Abstract
The aim of this review is to analyze the latest trends in the management of non-vestibular skull base and intracranial schwannomas in order to optimize tumor control and quality of life. Non-vestibular cranial nerve schwannomas are rare lesions, representing 5–10% of cranial nerve schwannomas. Management decisions should be individualized depending on tumor size, location and associated functional deficits. Generally, large sized schwannomas exerting significant mass effect with increased intracranial pressure are treated surgically. In some cases, even after optimal skull base resection, it is not possible to achieve a gross total resection because tumor location and extent and/or to reduce morbidity. Thus, subtotal resection followed by stereotactic radiosurgery or fractioned radiotherapy offers an alternative approach. In certain cases, stereotactic radiosurgery or radiotherapy alone achieves good tumor control rates and less morbidity to gross total resection. Finally, given the slow growth rate of most of these tumors, observation with periodic radiographic follow-up approach is also a reasonable alternative for small tumors with few, if any, symptoms.
Introduction
Schwannomas are primary peripheral nervous system tumors arising from Schwann cells. Schwann cells function to myelinate peripheral nerves beginning at the Obersteiner-Redlich zone, the transition from the central to the peripheral nervous system.Citation1 They may originate from any peripheral, cranial or autonomic nerve. Schwannomas are usually solitary neoplasms; multifocal presentation should raise the suspicion for neurofibromatosis type 1 and type 2 or schwannomatosis.Citation2
Sporadic non-vestibular cranial nerve schwannomas (NVCNS) are rare lesions, representing 5–10% of cranial nerve schwannomas.Citation3 Nerve sheath tumors account for 8% of primary intracranial tumors and more than 90% of intracranial nerve sheath tumors are vestibular schwannomas (VS).Citation4,Citation5 In descending frequency, NVCNS involve the trigeminal nerve, lower CNs, the facial nerve and lastly the oculomotor nerves.
NVCNS are characterized by slow growth. It has been estimated that over 50% of the NVCNS exhibit an annual growth rate of more than 5% of the initial volume. This ratio is higher than in sporadic vestibular schwannomas, but lower than in patients with neurofibromatosis type 2 (NF2).Citation6 Schwannomas usually remain benign, however there is a possibility of malignant transformation mainly in the setting of neurofibromatosis type 1 (NF1).Citation5,Citation7,Citation8
The morbidity associated with skull base and intracranial schwannomas and their treatment has decreased with the improvement of open surgical approaches to the skull base and with the development of endoscopic skull base surgery. However, the morbidity of resection is still relatively high depending on the size and location of the tumor.
The purpose of this review is to discuss the latest trends in the management of these lesions in order to optimize disease control and quality of life.
Trigeminal Schwannomas (TS)
Epidemiology
TS are a rare entity, accounting for 0.07–1% of all intracranial tumors. However, these tumors are the second most common intracranial schwannomas, after VS, and account for 3–9% of intracranial schwannomas.Citation9–Citation11 While most TS develop in the Gasserian ganglion within the middle fossa, TS can occur anywhere along the course of the trigeminal root, ganglion, and peripheral branches. Malignant TS are rare. Only 22 cases of histologically malignant TS have been reported in the literature.Citation12 A review of 514 patients surgically treated for TS reported malignancy in 1.5% of them.Citation9
Classification Systems
Several classification systems of TS have been employed, some of them are similar. The original classification of these tumors by Jefferson includes type A (mainly in the middle fossa), type B (mainly in the posterior fossa) and type C (dumbbell type, in both the middle and posterior fossae).Citation13 Type D was added later and includes those arising from one of the three divisions of the trigeminal nerve.Citation12,Citation14,Citation15 In a review of 455 cases the following incidence was observed: 40% type A, 22% type B, 32% type C and 6% type D.Citation9 A more complex classification into 6 different types has been proposed by Yoshida and Kawase:Citation16 type P (posterior fossa tumor); type M (middle fossa tumor); type E (extracranial tumor). The other 3 types refer to dumbbell type tumors in multiple fossae: type MP (middle and posterior fossae); type ME (middle fossa and extracranial space); type MPE (posterior fossa, middle fossa, and extracranial space). Employing this system, Yoshida and Kawase reported the location of 429 tumors: type M 38.5%; type P 23.5%; type E 5.4%; type MP 28.3%; type ME 3.5%; and type MPE 0.7%.Citation16 In summary, TS arising in the middle fossa are the most common.
Symptoms and Signs
Trigeminal nerve dysfunction is seen in most patients. The most common trigeminal nerve symptoms are pain, paresthesia, numbness and decreased motor function. Symptoms and signs are reflected in .Citation9–12,Citation15–Citation20 However, it has been reported that up to 18% of tumors can be found incidentally with magnetic resonance (MR) imaging performed for other reasons.Citation16
Table 1 Main Features of Non-Vestibular Intracranial Schwannomas
Management
Surgical resection is the mainstay of management, although the therapeutic modality used should be tailored individually according to tumor location and size, histology, patient comorbidities and symptoms.Citation21
describes the main open approaches.Citation11,Citation12,Citation18,Citation19,Citation22,Citation23 Lateral approaches via temporal craniotomy provide the shortest corridor to the Meckel´s cave.Citation12 Tumors originating intracranially from the second (V2) or third (V3) division of the trigeminal nerve are better approached through a subtemporal preauricular infratemporal fossa approach ().Citation22,Citation23 In tumors with a large extracranial component and anterior and inferior growth into the maxillary sinus, a facial translocation approach may be indicated ().Citation24,Citation25 Transnasal endoscopic access to type A TS offers advantages similar to open skull base approaches in terms that there is no need for temporal lobe exposure or retraction with either technique, and both provide direct access to the tumor (). Endoscopic approaches may provide a minimally invasive and safe approach to radically resect selected tumors.
Figure 1 Surgical approaches in trigeminal schwannomas: subtemporal-preauricular and transcochlear. (A) Type ME2 TS with massive involvement of the middle cranial fossa and the infratemporal fossa (*). (B) Postoperative CT showing the resection of the tumor through a subtemporal-preauricular approach. (C) T1-weighted MRI of a Type P TS in the posterior cranial fossa (*). (D) T1-weighted MRI with contrast showing total resection of the tumor through a transcochlear approach. The operative cavity is filled by fat (*).
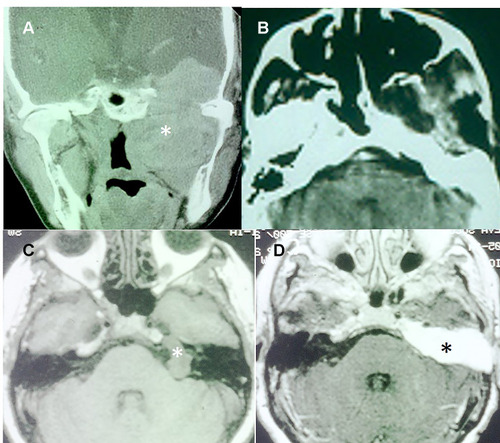
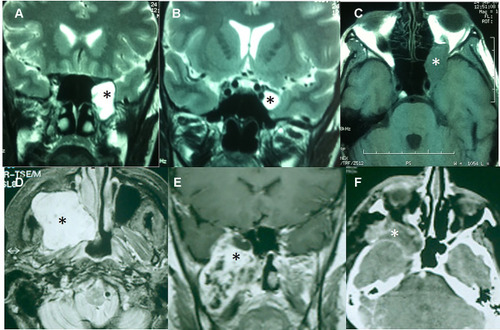
Figure 2 Surgical approaches in trigeminal schwannomas: EEA and facial translocation approach. (A) T2-weighted MRI of a Type ME1 TS. The tumor is in contact with the transition between ethmoidal cells and the sphenoid sinus (*). (B) Tumor into the Meckel’s cave (*). (C) Orbital progression in a T1-weighted MRI (*). This tumor was excised through an EEA. (D) T1-weighted MRI with contrast of an extensive dumbbell-shaped tumor (*) Type ME2 involving the middle and infratemporal fossae as well as the maxillary sinus. (E) T1-weighted MRI with contrast of a TS with a similar pattern of extension (*). (F) Postoperative CT scan of the anterior case showing the removal of the tumor. The operative cavity was obliterated with a temporalis muscle flap (*).
