Abstract
Background
Synovial sarcoma (SS) is a highly aggressive soft-tissue sarcoma (STS) with poor prognosis. Tyrosine kinase inhibitor (TKI) has shown a promising impact on advanced STS patients. This study aimed to evaluate the efficacy and safety of apatinib, an oral multi-TKI, which especially inhibited vascular endothelial growth factor receptor, as second-line therapy for patients with advanced SS.
Patients and Methods
This retrospective analysis included 21 advanced SS patients, who had a poor response to anthracycline-based chemotherapy alone or combined with ifosfamide at least one cycle. All the patients received an apatinib containing regimen between May 2016 and October 2019 in our institution. Apatinib 500–750 mg (250 mg for patients younger than 10) was given daily. Tumor responses were assessed by response evaluation criteria in solid tumors. Survival analysis was performed by the Kaplan–Meier test, and a safety profile was recorded.
Results
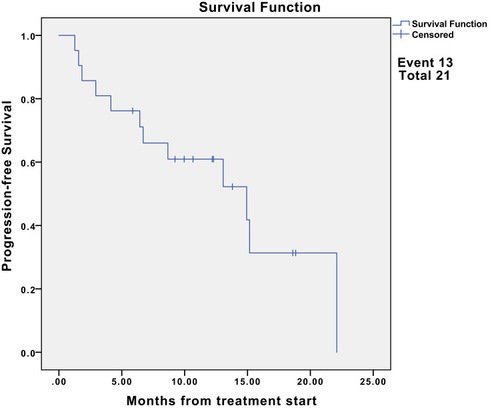
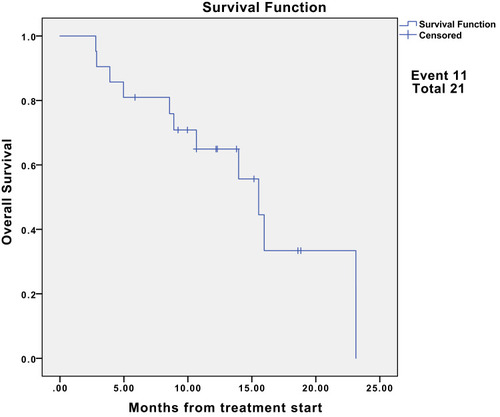
The median follow-up was 15.2 months (95% CI, 12.2-NE). Nine (42.9%) patients had partial response (PR), and eight (38.1%) had stable disease. The median progression-free survival (PFS) was 13.1 months (95% CI, 6.7-NE). The 6- and 12-month PFS rates were 76.2% (95% CI, 60.0–96.8) and 55.4% (95% CI, 37.3–82.3), respectively. Additionally, the median overall survival (OS) was 15.5 months (95% CI, 10.7-NE). The 6- and 12-month OS rates were 81.0% (95% CI, 65.8, 99.6) and 64.9% (95% CI, 46.9–90.0), respectively. Moreover, the objective response rate was 42.9% (9/21) for advanced SS patients. The disease control rate was 81.0% (17/21). For the nine patients with the best response of PR, the median duration of response was 7.7 months.
Conclusion
Apatinib was proved to be a potential second-line treatment option for advanced SS patients with chemo-resistance. Apatinib showed promising efficacy and acceptable safety profile in advanced SS, with considerable OS and particularly PFS. Indeed, further multicenter studies with a longer follow-up time are needed to fully determine the clinical application of apatinib in advanced SS.
