Abstract
The treatment of advanced non–small-cell lung cancer (NSCLC) has undergone a paradigm shift in the last decade. Molecular characterization of the disease has led to the rapid development of personalized medicine and swift delivery of targeted therapies to patients. The discovery of the anaplastic lymphoma kinase (ALK) gene in patients with NSCLC has resulted in rapid bench–bedside transition of several active drugs, with several others currently in clinical trials. After the first-generation ALK inhibitor crizotinib, next-generation ALK inhibitors have entered clinical applications for ALK-rearranged NSCLC. Ceritinib, alectinib, and brigatinib have all received approval for ALK-positive patients who have failed prior crizotinib, as well as first-line therapy in treatment-naïve patients based on favorable efficacy. Most recently, lorlatinib, a potent, newer-generation ALK inhibitor, has been approved as second- or third-line treatment. These advances have led to better patient outcomes, but concurrently have led to several crucial unanswered questions about optimal care for ALK-positive NSCLC patients. The ultimate acquisition of resistance to ALK-inhibitor therapy poses a challenge to ongoing research efforts, in addition to the routine management of these patients in the clinic. This review provides a summary of the clinical development of crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib and highlights current management paradigms, current and evolving clinical information, emerging clinical decision-making and sequencing of therapy in advanced, metastatic, or recurrent ALK-positive NSCLC.
Introduction
Lung cancer is the leading cause of cancer-related death in the US and worldwide, with <20% 5-year survival for newly diagnosed patients.Citation1 Lung cancers are classified into two main types: non–small cell lung cancer (NSCLC; 80%–85%) and small cell lung cancer (15%–20%).Citation2,Citation3 NSCLCs are further subdivided into three main types: adenocarcinoma (50%), squamous-cell carcinomas (30%), and large-cell carcinomas.Citation4 Increased understanding of molecular and biological aspects of cancer growth has led to the discovery of several oncogenic driver mutations, thereby dramatically changing treatment paradigms for patients with NSCLC over the past decade. Genetic alterations, such as epidermal growth factor receptor (EGFR) mutations (10%–15% in NSCLC in Europe and North America) and anaplastic lymphoma kinase (ALK) rearrangement are two examples of the targets in NSCLC that have revolutionized the concept of precision oncology.Citation4–Citation6 There can exist substantial variation in EGFR-mutation frequency when grouped by geographic region, and EGFR-mutation frequency has been reported to be 20%–76% in Asia–Pacific regions.Citation7 Rearrangement in ALK-receptor tyrosine kinase, a molecular subtype of NSCLC, occurs in 5%−7% of NSCLC patients.Citation8–Citation11 While in unselected NSCLC patients, overall frequency of ALK rearrangement is low, selection of patients based on clinicopathological features, such as no or light smoking history and adenocarcinoma histology results in higher frequencies (about 13%) of ALK-rearranged NSCLC.Citation16 There are an estimated 40,000 incident cases of ALK-positive NSCLC worldwide each year, and patient characteristics are quite dissimilar from the overall patient population with NSCLC.Citation11 ALK-positive NSCLC patients are generally younger (median age 52 years old), are never- to only light smokers, and primarily have adenocarcinoma histology.Citation12–Citation16 Clinicopathological findings of younger age, never- to light smoking history and adenocarcinoma-predominant histology in ALK-positive patients were confirmed in a large real-world retrospective analysis recently.Citation17
ALK-gene alterations were first reported in the 1990s through the cloning of translocation involving the short arm of chromosome 2 and long arm of chromosome 5— t(2;5) — discovered in a small number of anaplastic large-cell lymphomas.Citation18,Citation19 ALK translocation was next discovered in a subset of inflammatory myofibroblastic tumors,Citation20 and later in 2007 ALK-gene alternation/rearrangement was first described in patients with NSCLC.Citation20–Citation22 In NSCLC, this gene alteration was reported as a small inversion within the short arm of chromosome 2 (2p) that juxtaposed the 5′ end of the echinoderm microtuble-associated protein-like 4 (EML4) gene with the 3′ end of the ALK gene, resulting in the fusion oncogene EML4–ALK in NSCLC cells. Formation of the EML4–ALK fusion leads to activation, thereby potentiating proliferation and survival of the cancer cells.Citation11,Citation23 Diagnosis is most typically made using fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), or next-generation sequencing (NGS) of the tumor tissue.Citation24,Citation25 In the US, FISH, IHC, and NGS are approved companion diagnostic tests to identify ALK-positive NSCLC.Citation12
ALK Inhibitors
Before the discovery of the EML4–ALK fusion protein, conventional chemotherapy was used as the first line of therapy for all advanced or metastatic NSCLC. After the EML4–ALK discovery, crizotinib (first generation of ALK-directed therapy), a tyrosine kinase inhibitor (TKI) targeting ALK, ROS1, and MET was tested in a phase I trialCitation26 and became the first US FDA-approved ALK inhibitor for NSCLC. Ceritinib was the first of the second-generation ALK inhibitors tested, and was later approved after confirmation of its efficacy in both crizotinib-resistant and crizotinib-naïve patients. Soon after, two other ALK inhibitors — alectinib and brigatinib — were approved for ALK-positive patients who had failed prior crizotinib. While both are now approved in treatment-naïve patients, alectinib has become the preferred agent. Most recently, we have started learning more about the indisputable role of lorlatinib, a highly potent, next-generation ALK/ROS1 TKI. However, the benefit of ALK TKIs is limited by the emergence of drug resistance. Several mechanisms of resistance to ALK TKIs have now been discovered. In this review, we discuss each of the ALK inhibitors, mechanisms of acquired resistance of cancer cells to each of these inhibitors, their effectiveness in cases with brain metastases, and their role in optimal care of patients with advanced or metastatic ALK-rearranged NSCLC.
Crizotinib
The promising results from the aforementioned phase I studyCitation26 led to the phase III PROFILE 1007 trial, which compared crizotinib with either pemetrexed or docetaxel in the second-line setting in patients with locally advanced or metastatic ALK-rearranged NSCLC after progressing on one prior platinum-based regimen.Citation27 The primary end point of median progression-free survival (mPFS) was 7.7 months in the crizotinib group and 3.0 months in the pemetrexed or docetaxel group (for progression or death with crizotinib hazard ratio (HR) 0.49, 95% confidence interval (CI) 0.37–0.64; p<0.001). Prior to this, crizotinib had already received accelerated approval on August 26, 2011. Subsequently, the promising results from the PROFILE 1007 trial led to regular approval of crizotinib on November 20, 2013 in second line settings for ALK-rearranged lung cancer patients after progression on platinum doublet therapy.Citation28
The key phase III PROFILE 1014 trial aimed to assess the efficacy of the ALK inhibitor crizotinib compared with standard chemotherapy (pemetrexed plus platinum) as first-line treatment for advanced ALK-rearranged NSCLC.Citation29 This trial established crizotinib’s superiority over the standard first-line regimens. mPFS was significantly longer in the crizotinib arm (10.9 months) than the standard-chemotherapy arm (7 months; HR 0.45, 95% CI 0.35–0.60; p<0.001), and the overall response rate (ORR) was 74% in the crizotinib arm vs 45% in the standard-chemotherapy arm (p<0.001). Additionally, crizotinib was associated with greater reduction in lung cancer symptoms and improvement in quality of life. As a result of this study, crizotinib became the standard first-line agent in patients with ALK-positive NSCLC.Citation30 In 2014, the PARAMOUNT study demonstrated overall survival (OS) benefit with the use of maintenance pemetrexed in patients with advanced nonsquamous NSCLC.Citation31 These results were reported after the PROFILE 1014 trial had long been underway, but led to criticism of PROFILE 1014’s study design, which lacked the use of maintenance pemetrexed in the standard-chemotherapy arm. Nonetheless, this trial highlighted OS benefit in crizotinib, with median OS not reached (NR) with crizotinib (95% CI 45.8 months to NR) and 47.5 months with chemotherapy (95% CI 32.2 months to NR, HR 0.76, 95% CI 0.548–1.053; p=0.0978), probably not reaching statistical significance due to crossover allowed as part of the study design.