Although the use of transpetrosal approaches for type B TS is under debate, the retrosigmoid approach is usually employed for posterior fossa tumors ().Citation11,Citation12,Citation18 In dumbbell shaped tumors (type C) with both posterior and middle fossa extension, in addition to a subtemporal approach a pre- or retrosigmoid craniectomy may provide additional exposure to resect.Citation11,Citation18
Schwannomas frequently do not invade nerve fibers but compress them. However, the nerve fibers are frequently adherent to the tumor.Citation15 MRI sequences will soon allow preoperative determination of whether the trigeminal nerve fibers are medial or lateral to the tumor. Transcranial approaches might then be chosen for tumors that displace the trigeminal nerve medially while endonasal techniques may be selected for tumors displacing the nerve laterally.Citation26
The treatment aims of TS focus on improvement in neurologic symptoms, relief of mass effect, and preservation of cranial nerve function. After the application of skull base techniques, gross total resection (GTR) has been achieved in 69–87%. Near GTR (4–26% of patients) or subtotal tumor resection (STR) (7–21% of patients) are performed when there are difficulties dissecting tumor from cranial nerves, blood vessels, or the brainstem ().Citation11,Citation12,Citation15,Citation18,Citation19,Citation27 An improvement in facial pain post-operatively is seen between 45% and 100% of cases;Citation12,Citation15,Citation18,Citation19 trigeminal hypesthesia improved in 16% to 62%.Citation12,Citation15,Citation18,Citation19 Temporalis and pterygoid function will rarely, if ever, fully improve when present pre-operatively.Citation18,Citation19 Other cranial nerve deficits, such as diplopia caused by palsy of the abducens nerve, improved in 44–75% of patients,Citation12,Citation17–Citation19 However surgical injury to the abducens nerve (8%), trochlear nerve (3.7%), and oculomotor nerve (1.9%) has been reported.Citation28 Long tract and cerebellar signs disappeared in 78–100%.Citation15,Citation17–Citation19 Severe surgical complications are rare (<2% of cases) and include meningitis, cerebrospinal fluid (CSF) leak, arterial vasospasm, hematoma, and hydrocephalus.Citation9,Citation11,Citation18,Citation19 The overall mortality rates of surgical series reported since 1990 range from 0 to 3%.Citation11,Citation12,Citation19,Citation27,Citation29,Citation30 The sensory and motor outcomes of endoscopic approaches compare favorably with open surgical approaches. However, some patients may develop dry eye, as a result of vidian nerve sacrifice.Citation31
Table 2 Main Surgical Series of Intracranial Schwannomas
Stereotactic radiosurgery (SRS) is an alternative to surgery. Most of series report a tumor control rate greater than 80%, which is somewhat lower than that achieved with vestibular schwannomas.Citation3,Citation26,Citation32–Citation34 The incidence of tumor regression ranges from 34% to 87% and tumor stability from 8% to 50%.Citation32–Citation37 A significant symptomatic improvement following SRS varies from 31% to 72% across the series, whereas the incidence of symptomatic deterioration varies from 0% to 27%.Citation9 The factors affecting outcomes include tumor size and site, radiation dose and the radiological appearance of the mass (solid or cystic). The optimal tumor marginal dose appears to be 13–16 Gy. SRS is associated with a very low risk of a new neurologic deficit or other type of complications (less than 16% of patients) ().Citation32–Citation37 There has been speculation regarding the risk of malignancy after treatment with SRS. With a median follow-up of 8.1 years, 2 of 3251 (0.0006%) patients with benign tumors treated with SRS were diagnosed with suspected malignant transformation and 1 of 4905 (0.0002%) patients was considered a case of radiosurgery-associated intracranial malignancy, resulting in an incidence of 6.87 per 100,000 patient-years for malignant transformation and 2.26 per 100,000 patient-years for radiosurgery-associated intracranial malignancy, similar to the risk of developing a malignant CNS tumor in the general population.Citation38
Table 3 Main Stereotactic Radiosurgery Series of Intracranial Schwannomas
Small lesions with few, if any, symptoms may be managed with watchful waiting. Makarenko et al observed 8 patients with TS; an 11.1% progressed over a median of 7.1 years.Citation39
Lower Cranial Nerves Schwannomas (LCNS)
Epidemiology
Among NVCNS, LCNScomprise the second most common site after TS. LCNS can originate intracranially or in the cervical region. Intracranial LCNS usually extend to the jugular foramen or the hypoglossal canal. Jugular foramen schwannomas arising from cranial nerves IX, X or XI are rare and comprise 2.9% to 4% of all intracranial schwannomas, and represent 10–30% of all tumors observed around the jugular foramen.Citation40,Citation41 In a review of 204 patients, the tumor originated from the glossopharyngeal nerve (IX) in 47 cases (23.6%), in 26 cases (13%) from the vagal nerve (X) and in 11 cases (5.5%) from the accessory nerve (XI). In the remaining 58% of the cases, the origin of the tumor remained unknown.Citation40 Hypoglossal schwannomas (HS) constitute only about 1% of all intracranial schwannomas.Citation42
Classification
Pellet et al classified jugular foramen schwannomas into 4 classes.Citation43 Type A (primarily intracranial): minimal extension into the jugular foramen; type B (primarily within the bone): with or without an intracranial component; type C (primarily extracranial): only a minor extension into the jugular foramen or into the posterior fossa; and type D (dumbbell-shaped): intra- and extracranial extension. According to Pellet’s classification, type Dschwannomas are the most common (40–68%), followed by type A (25–32%), type B (15–22%) and type C (12–15%).Citation40,Citation44
HS have been classified by Kaye et alCitation45 in type A: intradural tumor; type B: dumbbell-shaped tumor; and type C: extracranial tumor. In 2 reviews of the literature consisting of 94 and 160 cases, the following distribution has been reported: type A, 29–32%; type B, 39–50%; and type C, 21–29%.Citation42,Citation46
Symptoms and Signs
Hearing impairment is the most frequent presenting symptom in intracranial and jugular foramen LCNS.Citation40,Citation41,Citation43,Citation47 Most of patients show a mid-frequency hearing loss, in contrast to the high-frequency hearing loss observed with VS.Citation47 Type A tumors may cause symptoms related to increased intracranial pressure, without or with minimal deficits of the lower cranial nerves, whereas a tumor expanding into the jugular foramen or upper cervical region might cause earlier damage of the nerves.Citation41 Other presenting symptoms are shown in .
