Abstract
Background
Preoperative imaging examination is the primary method for diagnosing metastatic gastrointestinal stromal tumor (GIST), but it is associated with a high rate of missed diagnosis. Therefore, it is important to establish an accurate model for predicting occult peritoneal metastasis (PM) of GIST.
Patients and Methods
GIST patients seen between April 2002 and December 2018 were selected from an institutional database. Using multivariate logistic regression analyses, we created a nomogram to predict occult PM of GIST and validated it with an independent cohort from the same center. The concordance index (C-index), decision curve analysis (DCA) and a clinical impact curve (CIC) were used to evaluate its predictive ability.
Results
A total of 522 eligible GIST patients were enrolled in this study and divided into training (n=350) and validation cohorts (n=172). Factors associated with occult PM were included in the model: tumor size (odds ratio [OR] 1.194 95% confidence interval [CI], 1.034–1.378; p=0.016), primary location (OR 7.365 95% CI, 2.192–24.746; p=0.001), tumor capsule (OR 4.282 95% CI, 1.209–15.166; p=0.024), Alb (OR 0.813 95% CI, 0.693–0.954; p=0.011) and FIB (OR 2.322 95% CI, 1.410–3.823; p=0.001). The C-index was 0.951 (95% CI, 0.917–0.985) in the training cohort and 0.946 (95% CI, 0.900–0.992) in the validation cohort. In the training cohort, the prediction model had a sensitivity of 82.8%, a specificity of 93.8%, a positive predictive value of 54.7%, and a negative predictive value of 98.4%; the validation cohort values were 94.7%, 85.0%, 43.9% and 99.2%, respectively. DCA and CIC results showed that the nomogram had clinical value in predicting occult PM in GIST patients.
Conclusion
Imaging and inflammatory indexes are significantly associated with microscopic metastases of GIST. A nomogram including these factors would have an excellent ability to predict occult PM.
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors in the gastrointestinal tract, most of which have kit- or pdgfra-activating mutations and express CD117.Citation1 Approximately 11–47% of patients have significant metastasis at the time of diagnosis, usually in the peritoneum.Citation2,Citation3 Therefore, early detection and diagnosis of peritoneal metastasis (PM) has important clinical significance for choosing the best treatment and avoiding progression of the disease.
Computed tomography (CT) is the primary noninvasive way to diagnose PM. Studies have shown that CT detection of PM has high specificity but low sensitivity (28–56%) in gastric cancer.Citation4,Citation5 Similarly, it is difficult for CT to detect the microscopic metastasis of GIST. Both the European Society for Medical Oncology (ESMO)Citation6 and the National Comprehensive Cancer Network (NCCN) guidelinesCitation7 recommended staging laparoscopy as the most reliable method to identify clinically occult PM. However, laparoscopy is an invasive procedure, and the selection of patients suitable for laparoscopic exploration is still controversial. Moreover, laparoscopy has been confirmed to be associated with tumor progression and metastases. It is possible that immune or metabolic disturbances due to the use of a pneumoperitoneum could contribute to this problem.Citation8–Citation10 Generally, conventional techniques for the preoperative detection of PM are invasive and lack sensitivity.
At present, nomograms are widely used to predict the prognosis of patients with bladder cancer, oropharyngeal cancer and metastatic GIST.Citation11–Citation13 Due to their convenience and quantitative characteristics, nomograms has great value in clinical practice.Citation14 Therefore, we aimed to identify risk factors for preoperative occult PM of GIST and to construct a comprehensive nomogram.