Mechanisms of Acquired Resistance to Crizotinib
Patients on crizotinib can develop resistance and relapse, with isolated central nervous system (CNS) progression, extracranial oligoprogression, or systemic progression, usually after a year.Citation32 The mechanisms of relapse have been categorized as ALK-dependent and ALK-independent.Citation11 Generally, ALK-dependent resistance occurs as a result of secondary mutations within the target kinase that reinduce kinase activation, despite the presence of the TKI.Citation33 The emergence of secondary mutations hinder the binding of the TKI to the target kinase, and thus unchecked kinase activation ensues.
Around a third of secondary resistance mutations are located in the ALK tyrosine-kinase domain, with the commonest being L1196M.Citation34 L1196M-mutation resistance (observed in 7% of crizotinib-resistant cases) is followed closely by the G1269A mutation (4%). The G1202R mutation, observed in 2% of cases, confers high-level resistance to crizotinib, as well as to next-generation ALK inhibitors. A second ALK-dominant mechanism of crizotinib resistance is amplification of the ALK-fusion gene, which occurs less frequently than secondary mutation acquisition.Citation33,Citation34 Non–ALK dominant mechanisms represent reactivation of bypass signaling pathways, such as EGFR, NRG1 overexpression, IGF-1R activation, or c-KIT, which prevent the tumor’s dependence upon ALK activation for continued growth and survival.Citation35 These two main mechanisms of ALK-TKI resistance to crizotinib and other next-generation ALK TKIs are highlighted in .
Table 1 Main Mechanisms of Resistance to First- and Next-Generation ALK TKIs
The PROFILE trials demonstrated that crizotinib achieves higher responses in systemic lesions in ALK-rearranged NSCLC than chemotherapy. However, it was not clear if crizotinib was effective in CNS metastasis.Citation29,Citation30,Citation36 The brain is a common sanctuary site of metastatic disease progression for patients on crizotinib, with about 70% of patients experiencing CNS progression.Citation37,Citation38 It is also known that crizotinib has a suboptimal effect on control of metastatic disease in the CNS.Citation39 Crizotinib is a known substrate of PgP, a key efflux pump in the blood–brain barrier (BBB), signifying that the BBB may preclude attainment of therapeutic levels in patients with CNS disease. Low concentrations of crizotinib in cerebrospinal fluid (CSF) compared with plasma-crizotinib concentrations have been demonstrated (lower CSF:plasma ratios, in the range of 0.06–0.26%). In animal models, the IC50 for crizotinib was determined to be 60–120 nM, which was well below the median steady-state plasma concentration of 570 nM/L achieved with the standard approved dose of 250 mg twice daily.Citation40,Citation41 In addition, anecdotal reports have highlighted poor CSF concentration of a meager 1.4 nM/L, signifying inadequate levels of crizotinib in the CNS.Citation40,Citation42 These findings explained the ineffectiveness of this drug in CNS metastatic disease.Citation42–Citation44
Ceritinib
In vitro enzymatic studies have demonstrated that ceritinib (a second-generation oral ALK inhibitor) is about 20 times as potent as crizotinib.Citation45,Citation46 It does not inhibit activity of MET kinase; however, it has inhibitory properties against other kinases, such as ROS1 and IGF-1R.Citation47 It has demonstrated activity and efficacy against ALK mutations arising after crizotinib exposure, namely L1196M, G1269A, I1171T, and S1206Y,Citation48 but failed to overcome two crizotinib-resistant ALK mutations — G1202R and F1174C (as illustrated in ).Citation46 In the phase I ASCEND-1 trial, 255 patients with locally advanced ALK-rearranged or metastatic NSCLC were enrolled. In the ALK-naïve patient population (n=83), ORR was noted to be 72% and median duration of response (DoR) 17 months. In the ALK inhibitor–pretreated patient cohort (n=163), ORR was noted to be 56% and median DoR 8.3 months. mPFS in ALK inhibitor-naïve patient population was 18.4 months and 6.9 months in patients with prior exposure to ALK inhibitors.Citation49 The phase II ASCEND-2 trial included 140 patients who had received two or more previous treatment regimens (with chemotherapy, one or more platinum doublets). The median DoR was 9.7 months and mPFS 5.7 months, similar to those reported in ASCEND-1.Citation50 The phase III ASCEND-5 trial evaluating ceritinib versus chemotherapy in patients with ALK-rearranged NSCLC (progressed on chemotherapy and crizotinib) met its primary end point of superior PFS in 231 patients with progressive disease (5.4 months for ceritinib vs 1.6 months for chemotherapy, HR 0.49,95% CI 0.36–0.67; p<0.0001).Citation51 In 2017, ASCEND-4, a global phase III trial, compared ceritinib with platinum–pemetrexed combination chemotherapy in newly diagnosed patients with metastatic ALK-rearranged NSCLC (n=376), demonstrating significant improvement in mPFS (16.6 months in the ceritinib arms vs 8.1 months in the chemotherapy arm, HR 0.55; p<0.00001).Citation52 The compelling results of ASCEND-4 led to US FDA approval of ceritinib 750 mg/day as a first-line agent in ALK-rearranged NSCLC on May 26, 2017. Later, in December 2017, the FDA-approved dose of ceritinib was changed from 750 mg/day under fasting conditions to 450 mg/day taken with food, based on the results of the ASCEND-8 study (a randomized phase I study of ceritinib 450 mg or 600 mg taken with a low-fat meal versus 750 mg in fasted state).Citation53 Ceritinib at 450 mg daily with food had similar plasma-drug concentrations and a more favorable gastrointestinal safety profile than ceritinib 750 mg daily in fasted patients, leading to the FDA decision to lower the ceritinib dose to 450 mg/day. In a recent safety and efficacy update on ASCEND-8, ceritinib at a dose of 450 mg with food showed consistent efficacy (shown in ) and less gastrointestinal toxicity.Citation54
Table 2 Major ALK-Inhibitor Clinical Trials for Second- and Next-Line Therapy in ALK-Rearranged Non–Small Cell Lung Cancer
In addition to being effective in the majority of patients who are resistant to crizotinib, ceritinib is also more efficacious than crizotinib in the treatment of brain metastasis. This was highlighted in the phase I and II trials ASCEND-1 and ASCEND-2, with ORR of 63% in patients who were ALK inhibitor–naïve (ASCEND-1)Citation49 and ORR of 45% in crizotinib-pretreated patients (ASCEND-2).Citation50 ASCEND-7 was designed specifically to study intracranial effects of ceritinib. This trial assigned patients based on prior treatment exposure. A total of 42 patients treated with an ALK inhibitor and brain radiotherapy were assigned to arm 1, 40 patients with prior ALK inhibitor only to arm 2, 12 patients with prior brain radiotherapy only to arm 3, and 44 patients not previously treated with brain radiotherapy or an ALK inhibitor to arm 4. In the recently reported results, intracranial ORRs of 39.3% (95% CI 21.5%–59.4%), 27.6% (95% CI 12.7%–47.2%), 28.6% (95% CI 3.7%–71.0%), and 51.5% (95% CI 33.5%–69.2%), respectively, for each of the four arms. This study confirmed the efficacy and safety of ceritinb in patients with active brain metastasis with or without a prior exposure to crizotinib.Citation55,Citation56 Similar to crizotinib, the efficacy of ceritinib can also be hindered by emergence of secondary resistance mutations. G1202R (found in only 2% of post-crizotinib samples) is a predominant resistance mechanism post-ceritinib, -alectinib, and -brigatinib (frequency of 21%–43%).Citation34 Additionally, F1174 mutations also confer resistance to ceritinib.Citation34 MEK reactivation is a key ALK-independent resistance mechanism post-ceritinib ().
