. GSDMB expression is linked to poor prognosis, poor therapeutic responses in terms of relapse-free survival and the development of distant metastasis in cancer, and GSDMB also has been used as a novel marker. Additionally, GSDMB could be a potential therapeutic target involved in the treatment of cancer. At present the researches of GSDMB in cancer is still not clear, there is abundant room for future research to investigate therapeutic targets for cancer treatment.
Table 2 GSDMB in Different Cancers
GSDMB and Other Disease
Previous studies of GSDMB also revealed a correlation between GSDMB and an increased susceptibility to asthma, acute lymphoblastic leukemia, inflammatory bowel disease (IBD) and fat. Studies of asthma showed that susceptibility to asthma is influenced by genes and environment, and chromosome 17q12-21 remains the most highly replicated and significant locus. People also proved that GSDMB is highly expressed in the bronchial epithelium of asthmatic human lungs and is reported as asthma susceptibility loci.Citation61,Citation62 Panganiban et al found a functional asthma variant in the GSDMB gene of the 17q21 locus.Citation46 Moffatt et al also demonstrated that variants at the ORMDL3/GSDMB locus are associated with childhood-onset disease.Citation63 Besides, studies of Kim et al indicated that genetic variations of GSDMB might be associated with the development of aspirin-exacerbated respiratory disease (AERD) and aspirin-induced bronchospasm.Citation40 In addition, Wiemels et al found two new genetic associations (at chromosomal locations 17q12 and 8q24.21) that impacted childhood acute lymphoblastic leukemia risk and could help us to clarify the etiology of this disease. They observed that the acute lymphoblastic leukemia association peak on chromosome 17q12 covers approximately 200 kb and includes IKZF3, single nucleotide polymorphism, and GSDMB is also present within 17q12. Thus, Wiemels et al found that GSDMB might impact gene expression locally and affect the risk of acute lymphoblastic leukemia.Citation64 Moreover, researchers found that multiple genes are implicated in altering IBD predisposition and there are potentially damaging mutations in the GSDMB gene in patients. Furthermore, people also proved that GSDMB affects the susceptibility to IBD via effects on apoptosis and cell proliferation.Citation65,Citation66 A study of the genetic determinants of body fat distribution showed that there are seven loci associated with ectopic-fat which included GSDMB, revealing the physiological roles for these genes in adipogenesis.Citation67
In short, people found that GSDMB plays an important role not only in cancers but also in other diseases. However, up to now there are a few studies discussing the specific mechanism of GSDMB in diseases and further research should be undertaken to investigate more GSDMB-associated diseases and whether GSDMB-mediated pyroptosis participates in them.
Summary and Prospects
This study set out to provide insights into the possible progress of how GSDMB participates in pyroptosis and the potential functions of GSDMB in cancer. GSDMB belongs to the GSDM family and may be able to uniquely bind liposomes containing sulfatide and to induce pyroptosis-like features. To date, researchers have posited three hypotheses about the pyroptosis pathway, including that GSDMB protein could be cleaved by caspase-1 to induce pyroptosis. In contrast, other researchers have found that GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. In addition, GSDMB protein could be cleaved by caspase-3/-6/-7 but whether it is possible to induce pyroptosis is not sure. Thus, further research should be undertaken to investigate whether GSDMB can induce cell pyroptosis and how GSDMB participates in the inflammatory regulation mechanistically. In addition, some studies have shown that GSDMB overexpression has been detected in several kinds of cancer cells. GSDMB has been considered an oncogene that appears to be involved in cancer progression and metastasis; there is a seeming contradiction here, as GSDMB participates in pyroptosis, but the role of GSDMB in programmed cell death suggests its dysfunction is itself oncogenic. In general, from our point of view, GSDMB is a downstream effector protein in the pathway of pyroptosis and the activation of pyroptosis depends on whether upstream pathway in this pathway is activated. We believe that once some stimulating factors activated caspases or other upstream regulation, GSDMB might be activated and induce pyroptosis. Therefore, GSDMB might be regarded as a potential target for cancer therapy and the trigger mechanism is the key to pyroptosis of GSDMB which may direct future research on therapeutic targets for cancer treatment. Overall, there is abundant room for further progress in determining the trigger mechanism of pyroptosis of GSDMB and how GSDMB participates in pyroptosis mechanistically and the role of GSDMB in cancer.
Acknowledgments
We are grateful to the teachers of the central cancer laboratory of Harbin Medical University for their kind help.