HS are characterized by tongue atrophy. Lower cranial nerves are affected in up to 50% of the cases, mainly in tumors with extension to the jugular foramen.Citation45,Citation46
Management
Schwannomas generally arise in nerves with a sensory component and are associated with sensory ganglia. In the case of the vagal nerve, tumors are likely to involve sensory nerves and it is possible to preserve their function with an intracapsular enucleation, which can increase the neural function preservation by more than 30%.Citation7,Citation48 Continuous vagal nerve monitoring during surgery is a new strategy developed with the aim of reducing postoperative morbidity.Citation49
summarizes the main surgical approaches used in LCNS resection according to their location.Citation40,Citation50–Citation52 For tumors confined intracranially (type A), a retrosigmoid approach may provide adequate exposure (). In tumors centered in the jugular foramen (type B) or with extracranial extension the modified infratemporal fossa approach type A (ITFA) provides the greatest exposure of the jugular foramen, carotid artery, and anterior temporal bone and it allows direct access to the posterior fossa ( and ).Citation51,Citation52 Other skull base techniques such as far lateral, transcondylar, and supracondylar approaches have been advocated for the surgical excision of HS ().Citation41,Citation45,Citation46
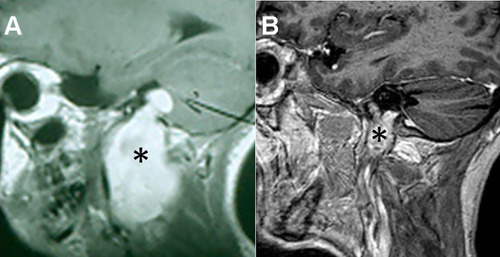
Figure 3 Surgical approaches in lower cranial nerve schwannomas. (A) T1-weighted MRI with contrast of a Type A cystic vagal schwannoma (*). (B) T1-weighted MRI with contrast of a Type A vagal schwannoma (*). (C) Retrosigmoid approach of the last case showing the tumor (1) and the cochleo-vestibular nerve (2). (D) Coronal T2-weighted MRI delimiting a Type B lower cranial nerve schwannoma in the jugular fossa (*). (E) Axial T1-weighted MRI with contrast of the same tumor (*). (F) Modified infratemporal fossa approach type (A) internal carotid artery (1), third portion of the facial nerve (2), tumor in the jugular fossa (3).
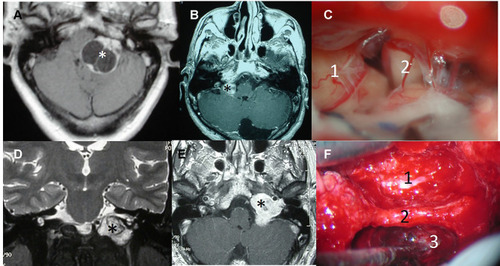
Figure 4 Lower cranial nerve schwannomas with intra- and extracranial extension. (A) T1-weighted MRI with contrast of a Type D vagal schwannoma (*). (B) T1-weighted MRI with contrast of a Type B hypoglossal schwannoma (*).
BakarCitation40 reviewed the outcomes obtained after the surgical treatment of 204 patients. He reported a GTR in 86.9% of the patients, near GTR in 3.3% of the patients and STR in 9.8% of the patients ().Citation40 Park et alCitation44 analyzed the results of surgery in 275 cases collected in large series of jugular foramen schwannomas and found postsurgical lower cranial nerve palsies in 34.9% of the patients. New neurological deficits are common, especially involving the facial nerve, which was reported in 6–34% of casesCitation40,Citation43,Citation47 Other reported complications include CSF leak (3–7%), aspiration pneumonia (1.5–6%), venous sinus thrombosis (3%), and meningitis (2%). Postoperative mortality ranges between 0.5% and 5%.Citation40,Citation46,Citation53
Near GTR followed by SRS or SRS alone offers an alternative approach in some patients ( and ). There are limited data based largely on extracranial schwannomas that fractionated external beam radiotherapy (RT) using doses of approximately 50 Gy at 1.8 Gy per fraction results in a high likelihood of local control.Citation54 RT usually induces cessation of lesion growth or reduction in size, rather than complete disappearance of the tumor, which is the goal of the surgical intervention. Side effects are negligible when doses are 50 Gy or less and modern precision RT techniques are used. This is additional option for patients requiring treatment who are unsuitable for surgery.
Table 4 Radical Resection versus More Conservative Management
Observation is another option in asymptomatic patients, particularly for those who are elderly and/or infirm.
Facial Nerve Schwannomas (FNS)
Classification
About 650 cases of FNS have been reported in the literature.Citation55 The site of origin can be anywhere along the course of the facial nerve from the glial-Schwann cell transition site at the cerebellopontine angle to an extracranial peripheral nerve location. They have been classified based on their site of origin into cerebellopontine angle (CPA), labyrinthine, geniculate, tympanic, mastoid, and parotid segments ().Citation56 The percentage involving each segment in a review of 438 cases was reported by Cornelius et al ().Citation55
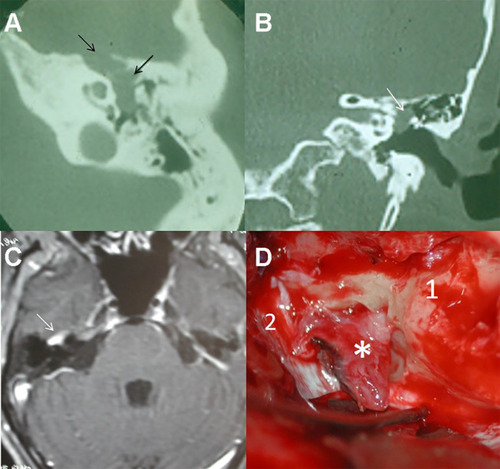
Figure 5 Facial nerve schwannomas. (A) The CT scan shows a tumor involving the tympanic and geniculate segments of the facial nerve (arrows), with displacement of the ossicular chain. (B) Small tumor limited to the tympanic segment (arrow). (C) T1-weighted MRI with contrast of a facial nerve schwannoma of the geniculate ganglion (arrow). (D) Transtemporal approach. Geniculate ganglion schwannoma (*), petrous pyramid (1), dura (2).
It is imperative to include FNS in the differential diagnosis of VS during preoperative planning and counseling. However, this is often difficult to achieve pre-operatively, even with a careful evaluation of the MRI and computed tomography (CT). FNS involving the CPA and/or the IAC segment of the FN cannot be differentiated from a VS on imaging unless the tumor extends to the labyrinthine segment of the FN. Erosion of superior part of the internal auditory canal and eccentricity of tumor in relation to porus acousticus are not reliable signs. However, facial paralysis is a sign that would support the origin in the facial nerve since VS presenting with facial paralysis is rare.