Alectinib
Alectinib is a highly potent second-generation ALK-specific TKI and exhibits suppressive activity against RET kinase.Citation57,Citation58 However, it lacks inhibitory properties against MET kinase and has little activity against ROS1 kinase.Citation59 In Japan, a phase I/II study of alectinib (AF-001JP) in ALK inhibitor–naïve ALK-rearranged NSCLC patients enrolled patients (n=24) in the phase I portion of the trial. A dose of 300 mg twice daily was identified as apt in the phase I portion (no dose-limiting toxicities or grade 4 adverse events with the maximal dose) and thus recommended for phase II.Citation60 An ORR of 93.5% (95% CI 82.1%–98.6%) was demonstrated in the 46 patients enrolled in the phase II portion. At the 3-year follow up of this study, reported in 2017, PFS was 62% (95% CI 45%–75%), OS rate 78%, and median PFS not reached.Citation61 In the studies that followed, 600 mg twice daily was recommended for phase II based on activity, tolerability, and pharmacokinetic data.Citation62 Efficacy of alectinib 600 mg twice daily was assessed in two phase II studies conducted in an ALK-rearranged, crizotinib-resistant patient population. The first of these two pilot phase II studies (NP28673) enrolled 138 patients, and showed an ORR of 50% (95% CI 41%–59%) with mPFS of 8.9 (95% CI 5.6–11.3) months.Citation63 The second trial (the North American NP28761) showed similar results, wherein 87 ALK-rearranged, crizotinib-resistant NSCLC patients were enrolled, demonstrating ORR of 48% (95% CI 36%–60%) and mPFS of 8.1 (95% CI 6.2–12.6) months.Citation64 The findings from these two pilot studies led to accelerated FDA approval of alectinib in the US in patients with ALK-rearranged, crizotinib-resistant NSCLC on December 11, 2015. After consistent benefit in phase II studies, more promising results for alectinib came to the forefront as a frontline therapy. The phase III J-ALEX trial included Japanese patients with ALK inhibitor–naïve ALK rearrangement–positive NSCLC who were randomized to either alectinib at 300 mg twice daily or crizotinib 250 mg twice daily as first-line therapy.Citation65 This trial enrolled 207 patients, and mPFS was 34.1 months for alectinib (recent update,Citation66 95% CI 22.1–NE) and 10.2 months for crizotinib (95% CI 8.2–12; HR0.37, 95% CI 0.26–0.52; p<0.0001). The similar global phase III ALEX trial compared alectinib with crizotinib in treatment-naïve, advanced ALK-rearranged NSCLC patients.Citation67 This trial enrolled a total of 303 patients (including those with asymptomatic CNS disease) randomized to receive either alectinib 600 mg twice daily or crizotinib 250 mg twice daily. mPFS in the alectinib arm was superior to crizotinib arm (34.8 months vs 10.9 months, HR 0.50, 95% CI 0.36–0.70; p<0.001) in a recent update.Citation68 Despite alectinib dose disparity in both ALEX trials, the superiority of alectinib over crizotinib was evident. Following this, alectinib was FDA-approved for first-line treatment of ALK-rearranged NSCLC on November 6, 2017 at a recommended dose of 600 mg orally twice daily with food.
In terms of activity against brain metastases, alectinib has proven superior to both crizotinib and ceritinib. Alectinib is not a substrate of PgP (unlike crizotinib and ceritinib), an essential efflux transporter located at the BBB. Alectinib was thus mechanistically thought to be a better penetrant of the BBB. A pooled analysis of two phase II studies showed an intracranial ORR of 64% (22% complete response) observed in 50 patients with measurable CNS disease.Citation69 In the ALEX trial, CNS-disease progression was more common in the crizotinib group as compared with the patient before enrollment, thereby making it possible to measure response to treatment in patients with baseline CNS disease: 59% of patients in the alectinib arm had a CNS response duration of >12 months compared with only 36% in the crizotinib arm.Citation67 In a recent update, for those with CNS metastases, mPFS was 25.4 months for alectinib versus 7.4 months for crizotinib (HR 0.37, 95% CI 0.23–0.58).Citation70
In addition to activity against L1196M-gatekeeper mutation, alectinib is also active against other secondary mutations, such as G1269A.Citation34,Citation57 Unfortunately, similarly to crizotinib and ceritinib, eventual resistance to alectinib is unavoidable. Common mutations seen after alectinib treatment are G1202R (as seen in ceritinib), I1171T/N/S (also seen post-crizotinib in 2% and ceritinib in 4% of cases), in addition to smaller percentages of a few others such as V1180L and L1196M ().Citation34 Genotyping results of paired tissue and plasma samples has demonstrated that G1202R, I1171T/N/S, and V1180L were prevalent at comparable percentages in both plasma and tissue samples; however, L1196M prevalence was much lower in tissue (2%) samples than in plasma (22%) genotyped samples.Citation71 L1196M-mutation paucity in tissue samples was believed to be residual from prior crizotinib exposure (as L1196M is the gatekeeper mutation that confers resistance to crizotinib), which likely was overcome by subsequent alectinib use. Overall, this suggests that the proportion of patients relapsing on alectinib due to secondary resistance mutations was similar between tissue and plasma samples. Therefore, it may be reasonable to detect putative resistance mutations in plasma upon progression on alectinib.