Disclosure
The authors report no conflicts of interest in this work.
References
- Saika K, Sobue T. [Cancer statistics in the world]. Gan To Kagaku Ryoho. 2013;40(13):2475–2480.24335357
- Feng R-M, Zong Y-N, Cao S-M, Xu R-H. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019;39(1). doi:10.1186/s40880-019-0368-6
- Cai Z, Liu Q. Understanding the Global Cancer Statistics 2018: implications for cancer control. Sci China Life Sci. 2019. doi:10.1007/s11427-019-9816-1
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi:10.1002/ijc.3193730350310
- Wu C, Li M, Meng H, et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci. 2019;62(5):640–647. doi:10.1007/s11427-018-9461-530900169
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.2155130620402
- Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection. Nat Rev Immunol. 2017;17(3):151–164. doi:10.1038/nri.2016.14728138137
- Xu YJ, Zheng L, Hu YW, Wang Q. Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta. 2018;476:28–37. doi:10.1016/j.cca.2017.11.00529129476
- Chaiteerakij R, Juran BD, Aboelsoud MM, et al. Association between variants in inflammation and cancer-associated genes and risk and survival of cholangiocarcinoma. Cancer Med. 2015;4(10):1599–1602. doi:10.1002/cam4.50126276523
- Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113–114. doi:10.1016/S0966-842X(00)01936-311303500
- Suzuki T, Franchi L, Toma C, et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3(8):e111. doi:10.1371/journal.ppat.003011117696608
- Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167–169. doi:10.1038/358167a01614548
- Fink SL, Cookson BT. Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol. 2007;9(11):2562–2570. doi:10.1111/j.1462-5822.2007.01036.x17714514
- Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202(8):1043–1049. doi:10.1084/jem.2005097716230474
- Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi:10.1038/nature1368325119034
- Kang SJ, Wang S, Hara H, et al. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J Cell Biol. 2000;149(3):613–622. doi:10.1083/jcb.149.3.61310791975
- Kayagaki N, Warming S, Lamkanfi M, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–121. doi:10.1038/nature1055822002608
- Pizato N, Luzete BC, Kiffer L, et al. Omega-3 docosahexaenoic acid induces pyroptosis cell death in triple-negative breast cancer cells. Sci Rep. 2018;8(1):1952. doi:10.1038/s41598-018-20422-029386662
- de Gassart A, Martinon F. Pyroptosis: caspase-11 unlocks the gates of death. Immunity. 2015;43(5):835–837. doi:10.1016/j.immuni.2015.10.02426588774
- Bollino D, Colunga A, Li B, Aurelian L. ΔPK oncolytic activity includes modulation of the tumour cell milieu. J Gen Virol. 2016;97(2):496–508. doi:10.1099/jgv.0.00035326602205
- de Vasconcelos NM, Van Opdenbosch N, Van Gorp H, Parthoens E, Lamkanfi M. Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ. 2018;26(1):146–161. doi:10.1038/s41418-018-0106-729666477
- Vande Walle L, Lamkanfi M. Pyroptosis. Curr Biol. 2016;26(13):R568–R572. doi:10.1016/j.cub.2016.02.01927404251
- Burgener SS, Leborgne NGF, Snipas SJ, Salvesen GS, Bird PI, Benarafa C. Cathepsin G inhibition by serpinb1 and serpinb6 prevents programmed necrosis in neutrophils and monocytes and reduces GSDMD-driven inflammation. Cell Rep. 2019;27(12):3646–3656 e3645. doi:10.1016/j.celrep.2019.05.06531216481
- Wang CJ, Tang L, Shen DW, et al. The expression and regulation of DFNA5 in human hepatocellular carcinoma DFNA5 in hepatocellular carcinoma. Mol Biol Rep. 2013;40(12):6525–6531. doi:10.1007/s11033-013-2581-824154762
- Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi:10.1038/nature2239328459430
- Tamura M, Tanaka S, Fujii T, et al. Members of a novel gene family, GSDM, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics. 2007;89(5):618–629. doi:10.1016/j.ygeno.2007.01.00317350798
- Wei Q, Zhu R, Zhu J, Zhao R, Li M. E2-induced activation of the NLRP3 inflammasome triggers pyroptosis and inhibits autophagy in HCC cells. Oncol Res. 2019;27(7):827–834. doi:10.3727/096504018X1546292075301230940293