Introduction
Synovial sarcoma (SS) is an aggressive malignancy of mesenchymal origin, accounting for approximately 8% to 10% of all soft-tissue sarcomas (STS).Citation1–Citation3 SS is marked by the presence of a pathognomonic translocation between chromosomes X and 18, t(X;18)(p11.2;q11.2),Citation4 and its histological characteristics can be identified including monophasic, biphasic, and poorly differentiated SS.Citation4 Although SS frequently arises in the extremities, its histological feature is not related to synovial tissue.Citation5 As the common non-rhabdomyosarcoma STS in children and young adults, the peak incidence of SS is adolescents and adults younger than 30 yearsCitation5,Citation7 Notably, SS has a high metastatic potential (approximately 24% of the patients at diagnosis) and a high locally recurrent rate (approximately 17% of patients).Citation8,Citation9
In general, SS is a high-grade sarcoma with a poor prognosis.Citation10 For local SS, the standard treatment was wide resection followed by radiation therapy in patients with high-risk disease (ie, deeply located tumor site, and tumor size>5 cm).Citation11 For advanced SS, anthracycline-based chemotherapy alone or combined with ifosfamide was regarded as the conventional frontline strategy.Citation12,Citation13 However, controversy has still existed related to the most effective therapy regimen.Citation2 Once disease progression or chemo-resistance happened, no appropriate systemic agent is targeting the histologic or genomic characteristics of SS. Therefore, recently, several clinical trials on different approved palliative options have been reported, such as trabectedin,Citation14 pazopanib,Citation15 and regorafenib.Citation3
Angiogenesis and multikinase inhibitors were recognized as active agents for advanced non-adipocytic sarcomas.Citation15,Citation16 Although pazopanib and regorafenib were reported to significantly improve progression-free survival (PFS) compared with placebo in advanced SS patients, these treatment strategies did not improve the overall survival (OS).Citation3,Citation16,Citation17 Therefore, alternative options are needed. Apatinib is an oral anti-angiogenesis tyrosine kinase inhibitor (TKI) highly and selectively inhibitor on vascular endothelial growth factor receptor (VEGFR)-2.Citation18 Promising efficacy of apatinib was reported in advanced gastric cancerCitation19 and metastatic STS.Citation20
No prior case series have described the efficacy and safety of apatinib in advanced SS. We further retrospectively studied the clinical outcomes of apatinib in 21 patients with advanced SS who have been previously treated at the Department of Orthopaedics of the West China Hospital.
Patients and Methods
Patients Eligibility
We examined advanced SS patients who were treated between May 2016 and October 2019 in our institution. The inclusion criteria were as follows: 1) histologically confirmed diagnosis of advanced SS according to the World Health Organization Classification of Tumours; 2) availability of complete data at the time of treatment including at least three measurable lesions according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; 3) intolerance or failure to anthracycline-based chemotherapy alone or combine with ifosfamide at least one cycle. This study was performed according to the principles embodied in the Declaration of Helsinki and the Institutional Review Board of Sichuan University West China Hospital. Written informed consent was obtained from all patients when they began treatment for apatinib. The study protocol followed all appropriate guidelines according to the Declaration of Helsinki.
Treatment Methods
Apatinib was orally administered at a dose of 750 mg for adults and 250 mg for children (less than 10 years of age) once a day for a 28-day cycle. In each cycle, one dose reduction (500–250 mg) was allowed for drug-related grade 3/4 adverse events (AEs). Treatment continued until disease progression or occurrence of unacceptable toxicities (grade 3/4 AEs) or patient refuel. Post-protocol treatment was discreetly managed by the patients and their physicians. The tumor response evaluation was based on computed tomographic or magnetic resonance imaging findings was performed every 2 months. Responses were assessed according to the RECIST 1.1.Citation21
Statistical Analysis
Standard descriptive and analytic methods were used to describe the patient population and their baseline characteristics. Overall survival (OS) was defined as the time from the initiation of apatinib treatment to the date of death or last follow-up. Progression-free-survival (PFS) was defined as the time from initiation of the apatinib treatment to the date of documented disease progression or death from any cause. And OS and PFS were calculated using the Kaplan–Meier product-limit method. Disease control rate (DCR) was defined as the percentage of patients achieving a complete response (CR), partial response (PR) and stable disease (SD); objective response rate (ORR) was defined as the percentage of patients showing CR and PR. Duration of response (DOR) was defined as the length of time that the tumor continues to respond to treatment without progressive disease (PD). In addition, the 95% confidence interval (CI)s of the DCR, ORR, and DOR were calculated using an asymptotic normal approximation. A two-tailed p < 0.05 was considered statistically significant. The statistical analysis was performed using SPSS version 23 software (SPSS Inc., Chicago, IL, USA).