Brigatinib
Brigatinib, another second-generation ALK inhibitor, differs from others in its wide range of inhibitory properties against tumors with resistance-associated mutations.Citation43,Citation72,Citation73 Brigatinib effectively inhibits ALK and ROS1, with higher selectivity over more than 250 kinases and also may have a role in treating osimertinib-refractory EGFR-mutant NSCLC as it inhibits C797S–T790M–activating-mutation (triple mutation)–mediated EGFR-TKI resistance in vitro and in vivo.Citation74 In a study by Zhang et al, cellular and in vivo activities of ALK TKIs were compared using engineered and cancer-derived cell lines.Citation75 This study demonstrated superior in vitro and in vivo potency of brigatinib compared with crizotinib (12-fold greater potency than crizotinib). The study also demonstrated a superior inhibitory profile against all known 17 secondary ALK mutations (including G1202R) tested in cellular assays and higher inhibitory properties compared with crizotinib, ceritinib, and alectinib. The role of this is yet to be determined in daily practice; however, there appears to be a signal in initial trials indicating favorable results.
After an earlier phase I/II trial,Citation76 the randomized phase II ALTA trial enrolled crizotinib-resistant patients (n=222, 74% were recipients of prior chemotherapy) with advanced ALK-rearranged NSCLC.Citation73 The primary end point ORR was 54% (similar to ceritinib and alectinib), but mPFS was 12.9 months (better than ceritinib and alectinib). Of note, mPFS with brigatinib when assessed by an independent review board was 15.6 months. Although cross-trial comparisons can be deceptive, brigatinib seems to have a PFS advantage over ceritinib and alectinib. This superior PFS may correspond to expanded inhibition of developed ALK resistance, but brigatinib’s efficacy after progression on alectinib and ceritinib remains to be determined. In a multicenter retrospective analysis, brigatinib demonstrated limited clinical activity in alectinib-refractory ALK-positive NSCLC.Citation77 Nonetheless, a phase II, open-label, single-arm, multicenter, international trial (NCT03535740) designed to assess the efficacy and safety of brigatinib in patients with ALK-positive NSCLC that have progressed on alectinib or ceritinib is under way.Citation78
In addition, brigatinib has notable CNS activity in spite of being a substrate for PgP. In the aforementioned phase II ALTA trial, in patients with prior exposure to crizotinib, 69% had CNS disease at baseline. The trial demonstrated ORR of 67% and median duration of CNS response of 16.6 months. In patients with any CNS disease at baseline, independent review board–assessed intracranial mPFS was 18.4 months. It received accelerated FDA approval on April 28, 2017 for ALK-rearranged NSCLC in patients who have progressed or are intolerant to crizotinib. ALTA-1L, the phase III trial, compared brigatinib with crizotinib in ALK inhibitor-–naïve ALK rearrangement–positive NSCLC to assess brigatinib’s role in the first-line setting. mPFS was not reached in the brigatinib arm at the time of data analysis or 9.8 months (9.0–12.9) in the crizotinib arm (HR for disease progression or death 0.49 [95% CI 0.33−0.74]; 12-month PFS 67% [95% CI 56%–75%] for brigatinib versus 43% [95% CI 32%–53%] for crizotinib).Citation79 Based on these results, the FDA recently approved brigatinib for the first-line treatment of patients with ALK-positive metastatic NSCLC on May 22, 2020.
Lorlatinib
Lorlatinib, a third-generation ALK inhibitor, was designed specifically to target mutations that drive resistance to other ALK inhibitors and to penetrate the BBB. This macrocyclic TKI of ALK and ROS1 effectively penetrates the BBB and retains potency against most ALK-resistance mutations known to develop during treatment with crizotinib and next-generation TKIs, including the G1202R solvent-front mutation.Citation34,Citation80,Citation81 In the phase I portion of a phase I/II study,Citation81,Citation82 lorlatinib demonstrated high efficacy, with 46% of patients with ALK-positive NSCLC achieving objective and durable responses (median DoR 12.4 months), many of whom had been recipients of several prior lines of therapy and had CNS involvement. Responses were evaluated for those patients who had received a second-generation TKI previously, as well as those who had prior exposure to crizotinib only. In addition, analysis of paired CSF and plasma samples showed high drug penetration into the CSF. This was demonstrated by pharmacokinetic analyses of paired blood and CSF, which demonstrated that the average ratio of CSF:plasma concentration of lorlatinib was 0.75 (75%), higher than the 0.03 ratio reported with crizotinib.Citation82 On the basis of phase I and preliminary phase II data, accelerated approval from the FDA was granted to lorlatinib on November 2, 2018 for the treatment of patients with ALK-rearranged advanced NSCLC after progression on crizotinib and at least one other ALK inhibitor. In a recent global phase II trial, lorlatinib demonstrated high intracranial activity in patients with advanced ALK-rearranged NSCLC who had been recipients of either crizotinib or other ALK inhibitors or were treatment-naïve.Citation81 The study enrolled 276 patients with histologically or cytologically ALK-rearranged or ROS1-positive advanced NSCLC with or without CNS disease, and assigned them to six experimental cohorts (EXP1–6) on the basis of prior therapy and ALK/ROS1 positivity.
The primary end point was response — overall and intracranial. In treatment-naïve patients (EXP1), objective response was achieved in 27 of 30. Three patients in this cohort had measurable baseline CNS lesions per independent centralized review, and intracranial tumor responses were observed in two (66.7%, 95% CI 9.4%–99.2%). ORR was 69% in crizotinib-treated patients, 33% in those treated with a non-crizotinib ALK inhibitor, and 39% in those treated with two or three previous ALK inhibitors. Lorlatinib thus represents an effective treatment strategy in heavily pretreated ALK-rearranged NSCLC patients and holds promise in the frontline setting. The future role of lorlatinib as a potent ALK inhibitor is promising. There exists a clear place for lorlatinib in the treatment of previously treated or refractory ALK-positive disease. The role of lorlatinib as first-line therapy was not answered in the first presented studies. The phase III CROWN trial comparing lorlatinib with crizotinib as first-line therapy is ongoing (NCT03052608), and results are eagerly awaited.
Sequence of Therapy
After progression on crizotinib, second-generation TKIs (ceritinib, alectinib, and brigatinib) were being used as second-line therapy. However, more recently the treatment paradigms have shifted, raising questions about the most optimal first-line therapy and selection of next-line therapies. summarizes the results from ALK inhibitors as first-line therapy in trials of crizotinib (PROFILE 1014),Citation29 ceritinib (ASCEND-4),Citation52 alectinib (J-ALEX and ALEX),Citation65,Citation67 and brigatinib (ALTA-1L).Citation79 Based on these results, alectinib has been widely adopted so far as a preferred first-line therapy for newly diagnosed ALK-rearranged NSCLC patients, due to its efficacy (including CNS activity) and safety profile. The National Comprehensive Cancer Network recommends use of any first- (crizotinib) or second-generation TKI (alectinib, ceritinib, or brigatinib) as first-line therapy for newly diagnosed ALK-rearranged NSCLC patients.Citation83 Treatment selection is also likely based on the experience and preference of the prescribing oncologist, as well as concerns for toxicity and tolerability. lists the pivotal trials that led to the (mostly initial) approval of the five ALK inhibitors discussed herein in second-line or above settings. highlights the efficacy of these ALK inhibitors for brain metastases in patients with ALK-rearranged NSCLC.