Patients and Methods
Study Population
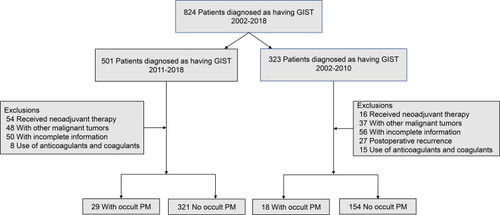
A retrospective analysis of GIST patients admitted to the Fujian Medical University Union Hospital from April 2002 to December 2018 was performed. A total of 522 patients were included for retrospective analysis. Patients who received radical treatment from January 2011 to December 2018 were divided into a training cohort (n=350), while patients from April 2002 to December 2010 were divided into a validation cohort (n=172). The inclusion criteria were as follows: (1) patients who underwent both CT scanning and laparoscopic or laparotomy exploration; (2) patients with no typical PM signs (diffuse omental nodules, irregular thickening with hyperenhancement of peritoneum, etc.) on CT; (3) patients with no distant metastasis; and (4) patients with no other malignant tumors. The exclusion criteria of this study are listed in .
Patients who received surgical treatment (laparoscopy or laparotomy) and whose diagnoses were confirmed by histology as GIST after operation were included. All patients were initially diagnosed with no PM by CT, but PM was later confirmed by laparotomy or laparoscopic exploration as occult PM. The basic information of patients with GIST, including age, sex, tumor size, primary location, tumor capsule, white blood cell count, neutrophil count, monocyte count, platelet count, fibrinogen (FIB), and albumin (Alb), were retrieved from electronic medical records. This study was approved by the Institutional Review Committee of the Fujian Medical University Union Hospital (2019YW024), and informed consent was obtained from all patients.
Markers of Systemic Inflammation
Hematology and laboratory examinations were performed 1 week before surgery. Parameters including neutrophil count, lymphocyte count, platelet count, and fibrinogen and Alb levels were examined. The prognostic nutritional index (PNI) was calculated as 10 × serum albumin (g/dl) + 0.005 × total lymphocyte count (per mm3).Citation15 The neutrophil-to-lymphocyte ratio (NLR) was calculated as the neutrophil count divided by lymphocyte count.Citation16 The platelet-to-lymphocyte ratio (PLR) was defined as the platelet count divided by the lymphocyte count.Citation17 The lymphocyte-to-monocyte ratio (LMR) was calculated as the lymphocyte count divided by the monocyte count.Citation15
Statistical Analysis
Student’s t-test was used for continuous data, and the Χ2 test was used for categorical variables. Univariable and multivariable analyses were undertaken to identify factors predictive of occult PM.
A nomogram based on tumor size, primary location, tumor capsule, FIB and Alb for the prediction of PM was constructed using the results of the multivariable analyses. For internal validation of the nomogram, bootstrapping with 1000 resamples was performed. Harrell’s concordance index (C-index) was used to measure the prediction performance of the nomogram. To further validate the prediction model, an independent external cohort was used. Calibration curves were plotted to assess the calibration of the model. Decision curve analysis (DCA)Citation18 and clinical impact curve (CIC) quantified the net benefits of different threshold probabilities to evaluate the clinical value of the nomogram.Citation19 The probability of PM was estimated based on the 95% confidence interval (95% CI) of binomial distribution.
All P values were two-sided, and differences with p < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 25.0 (SPSS, IBM Corp., Armonk, New York, USA) and R software version 3.2.5 (http://www.r-project.org). The packages included rms, rmda and survival.
Results
Clinicopathological Features
The training cohort included 350 consecutive patients, and the incidence of occult PM was 8.29% (29/350). The validation cohort included 172 patients, and the incidence of occult PM was 11.0% (19/172) in the validation cohort. The distribution of preoperative tumor size, primary location, tumor capsule, PNI, NLR, PLR, LMR, FIB, and Alb values between the two cohorts are given in .
Table 1 Demographic and Clinical Characteristics of Patients in Training and Validation Cohorts
Receiver Operating Characteristic (ROC) Curves and Optimal Cutoff Values for Predicting the Occult PM of GIST
Supplementary Figure 1 shows the ROC curves of preoperative blood parameters, including PLR, NLR, LMR, PNI; the area under the curve (AUC) values were 0.600, 0.606, 0.601, and 0.724, respectively (all p < 0.05 except for LMR, PLR and NLR) (Supplementary Table 1). The corresponding optimal cutoff values were 149.4, 3.6, 3.9, and 48.4, respectively.