Results
Patient Characteristics
Overall, 21 patients with metastatic or recurrent SS were treated with apatinib as at least a second-line chemotherapy. The baseline characteristic of the patients is summarized (). 47.6% (10) of the patients were male, and the median age was 31.5 ±14.0 years (range 8–71). The most common primary tumor sites were extremities (81.0%). All patients had undergone at least one first-line chemotherapy including: anthracycline-based agent alone (n=11, 52.4%) and anthracycline combined with ifosfamide (n=10, 47.6%). After first-line chemotherapy, 16 patients had distant metastasis, and 5 patients got local recurrence.
Table 1 Patient Characteristics
Efficacy
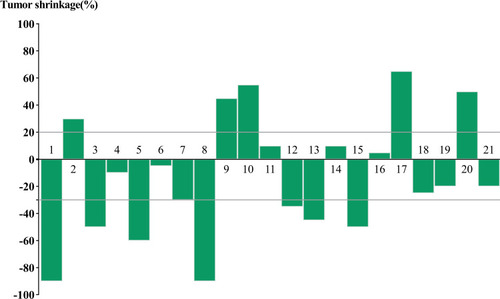
Among the 21 evaluable cases (), none of the patients were lost to follow-up and four patients died of lung metastasis. The median follow-up was 15.2 months (95% CI, 12.2-NE). Nine (42.9%) of 21 patients had PR, and eight (38.1%) had SD. We did notice dramatic tumor shrinkage (). The median PFS was 13.1 months (95% CI, 6.7-NE) (). The 6- and 12-month PFS rates were 76.2% (95% CI, 60.0–96.8) and 55.4% (95% CI, 37.3–82.3), respectively. Additionally, the median OS was 15.5 months (95% CI, 10.7-NE) (). The 6- and 12-month OS rates were 81.0% (95% CI, 65.8, 99.6) and 64.9% (95% CI, 46.9–90.0), respectively. Moreover, the ORR was 42.9% (9/21) for advanced SS patients. The DCR was 81.0% (17/21). For the nine patients with the best response of PR, the median DOR was 7.7 months.
Table 2 Clinical Characteristics of 21 Patients with Advanced SS Treated with Apatinib
Safety and Toxicity
The common grade ≥1 non-hematological toxicities were hand-foot skin reaction (n = 7, 33.3%) and oral ulcers (n = 7, 33.3%) and vomiting (n = 6, 28.6%). The only grade 4 reaction was pneumothorax treated by dose reduction. And one patient discontinued apatinib because of hand-foot skin reaction. Most adverse reactions were mild (grades 1/2) and easily controlled (). And all the AEs were causally related to apatinib. No deaths were related to the experimental treatment.
Table 3 Adverse Events
Discussion
Although SS is a relatively chemo-sensitive STS compared with adipocytic STS,Citation12 the efficacy of neoadjuvant chemotherapy in advanced SS remains controversial.Citation22 Various targeted regiments are used as second-line treatments after the failure of anthracycline-based chemotherapy, such as sorafenib,Citation23 pazopanib,Citation15,Citation16,Citation24-27 regorafenibCitation3,Citation17,Citation28,Citation29 and apatinibCitation20,Citation30 ().
Table 4 Targeted Agents for Advanced SS
Among them, multitargeted TKIs can be effective in patients with metastatic or recurrent SS, which have different affinities against VEGFR −1/2/3, platelet-derived growth factor -α/β, fibroblast growth factor receptor (FGFR) −1/3, stem cell factor (c-Kit) receptor, and implicated in oncogenesis (TIE2, RET, RAF).Citation15,Citation32 In previous studies, pazopanib and regorafenib had demonstrated acceptable antitumor activity with longer median PFS in metastatic or recurrent SS patients, compared with that of the patients treated by placebo. However, pazopanib and regorafenib did not improve the median OS in the trials.Citation15,Citation17 And, these drugs were not accessible to the Chinese investigators before January 2019.