Table 3 Major ALK Inhibitor Clinical Trials for First-Line Therapy in ALK-Rearranged Non–Small Cell Lung Cancer
Table 4 Efficacy of Various ALK Inhibitors for Brain Metastases in Patients with ALK-Rearranged NSCLC
However, as discussed, numerous ALK-resistant mutations arise after treatment with these TKIs,Citation34 raising concerns for optimal sequencing of therapy. Tumor progression on crizotinib is inevitable and common with other ALK inhibitors as well. It has also been reported that patients who have progressive disease on second-generation ALK inhibitors have a higher likelihood of harboring ALK-resistance mutations in their tumors than patients who have disease progression on crizotinib.Citation34,Citation84 The most commonly reported ALK mutation is the solvent-front mutation G1202R, found in 56% of patients who progress on second-generation ALK inhibitors.Citation34 Insight into acquired mutation patterns upon progression can help with sequencing of therapy. As an example, patients that progress on ceritinib acquiring the F1174C mutation may benefit from alectinib, and those with acquired V1180L mutation on alectinib may benefit from brigatinib ( illustrates putative resistance-mutation patterns for different TKIs). Until recently, limited data regarding treatment of patients previously treated with different ALK inhibitors existed, but recent results from a global phase II study of the third-generation ALK inhibitor lorlatinib showed a high ORR and high intracranial response rate for patients with advanced ALK-positive NSCLC.Citation81 Lorlatinib was developed to better penetrate the BBB and remain potent to acquired resistant mutations that developed during therapy with first- and second-generation agents (particularlyALKG1202R mutations). The fact that the solvent-front mutation G1202R is a resistant mutation acquired in most second-generation TKIs offers lorlatinib an indisputable role in patients progressed on several lines of TKI. In addition to targeting resistance mutations via use of new-generation TKIs for ALK-dependent tumors, improved understanding of basic mechanisms of ALK-independent resistance pathways are now informing the development of therapeutic strategies to counter resistance in the clinic. For instance, NRG1 (the ligand for HER3 and HER4 tyrosine kinases) overexpression can be abrogated by combined inhibition of ALK and HER2.Citation33 Similarly, MEK reactivation is an essential ALK-independent resistance mechanism post-ceritinib.Citation85 Combination with ALK and MEK inhibitors have reportedly resulted in improved responses, durability of response, and importantly suppression of TKI resistance.Citation33
It should be pointed out that the identification of ALK-resistance mutations underlies the importance of obtaining rebiopsy upon disease progression, either by tissue or liquid form, to guide further appropriate TKI treatment while gaining better understanding of resistance mechanisms. Validation studies testing for ALK mutations in liquid form have been conducted and are being utilized at some centers. Recently, a multicenter collaborative study utilized liquid-biopsy technology and found molecular aberrations at a rate at least as high as standard-of-care tissue genotyping, with high tissue concordance.Citation86 The overall concordance rate for ALK fusion was reported at 99%, with a positive predictive value of 100%. The sensitivity of liquid biopsy for detection of ALK fusion was 75%, with a false-negative rate (1 – sensitivity) of 25%. These findings, though not perfect, are definitely encouraging. Few shortcomings need to be improved upon before liquid testing for detection of molecular aberrations becomes a standard tool for monitoring patients on treatment and selecting the next therapeutic options. Until then, tissue genotyping remains the standard of care. Liquid biopsy can be particularly valuable when tissue for genotyping is insufficient, significant delays in diagnosis are expected, or contraindications to the tissue biopsy exist.
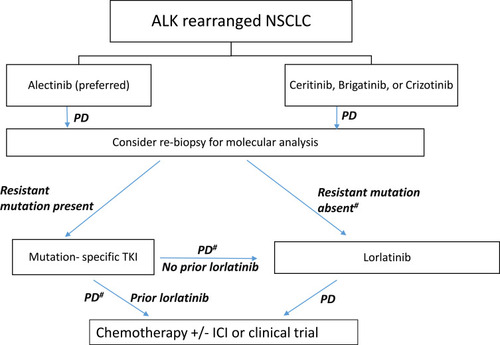
illustrates a current proposed treatment algorithm for ALK-rearranged NSCLC. The proposed algorithm is based on the recent data reviewed so far in this paper within the framework in accordance with National Comprehensive Cancer NetworkCitation83 and European Society for Medical Oncology guidelinesCitation87 and provides a useful treatment strategy in real-world practice. Our preference is to utilize rebiopsy-directed mutation-specific ALK inhibitors upon progression on first-line therapy, followed by lorlatinib (if not used previously) upon progression on mutation-specific TKIs. After progression on one or more second-generation TKIs, greater efficacy (ORR 62% and mPFS 7.3 months) of lorlatinib in patients with ALK mutations has been reported when compared with patients without ALK mutations.Citation88 This is likely due to less ALK dependence in the absence of putative mutations. In the absence of resistance mutation (and in the absence of alternate ALK-independent resistance pathways), upon progression on first-line TKIs, our preferred drug is lorlatinib as next-line therapy, due to evidence that suggests that even in mutation-negative patients, reasonable response rates (ORR 32%) and mPFS of 5.5 months can be attained.Citation88 Additionally, in patients with crizotinib-resistant disease, the efficacy of lorlatinib is comparable among patients with and without ALK mutations.Citation88 Nonetheless, further research is warranted to guide better treatment decision-making in patients with and without ALK-resistance mutations. Here again, the importance of rebiopsy, whether tissue- or liquid-based, upon each episode of progression cannot be emphasized more, so long as it is feasible in practice. The National Cancer Institute’s ALK Master Protocol (NCT03737994) will prospectively match patients to appropriate ALK TKIs on the basis of the underlying ALK-resistance mutation.Citation89 Once patients have progressed on lorlaitnib, the next line of therapy is chemotherapy with or without immunotherapy or consideration of participation in available clinical trials.
Figure 1 Treatment algorithm for ALK-rearranged non–small cell lung cancer.
Note: #Absence of ALK-dependent secondary mutations as well as ALK-independent alternate resistance pathways on rebiopsy.
Abbreviations: ICI, immunocheckpoint inhibitor; PD, progressive disease; TKI, tyrosine-kinase inhibitor.