Symptoms and Signs
The growth rate of FNS is slow. Thus, in a series of 13 patients treated expectantly with a mean follow-up period of 5 years, only 4 demonstrated tumor growth based on serial MRs. The average annual growth rate was 1.4 mm/year.Citation57
Patients frequently present with facial nerve weakness [House-Brackmann (HB) III or IV], as well as hearing loss.Citation58–Citation60 Tumors located at CPA usually present with sensorineural hearing loss (SNHL) and tinnitus instead of facial paresis. Patients with tumors originating from the labyrinthine segment tend to present with slowly progressive facial paresis and SNHL, whereas patients with tumors of the tympanic and mastoid segments show progressive facial paresis and conductive hearing loss. The distribution of presenting symptoms in 438 patients with FNS is depicted in .Citation55
Management
If the patient is asymptomatic and the tumor is small, conservative management with careful observation and follow-up with serial MRI are recommended. SRS may be an option for small and symptomatic tumors with good facial function (HB I or II). If the tumor is larger (more than 3 cm) or the patient has facial palsy (HB III or worse), surgical resection should be considered.Citation61 Alternatively, fractionated RT is an option if the anticipated morbidity of surgery is significant. The surgical approach depends on the tumor location and extent. The retrosigmoid approach permits access to the nerve from the brainstem to the IAC and allows resection of large tumors in the CPA. The translabyrinthine approach gives access to the entire nerve course from the brainstem to the stylomastoid foramen. This approach may only be chosen if hearing is not serviceable or if tumor volume or inner ear erosion makes hearing preservation impossible. A middle cranial fossa approach allows access to the nerve between IAC and the proximal tympanic segment. Combining this approach with a transmastoid approach the entire nerve from the IAC to the stylomastoid foramen can be exposed. Hearing preservation is possible by employing this route that make if possible, to repair ossicular chain defects.Citation55
There are different options to preserve the facial nerve function: nerve conservation, nerve resection with immediate grafting, or hypoglossal-facial nerve anastomosis.Citation59 Nerve preservation has been reported in 58–71% of the patients.Citation55–Citation57 However, facial nerve function was, in the majority of cases, a HB grade III, depending on surgical strategy ().Citation58,Citation60 Regardless of the type of facial nerve repair, patients can expect no better than an eventual HB grade III palsy.Citation58,Citation59
SRS achieves a good tumor control rates.Citation62,Citation63 Thus, a multi-institutional study reported 42 patients treated with SRS, with partial regression in 23 patients (54.7%) and stability in 19 patients (45.2%) (). The actuarial 5-year progression-free survival rate was 92%.Citation63
Oculomotor Nerve Schwannomas (OMNS)
Classification
There are only 54 cases of isolated OMNS reported in indexed literature.Citation64 The transition of the oculomotor nerve from the central to the peripheral nervous system occurs 0.6 mm distal to the brainstem. OMNS develop distal to this transition zone. OMNS have been divided into 4 types based on their location: cisternal, cavernous, cisterno-cavernous, and orbito-cavernous ().Citation65,Citation66
Symptoms and Signs
The clinical features depend on the location of the tumor with cisternal schwannomas showing isolated deficits of the nerve. When these tumors originate into the cavernous sinus, they can present with either a cavernous sinus syndrome or an orbital apex syndrome. Symptoms can vary from incomplete oculomotor palsy and exophthalmos to brainstem compression signs.Citation65 A systematic review has summarized the most frequent symptoms ().Citation64
Management
Treatment for OMNS remains multimodal, depending on clinical presentation, tumor size, histology, and location. A careful preoperative evaluation including MR guides in distinguishing different histologies. Although most tumors arising from the oculomotor nerve are well encapsulated schwannomas and, in a few cases, nerve sparing surgery may be possible, neurofibromas have also been described. Neurofibromas tend to invade the entire nerve fibers and always require the complete sacrifice of the nerve.Citation67
Often microsurgery remains the treatment of choice for the GTR of cisternal schwannomas after identification of the nerve of origin. However, when tumors are located in the cisterno-cavernous area, total removal of tumor and identification of the parent nerve is barely achieved in half of the cases and a combined approach with STR and SRS should be employed.Citation68 Tumors with small-to-medium sized volumes are preferably treated by SRS with a high rate of tumor control and stabilization and/or improvement of symptoms.Citation65
Functional recovery of the oculomotor nerve after STR is successful in less than 10% of the patients. Since the oculomotor nerve innervates multiple ocular muscles, the functional result after nerve anastomosis may be limited, and aberrant regeneration may interfere with coordinated binocular movements.Citation64
Trochlear Nerve Schwannomas (TNS)
Classification
A total of 85 cases reporting on the management of TNS have been published.Citation66 Three types of TNS have been described: cisternal type, cisterno-cavernous type, and the cavernous type. In more than half of the cases reported so far, the cisternal type was diagnosed.Citation65,Citation66
Symptoms and Signs
According to a systematic review from Torun et alCitation66 76% of the entire cohort presented with diplopia, which was the solitary symptom in over half of the cases, and 29% of cases presented with more severe symptoms, such as paresis, sensory changes, ataxia, and other cranial nerve palsies.Citation66
Management
All symptomatic patients with a lesion exerting significant mass effect should undergo decompression. The most widely employed microsurgical approach has been the subtemporal transtentorial approach. Nevertheless, nearly 80% of those with surgical resection had persistent trochlear nerve palsy at the last follow-up.
SRS is a good option for patients presented with diplopia only, and 75% of the patients show improved symptoms after therapy.Citation66
Patients who only have stable ocular misalignment due to trochlear nerve palsy can also be managed with prism glasses to alleviate diplopia or may choose to undergo strabismus surgery, combined with observation and serial MR imaging.Citation66
Abducens Nerve Schwannomas (ANS)
Classification
ANS form an extremely rare entity with only 33 surgical cases published to dateCitation69 and 16 cases treated by SRS.Citation65 The most common locations are shown in .Citation69,Citation70
Symptoms and Signs
The cardinal neurological sign of ANS is diplopia in the horizontal gaze. However, patients may be asymptomatic or present with headache, proptosis, other oculomotor and/or trigeminal signs, and signs of increased intracranial pressure.Citation65 Involvement of other cranial nerves has been noted in over half the patients.Citation69
Management
Surgical resection is indicated in symptomatic patients with neurological deficits, and most importantly to relieve any mass compression of surrounding structures.Citation69 Extra-cavernous extension involving the orbital apex, superior orbital fissure and petroclival junction limits complete resectability. GTR with graft reconstruction of the abducens nerve has been recommended,Citation69 but gross involvement of the cavernous sinus make more convenient to achieve a STR in order to avoid additional neurological damage. A frontotemporal transcavernous approach has been suggested for cavernous sinus and parasellar lesions, whereas the retrosigmoid approach is indicated for tumors involving the prepontine region.Citation71 In a systematic analysis, Sun et alCitation69 have reported abducens nerve recovery in less than half of the patients (45%). Tumor extension to the cavernous sinus was significantly associated with a lower likelihood of postsurgical recovery.
SRS (single dose of 12.0–12.5 Gy) is recommended for small and moderate size tumors. Tumor volume was reported to remain stable in 18%, reduced in 37%, and increased in 12% of the patients, respectively (not reported in 31%), treated with gamma knife SRS.Citation65
Olfactory Groove Schwannomas (OGS)
Histogenesis
Thus far, only 46 cases with an OGS have been reported.Citation8 Schwannomas of the olfactory bulb are uncommon as the olfactory nerve is unsheathed by Schwann cells. There have been various hypotheses proposed to explain the origin of OGS: the developmental hypothesis suggesting transformation of mesenchymal pial cells into ectodermal Schwann cells, and the non-developmental theory stating that these schwannomas may arise from Schwann cells of adjacent normal structures, such as Schwann cell hyperplasia occurring within the perivascular nerve plexuses.Citation59,Citation72 In contrast, neural crest cells have the ability to transform to other cells in the line or acquire abilities from some of these.Citation73
Symptoms and management
In most reported cases of OGS, the chief complaints were headache, vomiting, seizure, diplopia, hyposmia or anosmia ().Citation8,Citation59
Surgery, either transfrontal or endoscopic, is the preferred treatment for olfactory schwannoma. GTR done by careful extra-arachnoidal dissection is possible as the tumor is well encapsulated and a plane of cleavage from olfactory tract is often present thus allowing preservation.Citation59
Optic Nerve Schwannomas (ONS)
Histogenesis
Schwannomas partially located in the optic canal with an orbital and/or cisternal component are rare with only few cases reported, but those confined entirely within the optic canal are much rarer. As the optic nerve is in fact a part of the central nervous system, it is myelinated by central nervous system oligodendrocytes rather than Schwann cells.Citation1,Citation74 ONS originate from ectopic Schwann cells around the optic nerve or from the sympathetic nerves around the optic nerve in the orbit, probably originating from peripheral sympathetic nerves innervating the central retinal artery or the carotid artery.Citation1,Citation74
The microscopic appearance of schwannomas of the optic nerve is the same as for all schwannomas of peripheral nerve origin, thus distinguishing them from similar appearing lesions, such as optic nerve gliomas or meningiomas. Optic nerve sheath schwannomas probably cannot be diagnosed on clinical grounds alone, and their neuroimaging appearance mimics that of the more common optic nerve gliomas and the diagnosis is usually made at surgery.Citation75
Symptoms and Management
Clinical presentation of lesions arising in the optic nerve is nonspecific and ranges from insidious proptosis, severe visual acuity disturbance and/or visual field defect, retro-orbital pain and headaches, to in rare cases blindness. Since the optic canal is a narrow bony structure with a mean diameter of 4 mm, the optic nerve can be compressed by even a tiny tumor.