- Das S, Miller M, Broide DH. Chromosome 17q21 genes ORMDL3 and GSDMB in asthma and immune diseases. Adv Immunol. 2017;135:1–52.28826527
- Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–254. doi:10.1016/j.tibs.2016.10.00427932073
- Chao KL, Kulakova L, Herzberg O. Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc Natl Acad Sci U S A. 2017;114(7):E1128–E1137. doi:10.1073/pnas.161678311428154144
- Sarhan J, Liu BC, Muendlein HI, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115(46):E10888–E10897. doi:10.1073/pnas.180954811530381458
- Wang F, Liu W, Ning J, et al. Simvastatin suppresses proliferation and migration in non-small cell lung cancer via pyroptosis. Int J Biol Sci. 2018;14(4):406–417. doi:10.7150/ijbs.2354229725262
- Hergueta-Redondo M, Sarrio D, Molina-Crespo A, et al. Gasdermin B expression predicts poor clinical outcome in HER2-positive breast cancer. Oncotarget. 2016;7(35):56295–56308. doi:10.18632/oncotarget.1078727462779
- Komiyama H, Aoki A, Tanaka S, et al. Alu-derived cis-element regulates tumorigenesis-dependent gastric expression of Gasdermin B (GSDMB). Genes Genet Syst. 2010;85(1):75–83. doi:10.1266/ggs.85.7520410667
- Feng S, Fox D, Man SM. Mechanisms of gasdermin family members in inflammasome signaling and cell death. J Mol Biol. 2018;430(18Pt B):3068–3080. doi:10.1016/j.jmb.2018.07.00229990470
- Huh JW, Kim DS, Kang DW, et al. Transcriptional regulation of GSDML gene by antisense-oriented HERV-H LTR element. Arch Virol. 2008;153(6):1201–1205. doi:10.1007/s00705-008-0105-y18478180
- Sin HS, Huh JW, Kim DS, et al. Transcriptional control of the HERV-H LTR element of the GSDML gene in human tissues and cancer cells. Arch Virol. 2006;151(10):1985–1994. doi:10.1007/s00705-006-0764-516625320
- Ruan J. Structural insight of gasdermin family driving pyroptotic cell death. Adv Exp Med Biol. 2019;1172:189–205.31628657
- Ding J, Wang K, Liu W, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111–116. doi:10.1038/nature1859027281216
- Kim LH, Chang H, Namgoong S, et al. Genetic variants of the gasdermin B gene associated with the development of aspirin-exacerbated respiratory diseases. Allergy Asthma Proc. 2017;38(1):4–12. doi:10.2500/aap.2017.38.401428052796
- Suchanski J, Grzegrzolka J, Owczarek T, et al. Sulfatide decreases the resistance to stress-induced apoptosis and increases P-selectin-mediated adhesion: a two-edged sword in breast cancer progression. Breast Cancer Res. 2018;20(1):133. doi:10.1186/s13058-018-1058-z30400820
- Merten M, Beythien C, Gutensohn K, Kuhnl P, Meinertz T, Thiagarajan P. Sulfatides activate platelets through P-selectin and enhance platelet and platelet-leukocyte aggregation. Arterioscler Thromb Vasc Biol. 2005;25(1):258–263. doi:10.1161/01.ATV.0000149675.83552.8315528476
- Garcia J, Callewaert N, Borsig L. P-selectin mediates metastatic progression through binding to sulfatides on tumor cells. Glycobiology. 2007;17(2):185–196. doi:10.1093/glycob/cwl05917043066
- Suchanski J, Ugorski M. [The biological role of sulfatides]. Postep Hig Med Dosw. 2016;70:489–504. Polish. doi:10.5604/17322693.1201720
- Liu Z, Wang C, Rathkey JK, et al. Structures of the gasdermin D C-terminal domains reveal mechanisms of autoinhibition. Structure. 2018;26(5):778–784 e773. doi:10.1016/j.str.2018.03.00229576317
- Panganiban RA, Sun M, Dahlin A, et al. A functional splice variant associated with decreased asthma risk abolishes the ability of gasdermin B to induce epithelial cell pyroptosis. J Allergy Clin Immunol. 2018;142(5):1469–1478 e1462. doi:10.1016/j.jaci.2017.11.04029330013
- Shi P, Tang A, Xian L, et al. Loss of conserved GSDMA3 self-regulation causes autophagy and cell death. Biochem J. 2015;468(2):325–336. doi:10.1042/BJ2015020425825937
- Chen Q, Shi P, Wang Y, et al. GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J Mol Cell Biol. 2019;11(6):496–508. doi:10.1093/jmcb/mjy05630321352
- Chen Q, Shi P, Wang Y, et al. GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J Mol Cell Biol. 2018.