Angiogenesis plays a crucial role in tumor growth, invasion, and development of metastasis.Citation33 Apatinib is an orally small-molecule VEGFR‑TKI, which specifically and strongly inhibits VEGFR-2, decreasing VEGF-mediated endothelial cell migration, proliferation, and tumor microvascular formation.Citation19 In preclinical experiments, apatinib also impaired VEGF-stimulated proliferation, migration and tube formation by human umbilical vein endothelial cells, and blocked rat aortic ring budding in vitro, which may be associated with suppression of VEGFR-2-mediated phosphorylation of Ret, c-Kit, and c-src.Citation34 And apatinib has shown its anti-cancer effects in a variety type of tumors,Citation19 including STS.Citation20,Citation30,Citation31
There were few studies focus on the efficacy of apatinib in advanced SS. Xie et alCitation30 reported as a second-line or further-line option, apatinib was administrated for six patients with advanced SS following anthracycline-based chemo-resistance. The median DOR was 5.2 months, and the best response was PR. Chen et alCitation31 reported a case of advanced SS. Apatinib was administrated as a third-line treatment. The PFS and OS were 7.0 and 8.5 months, respectively. And no grade 3 or 4 side effects were observed. However, compared with previous studies, our data show a significant improvement in PFS (13.1 months) and OS (15.5 months), with similar DOR (7.7 months). And, apatinib shows relatively less and lower-grade AEs, when compared to pazopanib and regorafenib.Citation15,Citation17,Citation30 Therefore, in our study, most patients received apatinib 750 mg once daily for body surface area (BSA) >1.5.Citation30 The initial dose was relatively high, compared with the previous study. Additionally, although chemotherapy agents primarily act in the cell cycle, SS tumor cells are selected for insensitive chemotherapy, which contributes to the formation of chemotherapy-resistant SS. In the previous study, six advanced SS cases only had a median DOR of 5.2 months with apatinib treatment following poor response to at least two circles of anthracycline-based chemotherapy.Citation30 However, van der Graaf et alCitation15 reported pazopanib improved the median PFS (5.6 months) and OS (13.4 months) in advanced SS patients who previously had one circle chemotherapy. So apatinib was administered after the poor response of one circle chemotherapy in our study, with the notable result of nine PR and eight SD (). Therefore, we speculate the improvement of median OS, especially PFS, is extensively influenced by the relatively high dose and advanced treatment, when compared to early trials.Citation15,Citation17,Citation30
For the AEs, previous studies reported the most common toxicities were hand-foot skin reaction, fatigue, rash or desquamation, and hypertension for advanced STS.Citation20,Citation30 In our cases, the most common AEs in our study were hand-foot skin reaction (7/21[33.3%]), oral ulcers (7/21[33.3%]), and vomiting (6/21[28.6%]). Then, only one patient had to discontinue apatinib because of hand-foot skin reaction, and one patient received closed chest drainage following grade 4 pneumothorax. No other grade 3/4 toxicities were detected. However, as mainstream care for patients with non-adipocytic sarcomas, the use of pazopanib had induced several grade ≥3 toxicities including neutropenia, thrombocytopenia, pulmonary embolism, and nausea.Citation26,Citation27 Rapid and fatal acute heart failure had been even reported which induced by pazopanib.Citation35 The quality of life was significantly decreased in SS patients who administrated pazopanib because of AEs (ie, diarrhea, anorexia, nausea, vomiting, and asthenia).Citation15 Meanwhile, three of all the 14 patients discontinued regorafenib for the regorafenib-induced toxicities.Citation17 Hypertension, hand-foot skin reaction, and asthenia were the most common grade ≥3 toxicities for STS patients with regorafenib.Citation17 However, the overall toxicity and safety of apatinib were relatively better in advanced SS patients, when compared to pazopanib and regorafenib.
The study has several limitations. Firstly, it was a retrospective review of one institution, and there was no control for comparison. Secondly, we only enrolled 21 patients with a median follow-up time of 15.2 months. Moreover, regardless of the encouraging results, our study is lacking the relevant research on the mechanism of molecular biology in each patient. When making histological diagnose, our institution just concluded the typical immunohistochemical features of SS including: CD99 (+), vimentin (+), WT-1 (+), CK (-), HBME1 (-), TTF1 (-), Napsin A (-), p16 (-), NSE (-), Chromogranin A (-), Bcl-2 (+). However, our results are highly variable, whether the immunohistochemical features will impact on the clinical outcomes is unknown. Moreover, apatinib is known as a VEGFR‑TKI, but the definite mechanism of antitumor activity remains a question. Therefore, prospective studies with multicenter and large sample are needed for further evaluating the efficacy and safety of apatinib.