The tumor can be approached extradurally by drilling the anterior clinoid and unroofing the optic canal. Orbital tumors may be safely and effectively resected via a various number of approaches. Currently, a transorbital endoscopic approach alone or combined with an expanded endoscopic endonasal approach is proposed as a method to improve visualization and maximize instruments’ maneuverability. Nevertheless, traditional external approaches, with different skin or trans-conjunctival incisions, represent an effective and sound surgical option for the management of these lesions.Citation74,Citation76 SRS would be not be indicated because of the risk of optic neuropathy associated with a single large fraction of irradiation. Patients unsuitable for surgery may be considered for fractionated proton irradiation to provide a high likelihood of tumor control while minimizing the dose to the retina.
Discussion
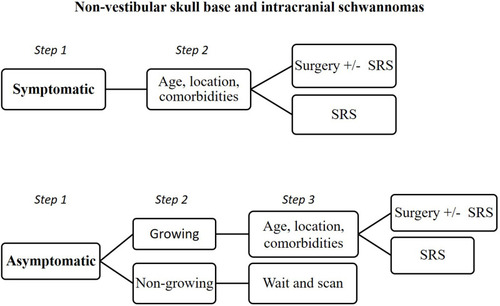
In the era of personalized medicine, trends in NVCNS management are giving way to a more individualized treatment plan based on the combination of surgery, radiation therapy and/or observation.
Because NVCNS are benign tumors, complete surgical excision while avoiding functional and aesthetic sequelae, is a primary objective. Despite the progress made in surgical approaches, resection is still associated with some morbidity in certain circumstances. The treatment goals for NVCNS are heavily dependent on the tumor presentation, location, histology and size.Citation67 In addition, patient characteristics such as age and comorbidities must be taken into account. Not all patients should be operated, and surgery would not be an option if the risks are greater than those of a non-surgical treatment or observation. When dealing with tumors involving critical structures (i.e.: cavernous sinus, optic nerve, jugular fossa, etc.), whose damage has a significant impact on quality of life, management decisions must be individualized. Although some authors have reported improvement of symptoms with GTR, in most cases the presenting symptoms persisted or worsened after surgical resection.Citation31
In older and in asymptomatic patients with small tumors, when imaging studies and examination strongly suggest a benign tumor, close surveillance may be considered even without histological evidence, to avoid the risks inherent in biopsy or surgical resection. Likewise, asymptomatic patients with slow-growing small tumors should be managed with caution because they are at high risk of cranial nerve damage if operated on. In these cases, surgery should be avoided if it is expected to cause high morbidity. Gradual loss of nerve function is usually better tolerated than sudden loss as a result of surgery, so observation or deferral of surgery is recommended in patients without neurological alterations.
Safer treatment options have been proposed in some cases. RT has been used as either a primary or an adjunct treatment for NVCNS and offers the advantages of a high tumor control rate and a low rate of radiation associated complications. In contrast to surgical resection, injuries to the brain, vascular, or other cranial nerves are unlikely. SRS may offer therapeutic advantages over conventional RT, particularly logistically because it employs one fraction as opposed to 5 to 6 weeks of daily fractionated RT. Additionally, a tighter margin is employed with SRS so that less normal tissue is irradiated. Disadvantages of SRS are that the risk of a neurologic deficit may be higher due to the large dose per fraction and the risk of a marginal miss may be increased.
Patients with small- to moderate-sized tumors with intact cranial nerve function are optimal candidates for SRS. Furthermore, symptoms related to cranial nerve dysfunction may improve after SRS. In addition, patients with previous contralateral neurological deficits of the lower cranial nerves may not be suitable for surgery because of the risk that bilateral deficits will cause problems with swallowing and laryngeal mobility. In these cases, SRS may be useful. However, it is important to note that sometimes small-sized and slow-growing tumors are very symptomatic, and the symptoms are refractory to medical treatment (i.e., TNS refractory to carbamazepine). In these cases, surgical treatment is safer than follow-up or SRS. RT has been also used for patients who present large tumors or tumors affecting critical areas with high surgical morbidity. Residual tumor after surgery should be treated and SRS or RT are good options with acceptable morbidity.
When it is not possible to achieve a GTR because of technical operative difficulties or when surgery causes significant morbidity, STR or near GTR followed by SRS is gaining acceptance. Concerning TS, Pan et alCitation36 reported that tumor growth control in 56 patients treated by using SRS, was obtained in 93% of the patients (including patients who had previously undergone STR). Cranial nerve preservation in patients with LCNS extending to the jugular foramen is challenging. Lower cranial neuropathies can severely affect quality of life requiring tracheostomy or gastrostomy. Park et alCitation44 reported on 13 patients with LCNS treated with GTR and 9 patients treated by STR followed by SRS. In the latter group, 4 patients (44%) showed a reduction in tumor size and 5 patients (56%) showed no evidence of tumor progression. Postoperative CN deficits were higher in the GTR group (23 vs. 4 CNs affected, respectively). In addition, patients treated by STR and SRS showed a statistically significant improvement in the level of dysphagia. Sedney et alCitation77 compared the outcomes of 53 LCNS patients who underwent a GTR with 28 patients receiving a more conservative resection. There was a statistically significant decrease in permanent deficits of CN IX/X with a conservative technique, whereas the recurrence rate was similar in both series (). The satisfactory clinical results obtained support that an appropriate strategy in the management of some LCNS is surgical resection to reduce tumor size followed by SRS in situations where GTR would likely cause significant morbidity.Citation78–Citation81 We believe that this alternative is potentially safe and effective and should be considered.