- Molina-Crespo A, Cadete A, Sarrio D, Gamez-Chiachio M. Intracellular delivery of an antibody targeting Gasdermin-B reduces HER2 breast cancer aggressiveness. Clin Cancer Res. 2019; 25(15):4846–4858. doi:10.1158/1078-0432.CCR-18-238131064780
- Hergueta-Redondo M, Sarrio D, Molina-Crespo A, et al. Gasdermin-B promotes invasion and metastasis in breast cancer cells. PLoS One. 2014;9(3):e90099. doi:10.1371/journal.pone.009009924675552
- Edgren H, Murumagi A, Kangaspeska S, et al. Identification of fusion genes in breast cancer by paired-end RNA-sequencing. Genome Biol. 2011;12(1):R6. doi:10.1186/gb-2011-12-1-r621247443
- Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–477.19107449
- Saeki N, Usui T, Aoyagi K, et al. Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Genes Chromosomes Cancer. 2009;48(3):261–271. doi:10.1002/gcc.2063619051310
- Saeki N, Komatsuzaki R, Chiwaki F, Yanagihara K, Sasaki H. A GSDMB enhancer-driven HSV thymidine kinase-expressing vector for controlling occult peritoneal dissemination of gastric cancer cells. BMC Cancer. 2015;15:439. doi:10.1186/s12885-015-1436-126016667
- Carl-McGrath S, Schneider-Stock R, Ebert M, Rocken C. Differential expression and localisation of gasdermin-like (GSDML), a novel member of the cancer-associated GSDMDC protein family, in neoplastic and non-neoplastic gastric, hepatic, and colon tissues. Pathology. 2008;40(1):13–24. doi:10.1080/0031302070171625018038310
- Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147(4):742–758. doi:10.1016/j.cell.2011.10.03322078876
- Chao A, Huang HJ, Lai CH. Human papillomavirus research on the prevention, diagnosis, and prognosis of cervical cancer in Taiwan. Chang Gung Med J. 2012;35(4):297–308. doi:10.4103/2319-4170.10614122913856
- Lutkowska A, Roszak A, Lianeri M, Sowinska A, Sotiri E, Jagodzinski PP. Analysis of rs8067378 Polymorphism in the risk of uterine cervical cancer from a polish population and its impact on gasdermin B expression. Mol Diagn Ther. 2017;21(2):199–207. doi:10.1007/s40291-017-0256-128120299
- Sun Q, Yang J, Xing G, Sun Q, Zhang L, He F. Expression of GSDML associates with tumor progression in uterine cervix cancer. Transl Oncol. 2008;1(2):73–83. doi:10.1593/tlo.0811218633457
- Bonnelykke K, Sleiman P, Nielsen K, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46(1):51–55. doi:10.1038/ng.283024241537
- Stein MM, Thompson EE, Schoettler N, et al. A decade of research on the 17q12-21 asthma locus: piecing together the puzzle. J Allergy Clin Immunol. 2018;142(3):749–764 e743. doi:10.1016/j.jaci.2017.12.97429307657
- Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–1221. doi:10.1056/NEJMoa090631220860503
- Wiemels JL, Walsh KM, de Smith AJ, Metayer C, Gonseth S. GWAS in childhood acute lymphoblastic leukemia reveals novel genetic associations at chromosomes 17q12 and 8q24.21. Nat Commun 2018;9(1):286.29348612
- Soderman J, Berglind L, Almer S. Gene expression-genotype analysis implicates GSDMA, GSDMB, and LRRC3C as contributors to inflammatory bowel disease susceptibility. Biomed Res Int. 2015;2015:834805. doi:10.1155/2015/83480526484354
- Christodoulou K, Wiskin AE, Gibson J, et al. Next generation exome sequencing of paediatric inflammatory bowel disease patients identifies rare and novel variants in candidate genes. Gut. 2013;62(7):977–984. doi:10.1136/gutjnl-2011-30183322543157
- Chu AY, Deng X, Fisher VA, et al. Multiethnic genome-wide meta-analysis of ectopic fat depots identifies loci associated with adipocyte development and differentiation. Nat Genet. 2017;49(1):125–130. doi:10.1038/ng.373827918534