Conclusions
In our study, the advanced SS patients with poor response to anthracycline-based chemotherapy revealed favorable short-term outcomes after using apatinib. Although the evidence level of this study seems preliminary, patients treated with apatinib indeed had a significant improvement on OS, and particularly on PFS. Meanwhile, the apatinib-induced toxicities were tolerable for most advanced SS patients. Apatinib was proved to be a potential second-line treatment option for advanced SS patients with chemo-resistance. Certainly, further long-term randomized controlled multicenter study with larger cases will fully determine the clinical application of apatinib in advanced SS.
Acknowledgments
We are thankful for the support of the nurse team from the Department of Orthopedics, West China Hospital. We are thankful for the patients enrolled in this study. The abstract of this paper was presented at the 40th SICOT Orthopaedic World Congress as an oral presentation talk with interim findings.
Disclosure
The authors report no conflicts of interest in this work.
References
- Baldi GG, Orbach D, Bertulli R, et al. Standard treatment and emerging drugs for managing synovial sarcoma: adult’s and pediatric oncologist perspective. Expert Opin Emerg Drugs. 2019;24(1):43–53. doi:10.1080/14728214.2019.159136730841761
- Riedel RF, Jones RL, Italiano A, et al. Systemic anti-cancer therapy in synovial sarcoma: a systematic review. Cancers. 2018;10:11. doi:10.3390/cancers10110417
- Berry V, Basson L, Bogart E, et al. REGOSARC: regorafenib versus placebo in doxorubicin-refractory soft-tissue sarcoma-A quality-adjusted time without symptoms of progression or toxicity analysis. Cancer. 2017;123(12):2294–2302. doi:10.1002/cncr.3066128295221
- Ladanyi M. Fusions of the SYT and SSX genes in synovial sarcoma. Oncogene. 2001;20(40):5755–5762. doi:10.1038/sj.onc.120460111607825
- Wang S, Song R, Sun T, et al. Survival changes in patients with Synovial Sarcoma, 19832012. J Cancer. 2017;8(10):1759–1768. doi:10.7150/jca.1734928819372
- Palmerini E, Staals EL, Alberghini M, et al. Synovial sarcoma: retrospective analysis of 250 patients treated at a single institution. Cancer. 2009;115(13):2988–2998. doi:10.1002/cncr.2437019452538
- Ferrari A, De Salvo GL, Brennan B, et al. Synovial sarcoma in children and adolescents: the European pediatric soft tissue sarcoma study group prospective trial (EpSSG NRSTS 2005). Ann Oncol. 2015;26(3):567–572. doi:10.1093/annonc/mdu56225488687
- Stacchiotti S, Van Tine BA. Synovial Sarcoma: current concepts and future perspectives. J Clin Oncol. 2018;36(2):180–187. doi:10.1200/JCO.2017.75.194129220290
- Bianchi G, Sambri A, Righi A, Dei Tos AP, Picci P, Donati D. Histology and grading are important prognostic factors in synovial sarcoma. Eur J Surg Oncol. 2017;43(9):1733–1739. doi:10.1016/j.ejso.2017.05.02028579008
- Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627–634. doi:10.1002/cncr.2038615274077
- von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, Version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(5):536–563. doi:10.6004/jnccn.2018.002529752328
- Sleijfer S, Ouali M, van Glabbeke M, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur J Cancer. 2010;46(1):72–83. doi:10.1016/j.ejca.2009.09.02219853437
- Tap WD, Papai Z, Van Tine BA, et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): an international, multicentre, open-label, randomised Phase 3 trial. Lancet Oncol. 2017;18(8):1089–1103. doi:10.1016/S1470-2045(17)30381-928651927
- Kawai A, Araki N, Sugiura H, et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: a randomised, open-label, Phase 2 study. Lancet Oncol. 2015;16(4):406–416. doi:10.1016/S1470-2045(15)70098-725795406
- van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–1886. doi:10.1016/S0140-6736(12)60651-522595799