The best timing for surgery in FNS is controversial: it depends on facial nerve function, hearing, size, intracranial mass effect and patient choice. If hearing remains good and there is no threat from intracranial mass effect, observation until HB III is possible and radical tumor excision may be performed then. The reason is that even for good preoperative facial function (HB I–II) and regardless of the grafting technique, no better postoperative result than HB-III can be expected, although it is possible to preserve the nerve in approximately 25% of cases if the tumor is located eccentrically on the nerve.Citation61 Liu and FaganCitation82 analyzed a series of 22 patients with FNS, of whom 12 underwent definitive excision and 10 were managed more conservatively. The best postoperative facial function in the group where the tumor was removal was a HB-III, while in the group treated conservatively, 8 had normal facial function. The role of “wait and see” approach has been evaluated by McMonagle et alCitation83 in a series of 53 patients with FNS where 20 patients were managed conservatively and 33 underwent surgery. There was a GTR in 21 cases and near GTR was obtained in 12 cases. HB grade remained stable in all patients in whom no removal or STR was undertaken, while it worsened in 55% of patients receiving GTR. They conclude that observation is preferred until facial function deteriorates to a HB-III (). Another alternative is RT to avoid facial nerve deterioration and have a high likelihood of tumor control. Wilkinson et alCitation84 reported a series of patients with FNS; through 1995, 85% of cases had surgical resection and none had observation only. Of the patients seen after 1995, 27% had surgical resection and grafting, 33% had bony decompression, 29% were managed with observation alone, and 11% had RT. Facial nerve grade was maintained or improved over the follow-up period in 78.9% of the decompression group and 100% of the observation and RT groups, compared to 54.8% of the resection group (P < 0.012). The trend is towards conservative management of these tumors.
Conclusion
NVCNS are rare tumors with a diversity of clinical manifestations, depending on the location and cranial nerve of origin. Management decisions are based on tumor size and functional deficits. The treatment of choice is total surgical resection, but SRS or RT are alternatives in those cases where surgical resection would be associated with substantial morbidity. Given the slow growth rate of most of these tumors, observation is also an alternative in asymptomatic patients ().
Disclosure
Johannes A Langendijk reports non-financial support from RaySearch, grants, personal fees, and consultancy fees paid to UMCG Research BV from IBA, and that the Department of Radiation Oncology has research collaboration with IBA, RaySearch, Elekta, Siemens, and VisionRT, outside the submitted work. The authors report no conflicts of interest in this work.
Acknowledgment
This article was written by members and invitees of the International Head and Neck Scientific Group (www.IHNSG.com).
References
- Ramey WL, Arnold SJ, Chiu A, Lemole MA. Rare case of optic nerve schwannoma: case report and review of the literature. Cureus. 2015;7(4):e265. doi:10.7759/cureus.26526180689
- Evans DG, Bowers NL, Tobi S, et al. Schwannomatosis: a genetic and epidemiological study. J Neurol Neurosurg Psychiatry. 2018;89(11):1215–1219. doi:10.1136/jnnp-2018-31853829909380
- D’Astous M, Ho AL, Pendharkar A, et al. Stereotactic radiosurgery for non-vestibular cranial nerve schwanommas. J Neurooncol. 2017;131(1):177–183. doi:10.1007/s11060-016-2286-727752881
- Colreavy MP, Lacy PD, Hughes J, et al. Head and neck schwannomas – a 10 year review. J Laryngol Otol. 2000;114(2):119–124. doi:10.1258/002221500190505810748827
- Guerrissi JO. Solitary benign schwannomas in major nerve systems of the head and neck. J Craniofac Surg. 2009;20(3):957–961. doi:10.1097/SCS.0b013e3181a14cbc19461342
- O’Reilly B, Mehanna H, Kishore A, Crowther JA. Growth rate of non-vestibular intracranial schwannomas. Clin Otolaryngol Allied Sci. 2004;29(1):94–97. doi:10.1111/j.1365-2273.2004.00770.x14961859
- Bondi S, Limardo P, Toma S, Bussi M. Non-vestibular head and neck schwannomas: a 10-year experience. Eur Arch Otorhinolaryngol. 2013;270(8):2365–2369. doi:10.1007/s00405-013-2520-223644938
- Wang B, Yuan J, Chen X, Xu H, Zhou Y, Dong P. Extracranial non-vestibular head and neck schwannomas. Saudi Med J. 2015;36(11):1363–1366. doi:10.15537/smj.2015.11.1231426593174
- MacNally SP, Rutherford SA, Ramsden RT, Evans DG, King AT. Trigeminal schwannomas. Br J Neurosurg. 2008;22(6):729–738. doi:10.1080/0268869080227217219085355
- Samii M, Migliori MM, Tatagiba M, Babu R. Surgical treatment of trigeminal schwannomas. J Neurosurg. 1995;82(5):711–718. doi:10.3171/jns.1995.82.5.07117714594
- Liu XD, Xu QW, Che XM, Yang DL. Trigeminal neurinomas: clinical features and surgical experience in 84 patients. Neurosurg Rev. 2009;32(4):435–444. doi:10.1007/s10143-009-0210-819633876
- Wanibuchi M, Fukushima T, Zomordi AR, Nonaka Y, Friedman AH. Trigeminal schwannomas: skull base approaches and operative results in 105 patients. Neurosurgery. 2012;70(1Suppl Operative):132–143. doi:10.1227/NEU.0b013e31822efb2121796003
- Jefferson G. The trigeminal neurinomas with some remarks on malignant invasion of the gasserian ganglion. Clin Neurosurg. 1953;1(1):11–54. doi:10.1093/neurosurgery/1.CN_suppl_1.1114379464
- Day JD, Fukushima T. The surgical management of trigeminal neuromas. Neurosurgery. 1998;42(2):233–240. doi:10.1097/00006123-199802000-000159482173
- Yang L, Hu L, Zhao W, Zhang H, Liu Q, Wang D. Endoscopic endonasal approach for trigeminal schwannomas: our experience of 39 patients in 10 years. Eur Arch Otorhinolaryngol. 2018;275(3):735–741. doi:10.1007/s00405-018-4871-129350272
- Yoshida K, Kawase T. Trigeminal neuromas extending into multiple fossae: surgical methods and review of the literature. J Neurosurg. 1999;91(2):202–211. doi:10.3171/jns.1999.91.2.020210433308
- Al-Mefty O, Ayoubi S, Gaber E. Trigeminal schwannomas: removal of dumbbell-shaped tumors through the expanded meckel cave and outcomes of cranial nerve function. J Neurosurg. 2002;96(3):453–463. doi:10.3171/jns.2002.96.3.045311883829
- Chen LF, Yang Y, Yu XG, et al. Operative management of trigeminal neuromas: an analysis of a surgical experience with 55 cases. Acta Neurochir (Wien). 2014;156(6):1105–1114. doi:10.1007/s00701-014-2051-724633987
- Goel A, Muzumdar D, Raman C. Trigeminal neuroma: analysis of surgical experience with 73 cases. Neurosurgery. 2003;52(4):783–790. doi:10.1227/01.NEU.0000053365.05795.0312657173