Establishment and Validation of a Nomogram for Predicting Occult PM
Logistic regression modeling identified five variables that were associated with occult PM: tumor size (OR 1.194 95% CI, 1.034–1.378; p=0.016), primary location (OR 7.365 95% CI, 2.192–24.746; p=0.001), tumor capsule (OR 4.282 95% CI, 1.209–15.166; p=0.024), Alb (OR 0.813 95% CI, 0.693–0.954; p=0.011) and FIB (OR 2.322 95% CI, 1.410–3.823; p=0.001) ().
Table 2 Risk Factors for Occult PM Identified by Multivariate Analysis
A nomogram containing tumor size, primary location, tumor capsule, Alb and FIB was constructed (). The Hosmer-Lemeshow test indicated a lack of significance (p=0.407), indicating a good fit. The C-index of the predicted nomogram was 0.951 (95% CI, 0.917–0.985) (). The calibration curve of the prediction model showed good consistency in the training cohort ().
Figure 2 Nomogram to estimate the risk of preoperative occult PM of GIST. To use the nomogram, find the position of each variable on the corresponding axis, draw a line to the points axis for the number of points, add the points from all of the variables, and draw a line from the total points axis to determine the preoperative occult PM of GIST probabilities at the lower line of the nomogram. Validation of the predictive performance of the nomogram in estimating the risk of preoperative occult PM of GIST (n = 350).
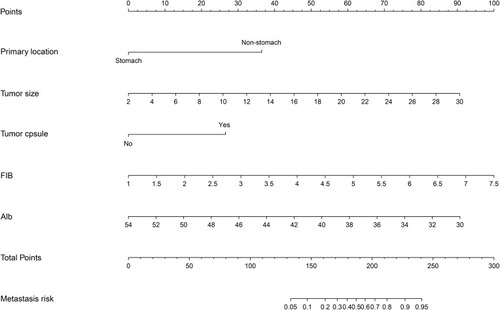
Figure 3 The accuracy of the model for identifying patients with occult PM was determined using AUC analysis for the training (A) and validation (B) cohorts. The distribution of the predicted probabilities of preoperative occult PM of GIST in the training (C) and validation (D) cohorts.
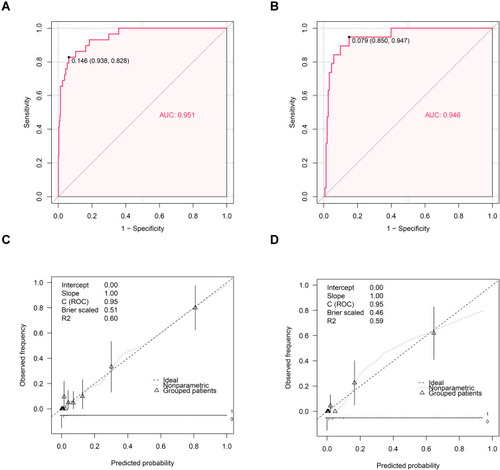
The P-value of the Hosmer-Lemeshow test in the validation cohort indicated a lack of significance (p=0.745). The C-index of the nomogram for predicting occult PM was 0.946 (95% CI, 0.900–0.992) (). The calibration curve in the validation cohort showed that there was good consistency between the prediction of occult PM of GIST and the actual situation observed ().
Clinical Utility
DCA was performed according to the nomogram that predicted the occult PM of GIST. This analysis indicated that when the threshold probability is within the range 4–100%, using the nomogram to predict occult PM adds more net benefit than the treat-all or treat-none strategies ().
Figure 4 The DCA of the nomogram for predicting preoperative occult PM of GIST was plotted. (A) The blue solid line assumes that all patients will have occult PM. The black solid line assumes that no patients will have occult PM. In this analysis, the decision curve provided a larger net benefit across the range of 4 to 100%. The CIC of the nomogram for predicting preoperative occult PM is shown. (B) The y-axis represents the net benefit.