- Yoo KH, Kim HS, Lee SJ, et al. Efficacy of pazopanib monotherapy in patients who had been heavily pretreated for metastatic soft tissue sarcoma: a retrospective case series. BMC Cancer. 2015;15:154. doi:10.1186/s12885-015-1160-x25885855
- Mir O, Brodowicz T, Italiano A, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17(12):1732–1742. doi:10.1016/S1470-2045(16)30507-127751846
- Mi YJ, Liang YJ, Huang HB, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70(20):7981–7991. doi:10.1158/0008-5472.CAN-10-011120876799
- Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase iii trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34(13):1448–1454. doi:10.1200/JCO.2015.63.599526884585
- Zhu B, Li J, Xie Q, Diao L, Gai L, Yang W. Efficacy and safety of apatinib monotherapy in advanced bone and soft tissue sarcoma: an observational study. Cancer Biol Ther. 2018;19(3):198–204. doi:10.1080/15384047.2017.141627529261005
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.02619097774
- von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, Version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(6):758–786. doi:10.6004/jnccn.2016.007827283169
- Maki RG, D’Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27(19):3133–3140. doi:10.1200/JCO.2008.20.449519451436
- Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol. 2009;27(19):3126–3132. doi:10.1200/JCO.2008.21.322319451427
- Nakamura T, Matsumine A, Kawai A, et al. The clinical outcome of pazopanib treatment in Japanese patients with relapsed soft tissue sarcoma: A Japanese Musculoskeletal Oncology Group (JMOG) study. Cancer. 2016;122(9):1408–1416. doi:10.1002/cncr.2996126970174
- Gelderblom H, Judson IR, Benson C, et al. Treatment patterns and clinical outcomes with pazopanib in patients with advanced soft tissue sarcomas in a compassionate use setting: results of the SPIRE study(). Acta Oncol. 2017;56(12):1769–1775. doi:10.1080/0284186X.2017.133277928723233
- Kim JH, Park HS, Heo SJ, et al. Differences in the efficacies of pazopanib and gemcitabine/docetaxel as second-line treatments for metastatic soft tissue Sarcoma. Oncology. 2019;96(2):59–69. doi:10.1159/00049259730336470
- Brodowicz T, Liegl-Atzwager B, Tresch E, et al. Study protocol of REGOSARC trial: activity and safety of regorafenib in advanced soft tissue sarcoma: a multinational, randomized, placebo-controlled, phase II trial. BMC Cancer. 2015;15:127. doi:10.1186/s12885-015-1143-y25884155
- Brodowicz T, Mir O, Wallet J, et al. Efficacy and safety of regorafenib compared to placebo and to post-cross-over regorafenib in advanced non-adipocytic soft tissue sarcoma. Eur J Cancer. 2018;99:28–36. doi:10.1016/j.ejca.2018.05.00829902612
- Xie L, Guo W, Wang Y, Yan T, Ji T, Xu J. Apatinib for advanced sarcoma: results from multiple institutions’ off-label use in China. BMC Cancer. 2018;18(1):396. doi:10.1186/s12885-018-4303-z29625604
- Chen S, Zhang K, Wan X, Chen Y, Ma S, Deng Q. The use of apatinib in treating primary pleural synovial sarcoma: A case report. Medicine. 2019;98(51):e18382. doi:10.1097/MD.000000000001838231861000
- Agulnik M, Attia S. Growing role of regorafenib in the treatment of patients with Sarcoma. Target Oncol. 2018;13(4):417–422. doi:10.1007/s11523-018-0575-029931504
- Ranieri G, Gadaleta CD, Patruno R, et al. A model of study for human cancer: spontaneous occurring tumors in dogs. Biological features and translation for new anticancer therapies. Crit Rev Oncol Hematol. 2013;88(1):187–197. doi:10.1016/j.critrevonc.2013.03.00523561333
- Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102(7):1374–1380. doi:10.1111/j.1349-7006.2011.01939.x21443688
- van Marcke C, Ledoux B, Petit B, Seront E. Rapid and fatal acute heart failure induced by pazopanib. BMJ Case Rep. 2015;2015.