Prospective Inhibitors and Trials in Progress
Enthusiasm for the new ALK inhibitors is high, and several new ALK inhibitors are in the pipeline. The ALK inhibitor ensartinib (X396) has shown activity and is well tolerated in ALK-rearranged NSCLC.Citation90 Additionally, phase III frontline studies, eg, comparing lorlatinib with crizotinib in the CROWN trial, are ongoing. However, there remains an unmet need to know more about on-target resistance mechanisms to the different ALK inhibitors. There also exists a need to understand off-target mechanisms underlying activation of alternate targetable molecular pathways during therapy with different ALK inhibitors. As such, the potential of eventually combining an ALK inhibitor with another targeted agent(s) might also be relevant in future to prevent or delay the development of resistance. Currently, the role of immunotherapy in combination with ALK inhibition is uncertain and not being studied actively. For example, a phase I/II trial in ALK-rearranged NSCLC patients evaluating a combination of ensartinib and durvalumab (NCT02898116) was terminated after enrolling just two patients, due to poor accrual. Another phase I/II study of nivolumab plus crizotinib for first-line treatment of ALK translocation–positive advanced NSCLC (CheckMate 370) was also closed prematurely, due to severe hepatotoxicity.Citation91 In the adjuvant setting, crizotinib is being studied after surgery for patients with stage IB–IIIA NSCLC in the ALCHEMIST trial (NCT02201992).Citation92 Another study in the adjuvant setting is evaluating the efficacy of alectinib versus standard adjuvant platinum-based chemotherapy (NCT03456076).Citation93 Other studies (NCT03088930, NCT04197076) evaluating ALK inihibitors in the neoadjuvant setting are also under way.Citation94,Citation95
Conclusion
Treatment options for ALK-rearranged NSCLC patients have advanced considerably in the past decade. Since the approval of the first ALK inhibitor, crizotinib, several newer generations of ALK inhibitors have proven their supremacy as first-line therapies and have efficacy against crizotinib resistance. Such factors as ability to overcome resistance-associated mutations and enhanced CNS penetration have played a crucial role in improving efficacy. Many of the agents have received accelerated FDA approval in recent years, and it will be prudent to study postmarketing survival trends of these drugs in the real world, as well as compare approved inhibitors head to head to better select front-line therapies for patients. Many such initiatives are under way, as discussed. However, while we are awaiting the results of future studies, it is reasonable to conclude that the recent rapid progress in ALK-rearranged NSCLC treatment has clearly shown incremental benefits to patients with ALK-positive NSCLC by providing more effective and less toxic therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.2159031912902
- Pikor LA, Ramnarine VR, Lam S, Lam WL. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer. 2013;82(2):179–189. doi:10.1016/j.lungcan.2013.07.02524011633
- Petersen I. The morphological and molecular diagnosis of lung cancer. Dtsch Arztebl Int. 2011;108(31–32):525–531. doi:10.3238/arztebl.2011.052521886665
- Khan M, Lin J, Liao G, et al. ALK inhibitors in the treatment of ALK positive NSCLC. Front Oncol. 2019;8(557). doi:10.3389/fonc.2018.00557.
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–285. doi:10.1097/JTO.0b013e318206a22121252716
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi:10.1126/science.109931415118125
- Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5(9):2892–2911.26609494
- Sasaki T, Rodig SJ, Chirieac LR, Janne PA. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer. 2010;46(10):1773–1780. doi:10.1016/j.ejca.2010.04.00220418096
- Xu H, Ma D, Yang G, et al. Sequential therapy according to distinct disease progression patterns in advanced ALK-positive non-small-cell lung cancer after crizotinib treatment. Chin J Cancer Res. 2019;31(2):349–356. doi:10.21147/j.issn.1000-9604.2019.02.0931156305
- Yang L, Ling Y, Guo L, et al. Detection of ALK translocation in non-small cell lung carcinoma (NSCLC) and its clinicopathological significance using the ventana immunohistochemical staining method: A single-center large-scale investigation of 1504 Chinese Han patients. Chin J Cancer Res. 2016;28(5):495–502. doi:10.21147/j.issn.1000-9604.2016.05.0427877008
- Verzura M, Batagelj E, Bagnes C, et al. Analysis of EML4-ALK rearrangement in non-small cell lung cancer in Argentina. Ann Diagn Pathol. 2018;34:77–81. doi:10.1016/j.anndiagpath.2018.02.00929661733
- Solomon B, Lovly C. Anaplastic lymphoma kinase (ALK) fusion oncogene positive non-small cell lung cancer In: Lilenbaum RC, editor. Uptodate. Waltham, MA: UpToDate Accessed 75, 2020.
- Chia PL, Mitchell P, Dobrovic A, John T. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin Epidemiol. 2014;6:423–432. doi:10.2147/CLEP.S6971825429239
- Takahashi T, Sonobe M, Kobayashi M, et al. Clinicopathologic features of non-small-cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol. 2010;17(3):889–897. doi:10.1245/s10434-009-0808-720183914
- Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol. 2008;3(1):13–17. doi:10.1097/JTO.0b013e31815e8b6018166835
- Bauer TM, Felip E, Solomon BJ, et al. Clinical management of adverse events associated with lorlatinib. Oncologist. 2019;24(8):1103–1110. doi:10.1634/theoncologist.2018-038030890623
- Patel A, Batra U, Prasad KT, et al. Real world experience of treatment and outcome in ALK-rearranged metastatic nonsmall cell lung cancer: A multicenter study from India. Curr Probl Cancer. 2020;44(3):100571. doi:10.1016/j.currproblcancer.2020.10057132234264
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–4253. doi:10.1200/JCO.2009.22.699319667264
- Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-hodgkin’s lymphoma. Science. 1994;263(5151):1281–1284. doi:10.1126/science.81221128122112
- Shaw A, Engelman J. ALK in lung cancer: past, present, and future. J Clin Oncol. 2013;31(8):1105–1111. doi:10.1200/JCO.2012.44.535323401436
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi:10.1038/nature0594517625570
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190–1203. doi:10.1016/j.cell.2007.11.02518083107
- Gandhi S, Chen H, Zhao Y, Dy GK. First-line treatment of advanced ALK-positive non-small-cell lung cancer. Lung Cancer (Auckl). 2015;6:71–82. doi:10.2147/LCTT.S6349128210152
- Weickhardt AJ, Aisner DL, Franklin WA, et al. Diagnostic assays for identification of anaplastic lymphoma kinase-positive non-small cell lung cancer. Cancer. 2013;119(8):1467–1477. doi:10.1002/cncr.2791323280244
- Ou SHI, Azada M, Hsiang DJ, et al. Next-generation sequencing reveals a novel NSCLC ALK f1174v mutation and confirms ALK G1202R mutation confers high-level resistance to alectinib (CH5424802/RO5424802) in ALK-rearranged NSCLC patients who progressed on crizotinib. J Thorac Oncol. 2014;9(4):549–553. doi:10.1097/JTO.000000000000009424736079
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. doi:10.1056/NEJMoa100644820979469