- Ramina R, Mattei TA, Sória MG, et al. Surgical management of trigeminal schwannomas. Neurosurg Focus. 2008;25(6):E6. doi:10.3171/FOC.2008.25.12.E6
- Dolenc VV. Frontotemporal epidural approach to trigeminal neurinomas. Acta Neurochir (Wien). 1994;130(1–4):55–65. doi:10.1007/BF014055037725943
- Sekhar LN, Schramm VL Jr, Jones NF. Subtemporal-preauricular infratemporal fossa approach to large lateral and posterior cranial base neoplasms. J Neurosurg. 1987;67(4):488–499. doi:10.3171/jns.1987.67.4.04883655886
- Llorente JL, Nazar G, Cabanillas R, Fernández de León R, Suárez C. Subtemporal-preauricular approach in the management of infratemporal and nasopharyngeal tumors. J Otolaryngol. 2006;35(3):173–179.16929993
- Janecka IP, Sen CN, Sekhar LN, Arriaga M. Facial translocation: a new approach to the cranial base. Otolaryngol Head Neck Surg. 1990;103(3):413–419. doi:10.1177/0194599890103003122122371
- Suárez C, Llorente JL, Muñoz C, García LA, Rodrigo JP. Facial translocation approach in the management of nasopharyngeal and sinonasal tumors. Laryngoscope. 2004;114(6):1047–1051. doi:10.1097/00005537-200406000-0001715179211
- Niranjan A, Barnett S, Anand V, Agazzi S. Multimodality management of trigeminal schwannomas. J Neurol Surg B Skull Base. 2016;77(4):371–378. doi:10.1055/s-0036-158113827441164
- Konovalov AN, Spallone A, Mukhamedjanov DJ, Tcherekajev VA, Makhmudov UB. Trigeminal neuromas: a series of 111 surgical cases from a single institution. Acta Neurochir. 1996;138(9):1027–1035. doi:10.1007/BF014123048911538
- Raza SM, Donaldson AM, Mehta A, Tsiouris AJ, Anand VK, Schwartz TH. Surgical management of trigeminal schwannomas: defining the role for endoscopic endonasal approaches. Neurosurg Focus. 2014;37(4):E17. doi:10.3171/2014.7.FOCUS14341
- Jeong SK, Lee EJ, Hue YH, Cho YH, Kim JH, Kim CJ. A suggestion of modified classification of trigeminal schwannomas according to location, shape, and extension. Brain Tumor Res Treat. 2014;2(2):62–68. doi:10.14791/btrt.2014.2.2.6225408927
- Sharma BS, Ahmad FU, Chandra PS, Mahapatra AK. Trigeminal schwannomas: experience with 68 cases. J Clin Neurosci. 2008;15(7):738–743. doi:10.1016/j.jocn.2006.09.00718396403
- Shin SS, Gardner PA, Stefko ST, Madhok R, Fernandez-Miranda JC, Snyderman CH. Endoscopic endonasal approach for non-vestibular schwannomas. Neurosurgery. 2011;69(5):1046–1057. doi:10.1227/NEU.0b013e3182287bb921673609
- Elsharkawy M, Xu Z, Schlesinger D, Sheehan JP. Gamma knife surgery for nonvestibular schwannomas: radiological and clinical outcomes. J Neurosurg. 2012;116(1):66–72. doi:10.3171/2011.8.JNS1121521962159
- Peker S, Bayrakli F, Kiliç T, Pamir MN. Gamma-knife radiosurgery in the treatment of trigeminal schwannomas. Acta Neurochir (Wien). 2007;149(11):1133–1137. doi:10.1007/s00701-007-1285-917728994
- Ryu J, Lee SH, Choi SK, Lim YJ. Gamma knife radiosurgery for trigeminal schwannoma: a 20-year experience with long-term treatment outcome. J Neurooncol. 2018;140(1):89–97. doi:10.1007/s11060-018-2934-129931615
- Snyder MH, Shepard MJ, Chen CJ, Sheehan JP. Stereotactic radiosurgery for trigeminal schwannomas: a 28-year single-center experience and review of the literature. World Neurosurg. 2018;119:e874–e881. doi:10.1016/j.wneu.2018.07.28930099184
- Pan L, Wang EM, Zhang N, et al. Long-term results of leksell gamma knife surgery for trigeminal schwannomas. J Neurosurg. 2005;102:220–224. doi:10.3171/sup.2005.102.s_supplement.022015662814
- Sun J, Zhang J, Yu X, et al. Stereotactic radiosurgery for trigeminal schwannoma: a clinical retrospective study in 52 cases. Stereotact Funct Neurosurg. 2013;91(4):236–242. doi:10.1159/00034525823548989
- Wolf A, Naylor K, Tam M, et al. Risk of radiation-associated intracranial malignancy after stereotactic radiosurgery: a retrospective, multicentre, cohort study. Lancet Oncol. 2019;20(1):159–164. doi:10.1016/S1470-2045(18)30659-430473468
- Makarenko S, Ye V, Akagami R. Natural history, multimodal management, and quality of life outcomes of trigeminal schwannomas. J Neurol Surg B Skull Base. 2018;79(6):586–592. doi:10.1055/s-0038-165150330456029
- Bakar B. The jugular foramen schwannomas: review of the large surgical series. J Korean Neurosurg Soc. 2008;44(5):285–294. doi:10.3340/jkns.2008.44.5.28519119464
- Chibbaro S, Mirone G, Makiese O, Bresson D, George B. Dumbbell-shaped jugular foramen schwannomas: surgical management, outcome and complications on a series of 16 patients. Neurosurg Rev. 2009;32(2):151–159. doi:10.1007/s10143-009-0188-219189142
- Nonaka Y, Grossi PM, Bulsara KR, Taniguchi RM, Friedman AH, Fukushima T. Microsurgical management of hypoglossal schwannomas over 3 decades: a modified grading scale to guide surgical approach. Neurosurgery. 2011;69(2Suppl Operative):121–140.
- Pellet W, Cannoni M, Pech A. The widened transcochlear approach to jugular foramen tumors. J Neurosurg. 1988;69(6):887–894. doi:10.3171/jns.1988.69.6.08873193194
- Park ES, Lee EJ, Park JB, et al. A single-institution retrospective study of jugular foramen schwannoma. management: radical resection versus subtotal intracranial resection through a retrosigmoid suboccipital approach followed by radiosurgery. World Neurosurg. 2016;88:552–562. doi:10.1016/j.wneu.2015.10.04226520430
- Kaye AH, Hahn JF, Kinney SE, Hardy RW Jr, Bay JW. Jugular foramen schwannomas. J Neurosurg. 1984;60(5):1045–1053. doi:10.3171/jns.1984.60.5.10456716139
- Bindal S, El Ahmadieh TY, Plitt A, et al. Hypoglossal schwannomas: a systematic review of the literature. J Clin Neurosci. 2019;62:162–173. doi:10.1016/j.jocn.2018.11.03730472335
- Vorasubin N, Sang UH, Mafee M, Nguyen QT. Glossopharyngeal schwannomas: a 100 year review. Laryngoscope. 2009;119(1):26–35. doi:10.1002/lary.2004519117318
- Behuria S, Rout TK, Pattanayak S. Diagnosis and management of schwannomas originating from the cervical vagus nerve. Ann R Coll Surg Eng. 2015;97(2):92–97. doi:10.1308/003588414X14055925058355
- Sandler ML, Sims JR, Sinclair C, et al. Vagal schwannomas of the head and neck: a comprehensive review and a novel approach to preserving vocal cord innervation and function. Head Neck. 2019;41(7):2450–2466. doi:10.1002/hed.2575830957342
- Sanna M, Mazzoni A, Saleh EA, Taibah AK, Russo A. Lateral approaches to the median skull base through the petrous bone: the system of the modified transcochlear approach. J Laryngol Otol. 1994;108(12):1036–1044. doi:10.1017/S00222151001288417861077
- Fisch U. Infratemporal fossa approach to tumours of the temporal bone and base of the skull. J Laryngol Otol. 1978;92(11):949–967. doi:10.1017/S0022215100086382213516