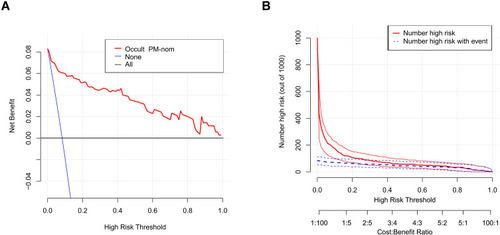
Based on the DCA, a CIC using the cost:benefit ratio to evaluate the nomogram can quickly help us understand the significance of the nomogram in predicting the occult PM of GIST. shows the estimated number of patients who would be declared at high risk of occult PM for each risk threshold and visually shows the proportion of those who are true positives. For example, if a 20% risk threshold was used, then of 1000 people screened, approximately 100 would be deemed at high risk of occult PM, with approximately 50 of these being true cases.
Moreover, in the training and validation cohorts, the AUC values were 0.951 and 0.946, respectively, and the corresponding best cutoff values were 0.146 and 0.079, respectively. The prediction model had a sensitivity of 82.8%, a specificity of 93.8%, a positive predictive value of 54.7%, and a negative predictive value of 98.4% in the training cohort. In the validation cohort, the sensitivity, specificity, positive predictive value and negative predictive value were 94.7%, 85.0%, 43.9% and 99.2%, respectively.
Discussion
Occult PM is a special metastasis situation in which CT-diagnosed PM-negative patients are confirmed as PM-positive during subsequent laparoscopies. Because laparoscopic exploration is invasive and changes in abdominal carbon dioxide pressure may lead to tumor progression,Citation8,Citation9 many researchers believe that it is necessary to establish a simple, efficient and noninvasive method for preoperative prediction of occult PM. While prediction models have been used to identify the occult PM of patients with certain malignancies, such as gastric, pancreatic and ovarian cancer, no data currently exist concerning the occult PM of patients with GIST.Citation20–Citation22 The ability to predict the likelihood of occult PM at the time of diagnosis is important for several reasons. Preoperative histological confirmation of GIST with a high risk of PM can avoid unnecessary or inappropriate surgery.Citation23 Moreover, related studies have shown that transluminal or transcutaneous biopsy of GIST does not influence oncological outcome in GIST patientsCitation24 and that tumor rupture caused by surgery is a poor prognostic factor.Citation25–Citation27
A nomogram was developed and validated for the prediction of occult PM in patients with GIST. The nomogram incorporates five factors, namely, tumor size, primary location, tumor capsule, FIB and Alb. It has high accuracy in predicting the risk of occult PM before surgery in patients with GIST and has potential clinical application value.
The tumor size of GIST is related to the degree of malignancy of the tumor, and patients with a larger tumor size have a higher risk of recurrence and metastasis.Citation28 Compared with patients with stomach GIST, patients with non-stomach GIST (small intestine, retroperitoneum) have a higher risk of metastasis.Citation25 Preoperative imaging showed that extensive tumor capsule and necrosis were also associated with the risk of tumor recurrence and metastasis.Citation29 In our study, tumor size, tumor location and tumor capsule status were strongly predictive of occult PM in the multivariable analysis. Therefore, we incorporated the tumor size, primary site and tumor capsule status into the nomogram and demonstrated excellent predictive ability.
In 1863, Virchow initially reported a link between malignancy and inflammation.Citation30 Systemic inflammation is considered to play an important role in tumor progression and metastasis.Citation31 Studies have shown that markers of systemic inflammation, including the NLR, LMR and PLR, are closely related to the development of tumors.Citation32–Citation34 However, in our study, we found that the increase or decrease in serum NLR, LMR, and PLR were not independent predictors of occult PM of GIST. Therefore, these markers of systemic inflammation were not included during the development of the present model.
FIB is an important factor that regulates blood coagulation in humans.Citation35 In the process of blood coagulation, FIB can promote tumor cell adhesion, mediate the systemic inflammatory response, and promote the colonization and metastasis of circulating cancer cells.Citation36 Previous studies have shown that abnormally elevated FIB increases the ability of tumor cells to invade and metastasize, which results in a poor prognosis of cancer patients.Citation37 In addition, some studies have found that the FIB/Alb ratio can predict the prognosis of tumor patients, and the higher the FIB/Alb ratio is, the higher the risk of recurrence and metastasis.Citation38–Citation40 For this reason, in the present study, we evaluated the ability of FIB and Alb to predict occult PM in GIST. The results of this study revealed that these two indicators have sufficient predictive strength in the multivariable analysis of the nomogram.