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–2394. doi:10.1056/NEJMoa121488623724913
- Kazandjian D, Blumenthal GM, Chen H-Y, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist. 2014;19(10):e5–11. doi:10.1634/theoncologist.2014-024125170012
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. doi:10.1056/NEJMoa140844025470694
- Chuang JC, Neal JW. Crizotinib as first line therapy for advanced ALK-positive non-small cell lung cancers. Transl Lung Cancer Res. 2015;4(5):639–641. doi:10.3978/j.issn.2218-6751.2015.03.0626629437
- Paz-Ares LG, de Marinis F, Dediu M, et al. Paramount: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(23):2895–2902. doi:10.1200/JCO.2012.47.110223835707
- Rothenstein JM, Chooback N. ALK inhibitors, resistance development, clinical trials. Curr Oncol. 2018;25(Suppl 1):S59–S67. doi:10.3747/co.25.376029910648
- Lin J, Riely G, Shaw A. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. 2017;7(2):137–155. doi:10.1158/2159-8290.CD-16-112328122866
- Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6(10):1118–1133. doi:10.1158/2159-8290.CD-16-059627432227
- Dagogo-Jack I, Shaw AT. Crizotinib resistance: implications for therapeutic strategies. Ann Oncol. 2016;27 Suppl 3:iii42–iii50. doi:10.1093/annonc/mdw30527573756
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase I study. Lancet Oncol. 2012;13(10):1011–1019. doi:10.1016/S1470-2045(12)70344-322954507
- Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial efficacy of crizotinib versus chemotherapy in patients with advanced ALK-positive non-small-cell lung cancer: results from profile 1014. J Clin Oncol. 2016;34(24):2858–2865. doi:10.1200/JCO.2015.63.588827022118
- Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–1888. doi:10.1200/JCO.2014.59.053925624436
- Toyokawa G, Seto T, Takenoyama M, Ichinose Y. Insights into brain metastasis in patients with ALK+ lung cancer: is the brain truly a sanctuary? Cancer Metastasis Rev. 2015;34(4):797–805. doi:10.1007/s10555-015-9592-y26342831
- Klempner SJ, Ou SH. Anaplastic lymphoma kinase inhibitors in brain metastases from ALK+ non-small cell lung cancer: hitting the target even in the CNS. Chin Clin Oncol. 2015;4(2):20.26112806
- Ou SH. Crizotinib: A novel and first-in-class multitargeted tyrosine kinase inhibitor for the treatment of anaplastic lymphoma kinase rearranged non-small cell lung cancer and beyond. Drug Des Devel Ther. 2011;5:471–485. doi:10.2147/DDDT.S19045
- Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–e445. doi:10.1200/JCO.2010.34.131321422405
- Jain RK, Chen H. Spotlight on brigatinib and its potential in the treatment of patients with metastatic ALK-positive non-small cell lung cancer who are resistant or intolerant to crizotinib. Lung Cancer (Auckl). 2017;8:169–177. doi:10.2147/LCTT.S12650729075144
- Kaneda H, Okamoto I, Nakagawa K. Rapid response of brain metastasis to crizotinib in a patient with ALK rearrangement-positive non-small-cell lung cancer. J Thorac Oncol. 2013;8(4):e32–e33. doi:10.1097/JTO.0b013e318284377123486271
- Santarpia M, Daffina MG, D’Aveni A, et al. Spotlight on ceritinib in the treatment of ALK+ NSCLC: design, development and place in therapy. Drug Des Devel Ther. 2017;11:2047–2063. doi:10.2147/DDDT.S113500
- Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4(6):662–673. doi:10.1158/2159-8290.CD-13-084624675041
- Massarelli E, Papadimitrakopoulou V. Ceritinib for the treatment of late-stage (metastatic) non–small cell lung cancer. Clin Cancer Res. 2015;21(4):670–674. doi:10.1158/1078-0432.CCR-14-129125564153
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–1197. doi:10.1056/NEJMoa131110724670165
- Kim DW, Mehra R, Tan DSW, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase I trial. Lancet Oncol. 2016;17(4):452–463. doi:10.1016/S1470-2045(15)00614-226973324
- Mok T, Spigel D, Felip E, et al. ASCEND-2: A single-arm, open-label, multicenter phase ii study of ceritinib in adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with chemotherapy and crizotinib (crz). J Clin Oncol. 2015;33(15_suppl):8059. doi:10.1200/jco.2015.33.15_suppl.8059
- Shaw AT, Kim TM, Crino L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): A randomised, controlled, open-label, Phase 3 trial. Lancet Oncol. 2017;18(7):874–886. doi:10.1016/S1470-2045(17)30339-X28602779
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet. 2017;389(10072):917–929. doi:10.1016/S0140-6736(17)30123-X28126333
- Cho BC, Kim DW, Bearz A, et al. ASCEND-8: A randomized Phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)-rearranged metastatic non-small cell lung cancer (NSCLC). J Thorac Oncol. 2017;12(9):1357–1367. doi:10.1016/j.jtho.2017.07.00528729021
- Cho BC, Obermannova R, Bearz A, et al. Efficacy and safety of ceritinib (450 mg/d or 600 mg/d) with food versus 750-mg/d fasted in patients with ALK receptor tyrosine kinase (ALK)-positive NSCLC: primary efficacy results from the ASCEND-8 study. J Thorac Oncol. 2019;14(7):1255–1265. doi:10.1016/j.jtho.2019.03.00230851442
- European Society for Medical Oncology. Ceritinib targets brain metastases in patients with ALK-positive NSCLC. Available from: https://www.esmo.org/oncology-news/Ceritinib-Targets-Brain-Metastases-in-Patients-with-ALK-positive-NSCLC. Accessed 75, 2020.
- Chow LQ, Barlesi F, Bertino EM, et al. Results of the ASCEND-7 phase II study evaluating ALK inhibitor (ALKi) ceritinib in patients (pts) with ALK+ non-small cell lung cancer (NSCLC) metastatic to the brain. Ann Oncol. 2019;30(Supplement_5):v602–v603. doi:10.1093/annonc/mdz260
- Kinoshita K, Asoh K, Furuichi N, et al. Design and synthesis of a highly selective, orally active and potent anaplastic lymphoma kinase inhibitor (CH5424802). Bioorg Med Chem. 2012;20(3):1271–1280. doi:10.1016/j.bmc.2011.12.02122225917
- Kodama T, Tsukaguchi T, Yoshida M, Kondoh O, Sakamoto H. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett. 2014;351(2):215–221. doi:10.1016/j.canlet.2014.05.02024887559
- Ou SH, Janne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol. 2014;25(2):415–422. doi:10.1093/annonc/mdt57224478318
- Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (af-001jp study): A single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013;14(7):590–598. doi:10.1016/S1470-2045(13)70142-623639470
- Tamura T, Kiura K, Seto T, et al. Three-year follow-up of an alectinib phase I/II study in ALK-positive non-small-cell lung cancer: af-001jp. J Clin Oncol. 2017;35(14):1515–1521. doi:10.1200/JCO.2016.70.574928296581
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (af-002jg): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15(10):1119–1128. doi:10.1016/S1470-2045(14)70362-625153538
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: A phase II global study. J Clin Oncol. 2016;34(7):661–668. doi:10.1200/JCO.2015.63.944326598747
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: A single-group, multicentre, Phase 2 trial. Lancet Oncol. 2016;17(2):234–242. doi:10.1016/S1470-2045(15)00488-X26708155
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29–39. doi:10.1016/S0140-6736(17)30565-228501140