- Suárez C, Sevilla MA, Llorente JL. Temporal paragangliomas. Eur Arch Otorhinolaryngol. 2007;264(7):719–731. doi:10.1007/s00405-007-0267-317333230
- Bulsara KR, Sameshima T, Friedman AH, Fukushima T. Microsurgical management of 53 jugular foramen schwannomas: lessons learned incorporated into a modified grading system. J Neurosurg. 2008;109(5):794–803. doi:10.3171/JNS/2008/109/11/079418976067
- Kharod SM, Herman MP, Amdur RJ, Mendenhall WM. Fractionated radiation therapy for benign nonacoustic schwannomas. Am J Clin Oncol. 2018;41(1):13–17. doi:10.1097/COC.000000000000021926270440
- Cornelius JF, Sauvaget E, Huy PT, George B. Surgical treatment of facial nerve schwannomas. Prog Neurol Surg. 2008;21:119–130.18810209
- Sherman JD, Dagnew E, Pensak ML, van Loveren HR, Tew JM Jr. Facial nerve neuromas: report of 10 cases and review of the literature. Neurosurgery. 2002;50(3):450–456. doi:10.1097/00006123-200203000-0000411841711
- Perez R, Chen JM, Nedzelski JM. Intratemporal facial nerve schwannoma: a management dilemma. Otol Neurotol. 2005;26(1):121–126. doi:10.1097/00129492-200501000-0002215699732
- Lahlou G, Nguyen Y, Russo FY, Ferrary E, Sterkers O, Bernardeschi D. Intratemporal facial nerve schwannoma: clinical presentation and management. Eur Arch Otorhinolaryngol. 2016;273(11):3497–3504. doi:10.1007/s00405-015-3850-z26676873
- Deora H, Srinivas D, Beniwal M, Vikas V, Rao KVLN, Somanna S. Rare cranial nerve schwannomas: a retrospective review of nontrigeminal, nonvestibular cranial nerve schwannomas. J Neurosci Rural Pract. 2018;9(2):258–263. doi:10.4103/jnrp.jnrp_469_1729725180
- Lu R, Li S, Zhang L, Li Y, Sun Q. Stripping surgery in intratemporal facial nerve schwannomas with poor facial nerve function. Am J Otolaryngol. 2015;36(3):338–341. doi:10.1016/j.amjoto.2014.12.00325659624
- Xu F, Pan S, Alonso F, Dekker SE, Bambakidis NC. Intracranial facial nerve schwannomas: current management and review of literature. World Neurosurg. 2017;100:444–449. doi:10.1016/j.wneu.2016.09.08227693767
- Hasegawa T, Kato T, Kida Y, et al. Gamma knife surgery for patients with facial nerve schwannomas: a multiinstitutional retrospective study in Japan. J Neurosurg. 2016;124(2):403–410. doi:10.3171/2015.3.JNS14267726361275
- Kida Y, Yoshimoto M, Hasegawa T. Radiosurgery for facial schwannoma. J Neurosurg. 2007;106(1):24–29. doi:10.3171/jns.2007.106.1.2417236484
- Furtado SV, Hegde AS. Management of oculomotor nerve schwannomas in two different locations: surgical nuances and comprehensive review. Neurosurg Rev. 2012;35(1):27–34. doi:10.1007/s10143-011-0344-321789570
- Peciu-Florianu I, Tuleasca C, Comps JN, et al. Radiosurgery in trochlear and abducens nerve schwannomas: case series and systematic review. Acta Neurochir (Wien). 2017;159(12):2409–2418. doi:10.1007/s00701-017-3348-029022157
- Torun N, Laviv Y, Jazi KK, et al. Schwannoma of the trochlear nerve -an illustrated case series and a systematic review of management. Neurosurg Rev. 2018;41(3):699–711. doi:10.1007/s10143-016-0783-y27586875
- Matano F, Di Russo P, Okano A, et al. Oculomotor neurofibroma: a different histology implying an unsatisfying clinical outcome. World Neurosurg. 2020;139:31–38. doi:10.1016/j.wneu.2020.03.19132289509
- Celli P, Ferrante L, Acqui M, Mastronardi L, Fortuna A, Palma L. Neurinoma of the third, fourth, and sixth cranial nerves: a survey and report of a new fourth nerve case. Surg Neurol. 1992;38(3):216–224. doi:10.1016/0090-3019(92)90172-J1440207
- Sun H, Sharma K, Kalakoti P, et al. Factors associated with abducens nerve recovery in patients undergoing surgical resection of sixth nerve schwannoma: a systematic review and case illustration. World Neurosurg. 2017;104:883–899. doi:10.1016/j.wneu.2017.04.14628465275
- Iida Y, Sakata K, Kobayashi N, Tatezuki J, Manaka H, Kawasaki T. Orbital abducens nerve schwannoma: a case report and review of the literature. NMC Case Rep J. 2016;3(4):107–109. doi:10.2176/nmccrj.cr.2015-025928664009
- Tung H, Chen T, Weiss MH. Sixth nerve schwannomas. Report of two cases. J Neurosurg. 1991;75(4):638–641. doi:10.3171/jns.1991.75.4.06381885983
- Sunaryo PL, Svider PF, Husain Q, Choudhry OJ, Eloy JA, Liu JK. Schwannomas of the sinonasal tract and anterior skull base: a systematic review of 94 cases. Am J Rhinol Allergy. 2014;28(1):39–49. doi:10.2500/ajra.2014.28.397824717879
- Mikkelsen LH, Heegaard S. Mucosal melanoma In: Riker A, editor. Melanoma. Switzerland: Springer International Publishing; 2018:253–272.
- Miyamura S, Yamaguchi S, Takeda M, et al. Pure intra-optic canal schwannoma: report of two cases. Asian J Neurosurg. 2017;12(4):797–800. doi:10.4103/1793-5482.18507129114316
- Miller N. Primary tumours of the optic nerve and its sheath. Eye. 2004;18(11):1026–1037. doi:10.1038/sj.eye.670159215534587
- Locatelli D, Dallan I, Castelnuovo P. Surgery around the orbit: how to select an approach. J Neurol Surg B Skull Base. 2020;81(4):409–421. doi:10.1055/s-0040-171389333072481
- Sedney CL, Nonaka Y, Bulsara KR, Fukushima T. Microsurgical management of jugular foramen schwannomas. Neurosurgery. 2013;72(1):42–46. doi:10.1227/NEU.0b013e3182770e7423096422
- Suri A, Bansal S, Sharma BS, et al. Management of hypoglossal schwannomas: single institutional experience of 14 cases. J Neurol Surg B Skull Base. 2014;75(3):159–164. doi:10.1055/s-0033-135692425072009
- Kano H, Meola A, Yang HC, et al. Stereotactic radiosurgery for jugular foramen schwannomas: an international multicenter study. J Neurosurg. 2018;129(4):928–936. doi:10.3171/2017.5.JNS16289429125412
- Hasegawa T, Kato T, Kida Y, et al. Gamma knife surgery for patients with jugular foramen schwannomas: a multiinstitutional retrospective study in Japan. J Neurosurg. 2016;125(4):822–831. doi:10.3171/2015.8.JNS15115626799304
- Martin JJ, Kondziolka D, Flickinger JC, Mathieu D, Niranjan A, Lunsford LD. Cranial nerve preservation and outcomes after stereotactic radiosurgery for jugular foramen schwannomas. Neurosurgery. 2007;61(1):76–81. doi:10.1227/01.neu.0000279726.90650.6d17621021
- Liu R, Fagan P. Facial nerve schwannoma: surgical excision versus conservative management. Ann Otol Rhinol Laryngol. 2001;110(11):1025–1029. doi:10.1177/00034894011100110611713912
- McMonagle B, Al-Sanosi A, Croxson G, Fagan P. Facial schwannoma: results of a large case series and review. J Laryngol Otol. 2008;122(11):1139–1150. doi:10.1017/S002221510700066718177538
- Wilkinson EP, Hoa M, Slattery WH 3rd, et al. Evolution in the management of facial nerve schwannoma. Laryngoscope. 2011;121(10):2065–2074. doi:10.1002/lary.2214121898431