To further verify the clinical value of the nomogram, we used DCA and CICs to analyze the clinical value of the predictive model. DCA is a new method used to evaluate diagnostic trials, predictive models and molecular markers, and it uses threshold probability to express the net clinical benefit.Citation14 In the present study, DCA showed the application of the nomogram in the prediction of occult PM of GIST within an appropriate range. CICs can visually show the estimated number of people who would be considered high risk and the number of cases with real occult PM.Citation19 For example, if a risk threshold of 20% is used, of the 1000 people screened, approximately half of the high-risk population are truly cases of occult PM. In the training cohort, the sensitivity was 82.8%, and the specificity was 93.8%, while the sensitivity and specificity of the validation cohort were 94.7% and 85.0%, respectively. Therefore, the predictive model has good clinical application value.
However, this study has some limitations. This is a single-center small-sample retrospective study, and a greater sample size is needed to improve the nomogram. The ideal nomogram should be subjected to external validation from the different centers to evaluate the effectiveness of the prediction model, but we only performed a self-test with 1000 resamples of the same population and validated the nomogram at the same institution. Moreover, due to the different races of patients in the East and West, this prediction model may only be applicable to patients in the East. Although this nomogram has some shortcomings, it can easily and effectively predict the probability of occult PM in patients with GIST and can guide clinical decision-making and personalized treatment for patients.
Conclusion
The present study constructed and validated a nomogram to predict preoperative occult PM of GIST based on tumor size, primary location, tumor capsule status, FIB and Alb. This tool can assist clinicians and patients in predicting potential occult PM and avoiding unnecessary surgery. For future studies, we should expand the sample size and add additional centers to validate this nomogram.
Abbreviations
GIST, gastrointestinal stromal tumors; PM, occult peritoneal metastasis; DCA, decision curve analysis; CIC, clinical impact curve; IQR, interquartile range; PNI, prognostic nutritional index; NLR, neutrophil-lymphocyte ratio; PLR, platelet–lymphocyte ratio; LMR, lymphocyte–monocyte ratio; Alb, albumin; FIB, fibrinogen.
Data Sharing Statement
The dataset analyzed for this study is available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study obtained approval from the Independent Ethics Committee of Fujian Medical University Union Hospital (2019YW024) to identify patients diagnosed with GIST in our center. Written informed consent was provided by the patients for their information and specimens to be stored in the hospital database and used in research. Patient records were anonymized and de-identified before analysis.
Consent for Publication
Written consent was given by the patients and their relatives to use their information in a research study and publish it.