- Seto T, Nishio M, Hida T, et al. Final PFS analysis and safety data from the phase III J-ALEX study of alectinib (alc) vs. crizotinib (crz) in ALK-inhibitor naïve ALK-positive non-small cell lung cancer (ALK+ NSCLC). J Clin Oncol. 2019;37(15_suppl):9092. doi:10.1200/JCO.2019.37.15_suppl.9092
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–838. doi:10.1056/NEJMoa170479528586279
- Camidge DR, Dziadziuszko R, Peters S, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol. 2019;14(7):1233–1243. doi:10.1016/j.jtho.2019.03.00730902613
- Gadgeel SM, Shaw AT, Govindan R, et al. Pooled analysis of CNS response to alectinib in two studies of pretreated patients with ALK-positive non-small-cell lung cancer. J Clin Oncol. 2016;34(34):4079–4085. doi:10.1200/JCO.2016.68.463927863201
- Mok TSK, Shaw AT, Camidge RD, et al. The updated OS and safety data from the randomised, phase III ALEX study of alectinib (alc) versus crizotinib (crz) in untreated advanced ALK+ NSCLC. Ann Oncol. 2019;30(Supplement_5):v607. doi:10.1093/annonc/mdz260.006
- Dagogo-Jack I, Rooney M, Lin JJ, et al. Treatment with next-generation ALK inhibitors fuels plasma ALK mutation diversity. Clin Cancer Res. 2019;25(22):6662–6670. doi:10.1158/1078-0432.CCR-19-143631358542
- Huang WS, Liu S, Zou D, et al. Discovery of brigatinib (ap26113), a phosphine oxide-containing, potent, orally active inhibitor of anaplastic lymphoma kinase. J Med Chem. 2016;59(10):4948–4964. doi:10.1021/acs.jmedchem.6b0030627144831
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: A randomized, multicenter phase II trial. J Clin Oncol. 2017;35(22):2490–2498. doi:10.1200/JCO.2016.71.590428475456
- Uchibori K, Inase N, Araki M, et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun. 2017;8:14768. doi:10.1038/ncomms1476828287083
- Zhang S, Anjum R, Squillace R, et al. The potent ALK inhibitor brigatinib (ap26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in preclinical models. Clin Cancer Res. 2016;22(22):5527–5538. doi:10.1158/1078-0432.CCR-16-056927780853
- Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: A single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(12):1683–1696. doi:10.1016/S1470-2045(16)30392-827836716
- Lin J, Zhu V, Schoenfeld A, et al. Brigatinib in patients with alectinib-refractory ALK-positive NSCLC. J Thorac Oncol. 2018;13(10):1530–1538. doi:10.1016/j.jtho.2018.06.00529935304
- Kim ES, Ou S-HI, Barlesi F, et al. Phase 2 study of brigatinib in patients (pts) with anaplastic lymphoma kinase (ALK)−positive, advanced non–small cell lung cancer (NSCLC) that progressed on alectinib or ceritinib. J Clin Oncol. 2019;37(15_suppl):TPS9115. doi:10.1200/JCO.2019.37.15_suppl.TPS9115
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379(21):2027–2039. doi:10.1056/NEJMoa181017130280657
- Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10r)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2h-8,4-(m etheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (pf-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ros1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57(11):4720–4744. doi:10.1021/jm500261q24819116
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19(12):1654–1667. doi:10.1016/S1470-2045(18)30649-130413378
- Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18(12):1590–1599. doi:10.1016/S1470-2045(17)30680-029074098
- National Comprehensive Cancer Network. Non-small cell lung cancer Guideline. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 75, 2020.
- Gadgeel SM. Sequencing of ALK inhibitors in ALK+ non-small cell lung cancer. Curr Treat Options Oncol. 2017;18(6):36. doi:10.1007/s11864-017-0479-828534251
- Crystal A, Shaw A, Sequist L, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science. 2014;346(6216):1480–1486. doi:10.1126/science.125472125394791
- Leighl NB, Page RD, Raymond VM, et al. Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell lung cancer. Clin Cancer Res. 2019;25(15):4691–4700. doi:10.1158/1078-0432.CCR-19-062430988079
- European Society for Medical Oncology. Management of advanced/metastatic NSCLC. Available from: https://www.esmo.org/guidelines/lung-and-chest-tumours/metastatic-non-small-cell-lung-cancer/management-of-advanced-metastatic-nsclc. Accessed 75, 2020.
- Shaw A, Solomon B, Besse B, et al. ALK Resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol. 2019;37(16):1370–1379. doi:10.1200/JCO.18.0223630892989
- Targeted treatment for ALK positive patients who have previously been treated for non-squamous non-small cell lung cancer. Available from: ClinicalTrials.gov. Accessed 75, 2020.
- Horn L, Infante JR, Reckamp KL, et al. Ensartinib (x-396) in ALK-positive non-small cell lung cancer: results from a first-in-human phase I/II, multicenter study. Clin Cancer Res. 2018;24(12):2771–2779. doi:10.1158/1078-0432.CCR-17-239829563138
- Spigel DR, Reynolds C, Waterhouse D, et al. Phase 1/2 study of the safety and tolerability of nivolumab plus crizotinib for the first-line treatment of anaplastic lymphoma kinase translocation - positive advanced non-small cell lung cancer (CheckMate 370). J Thorac Oncol. 2018;13(5):682–688. doi:10.1016/j.jtho.2018.02.02229518553
- Crizotinib in treating patients with stage Ib-IIIa non-small cell lung cancer that has been removed by surgery and ALK fusion mutations (an ALCHEMIST treatment trial). Available from: ClinicalTrials.gov. Accessed 75, 2020.
- A study comparing adjuvant alectinib versus adjuvant platinum-based chemotherapy in patients with ALK positive non-small cell lung cancer. Available from: ClinicalTrials.gov. Accessed 75, 2020.
- Evaluating crizotinib in the neoadjuvant setting in patients with non-small cell lung cancer. Available from: ClinicalTrials.gov. Accessed 75, 2020.
- Neoadjuvant therapy in resectable non-small cell lung cancer stages IIIA. Available from: ClinicalTrials.gov. Accessed 75, 2020.
- Lovly CM, Iyengar P, Gainor JF. Managing resistance to EFGR- and ALK-targeted therapies. Am Soc Clin Oncol Educ Book. 2017;37:607–618. doi:10.14694/EDBK_17625128561721
- Katayama R. Therapeutic strategies and mechanisms of drug resistance in anaplastic lymphoma kinase (ALK)-rearranged lung cancer. Pharmacol Ther. 2017;177:1–8. doi:10.1016/j.pharmthera.2017.02.01528185914
- Tran PN, Klempner SJ. ALK on my mind: alectinib takes an early lead in managing intracranial disease in non-small cell lung cancer with ALK rearrangements. Ann Transl Med. 2017;5(7):173. doi:10.21037/atm.2017.03.4728480209
- Bauer T, Shaw A, Johnson M, et al. Brain penetration of lorlatinib: cumulative incidences of CNS and non-CNS progression with lorlatinib in patients with previously treated ALK-positive non-small-cell lung cancer. Target Oncol. 2020;15(1):55–65. doi:10.1007/s11523-020-00702-432060867
- Recondo G, Mezquita L, Facchinetti F, et al. Diverse resistance mechanisms to the third-generation ALK inhibitor lorlatinib in ALK-rearranged lung cancer. Clin Cancer Res. 2020;26(1):242–255. doi:10.1158/1078-0432.CCR-19-110431585938
- Wilson FH, Johannessen CM, Piccioni F, et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell. 2015;27(3):397–408. doi:10.1016/j.ccell.2015.02.00525759024
- Lovly CM, McDonald NT, Chen H, et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat Med. 2014;20(9):1027–1034. doi:10.1038/nm.366725173427