Acknowledgments
The authors thank all the medical staff who contributed to the maintenance of the medical record database. Shao-jun Xu and Guo-sheng Lin are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382(9896):973–983. doi:10.1016/S0140-6736(13)60106-323623056
- DeMatteo RP, Lewis JJ, Leung D, S S. M, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231(1):51–58. doi:10.1097/00000658-200001000-0000810636102
- Cassier PA, Ducimetière F, Lurkin A, et al. A prospective epidemiological study of new incident GISTs during two consecutive years in Rhône Alpes region: incidence and molecular distribution of GIST in a European region. Br J Cancer. 2010;103(2):165–170. doi:10.1038/sj.bjc.660574320588273
- Kim SJ, Kim -H-H, Kim YH, et al. Peritoneal metastasis: detection with 16- or 64-detector row CT in patients undergoing surgery for gastric cancer. Radiology. 2009;253(2):407–415. doi:10.1148/radiol.253208227219789243
- Burbidge S, Mahady K, Naik K, S B, K M, K N. The role of CT and staging laparoscopy in the staging of gastric cancer. Clin Radiol. 2013;68(3):251–255. doi:10.1016/j.crad.2012.07.01522985749
- Casali PG, Abecassis N, Aro HT, et al. Gastrointestinal stromal tumours: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv68–iv78. doi:10.1093/annonc/mdy09529846513
- von Mehren M, Benjamin RS, Bui MM, et al. Soft tissue sarcoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10(8):951–960. doi:10.6004/jnccn.2012.009922878820
- Pidgeon P, Harmey H, Kay E, Costa MD, Redmond HP, Bouchier-Hayes DJ. The role of endotoxin/lipopolysaccharide in surgically induced tumour growth in a murine model of metastatic disease. Br J Cancer. 1999;81(8):1311–1317. doi:10.1038/sj.bjc.669436910604727
- Volz J, Köster S, Spacek Z, Paweletz N. Characteristic alterations of the peritoneum after carbon dioxide pneumoperitoneum. Surg Endosc. 1999;13(6):611–614. doi:10.1007/s00464990105210347302
- Neuhaus SJ, Watson DI, Ellis T, Rofe AM, Mathew G, Jamieson GG. Influence of gases on intraperitoneal immunity during laparoscopy in tumor-bearing rats. World J Surg. 2000;24(10):1227–1231. doi:10.1007/s00268001024211071467
- Kluth LA, Black PC, Bochner B, et al. Prognostic and prediction tools in bladder cancer: a comprehensive review of the literature. Eur Urol. 2015;68(2):238–253. doi:10.1016/j.eururo.2015.01.03225709027
- Fakhry C, Zhang Q, Nguyen-Tân PF, et al. Development and validation of nomograms predictive of overall and progression-free survival in patients with oropharyngeal cancer. J Clin Oncol. 2017;35(36):4057–4065. doi:10.1200/JCO.2016.72.074828777690
- Lee CK, Goldstein D, Gibbs E, et al. Development and validation of prognostic nomograms for metastatic gastrointestinal stromal tumour treated with imatinib. Eur J Cancer. 2015;51(7):852–860. doi:10.1016/j.ejca.2015.02.01525801699
- Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–180. doi:10.1016/S1470-2045(14)71116-725846097
- Park JW, Chang HJ, Yeo HY, et al. The relationships between systemic cytokine profiles and inflammatory markers in colorectal cancer and the prognostic significance of these parameters. Br J Cancer. 2020:1–19.
- Patel A, Ravaud A, Motzer RJ, et al. Neutrophil-to-lymphocyte ratio as a prognostic factor of disease-free survival in post-nephrectomy high-risk loco-regional RCC: analysis of the S-TRAC trial. Clin Cancer Res. 2020;26:4863–4868.32546645
- Lee S, Hoberstorfer T, Wadowski PP, et al. Platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios predict target vessel restenosis after infrainguinal angioplasty with stent implantation. J Clin Med. 2020;9(6):1729.
- Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26(6):565–574. doi:10.1177/0272989X0629536117099194
- Kerr KF, Brown MD, Zhu K, Janes H. Assessing the clinical impact of risk prediction models with decision curves: guidance for correct interpretation and appropriate use. J Clin Oncol. 2016;34(21):2534–2540. doi:10.1200/JCO.2015.65.565427247223
- Ayhan A, Gultekin M, Celik NY, et al. Occult metastasis in early ovarian cancers: risk factors and associated prognosis. Am J Obstet Gynecol. 2007;196(1):81.e81–81.e86. doi:10.1016/j.ajog.2006.08.043
- Li Z-Y, Tang L, Li Z-M, et al. Four-point computed tomography scores for evaluation of occult peritoneal metastasis in patients with gastric cancer: a region-to-region comparison with staging laparoscopy. Ann Surg Oncol. 2020;27(4):1103–1109. doi:10.1245/s10434-019-07812-y31965376
- Dong D, Tang L, Li ZY, et al. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann Oncol. 2019;30(3):431–438. doi:10.1093/annonc/mdz00130689702
- van Houdt WJ, IJzerman NS, Marjolein Schrijver A, et al. Oncological outcome after diagnostic biopsies in gastrointestinal stromal tumors: a retrospective cohort study. Ann Surg. 2019. doi:10.1097/SLA.0000000000003744
- Eriksson M, Reichardt P, Hall KS, et al. Needle biopsy through the abdominal wall for the diagnosis of gastrointestinal stromal tumour – does it increase the risk for tumour cell seeding and recurrence? Eur J Cancer. 2016;59:128–133.27033260
- Joensuu H, Vehtari A, Riihimäki J. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13(3):265–274. doi:10.1016/S1470-2045(11)70299-622153892
- Nishida T, Hølmebakk T, Raut CP, Rutkowski P. Defining tumor rupture in gastrointestinal stromal tumor. Ann Surg Oncol. 2019;26(6):1669–1675. doi:10.1245/s10434-019-07297-930868512
- Hølmebakk T, Bjerkehagen B, Boye K, Ø B, Stoldt S, Sundby Hall K. Definition and clinical significance of tumour rupture in gastrointestinal stromal tumours of the small intestine. Br J Surg. 2016;103(6):684–691.26988241
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466–1478.17090188
- Li H, Ren G, Cai R, Chen J, Wu X, Zhao J. A correlation research of Ki67 index, CT features, and risk stratification in gastrointestinal stromal tumor. Cancer Med. 2018;7(9):4467–4474. doi:10.1002/cam4.173730123969
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi:10.1016/S0140-6736(00)04046-011229684
- McMillan DC. The systemic inflammation-based Glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39(5):534–540. doi:10.1016/j.ctrv.2012.08.00322995477
- Kim EY, Lee JW, Yoo HM, Park CH, Song KY. The platelet-to-lymphocyte ratio versus neutrophil-to-lymphocyte ratio: which is better as a prognostic factor in gastric cancer? Ann Surg Oncol. 2015;22(13):4363–4370. doi:10.1245/s10434-015-4518-z25805235
- Sun Y, Zhang L. The clinical use of pretreatment NLR, PLR, and LMR in patients with esophageal squamous cell carcinoma: evidence from a meta-analysis. Cancer Manag Res. 2018;10:6167–6179. doi:10.2147/CMAR.S17103530538564
- Li K-J, Xia X-F, Su M, Zhang H, Chen W-H, Zou C-L. Predictive value of lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in patients with oesophageal cancer undergoing concurrent chemoradiotherapy. BMC Cancer. 2019;19(1):1004. doi:10.1186/s12885-019-6157-431655563
- Lee SE, Lee JH, Ryu KW, et al. Preoperative plasma fibrinogen level is a useful predictor of adjacent organ involvement in patients with advanced gastric cancer. J Gastric Cancer. 2012;12(2):81–87. doi:10.5230/jgc.2012.12.2.8122792520
- Arigami T, Uenosono Y, Matsushita D, et al. Combined fibrinogen concentration and neutrophil-lymphocyte ratio as a prognostic marker of gastric cancer. Oncol Lett. 2016;11(2):1537–1544. doi:10.3892/ol.2015.404926893776
- Perisanidis C, Psyrri A, Cohen EE, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):960–970. doi:10.1016/j.ctrv.2015.10.00226604093
- Wang Y-Y, Liu Z-Z, Xu D, Liu M, Wang K, Xing B-C. Fibrinogen-albumin ratio index (FARI): a more promising inflammation-based prognostic marker for patients undergoing hepatectomy for colorectal liver metastases. Ann Surg Oncol. 2019;26(11):3682–3692. doi:10.1245/s10434-019-07586-331264117
- Zou YX, Qiao J, Zhu HY, et al. Albumin-to-fibrinogen ratio as an independent prognostic parameter in untreated chronic lymphocytic leukemia: a retrospective study of 191 cases. Cancer Res Treat. 2019;51(2):664–671.30064197
- Li SQ, Jiang YH, Lin J, et al. Albumin-to-fibrinogen ratio as a promising biomarker to predict clinical outcome of non-small cell lung cancer individuals. Cancer Med. 2018;7(4):1221–1231. doi:10.1002/cam4.142829533009