Abstract
Purpose
Based on a multi-centered and a large sample size, this study aims to analyze the relationship between preoperative and postoperative serum CEA and recurrence of rectal cancer without preoperative therapy.
Methods
This retrospective cohort study enrolled stage I to III rectal cancer patients without preoperative therapy (N = 1,022) who received radical resection of rectal cancer from 2 hospitals in China. Based on the preoperative and postoperative serum carcinoembryonic antigen, the patients were subdivided into 3 groups ie, normal preoperative CEA (≤5.0 ng/mL, N = 627), elevated preoperative (>5.0 ng/mL) but normalized postoperative CEA (normalized postoperative CEA, N = 255), as well as elevated preoperative and postoperative CEA (elevated postoperative CEA, N = 67). The generalized additive model was used to assess the relationship between carcinoembryonic antigen and the risk of recurrence. Further, the Cox regression model was used to evaluate the relationship between carcinoembryonic antigen and 3-year recurrence-free survival (RFS) after adjusting for potential confounders.
Results
The 3-year RFS of patients with elevated postoperative CEA was 45.8% (95% CI, 35.2% −59.5%), which was significantly lower compared to the other two groups of patients (normalized postoperative CEA: 75.9%, 95% CI, 70.8%-81.4%; and normal preoperative CEA: 84.9%, 95% CI, 82.2%-87.8%) (P <0.001). Based on multivariable Cox model analysis, the elevated postoperative CEA was a prognostic factor for 3 years RFS (hazard ratio [HR], 3.08; 95% CI, 2.05–4.66; P<0.001). At the same time, normalized postoperative CEA was insignificantly correlated with 3-year RFS (HR, 1.38; 95% CI, 1.00–1.92; P = 0.05) and was not an independent risk factor.
Conclusion
We found that preoperative and postoperative serum CEA of rectal cancer patients were related to the 3-year recurrence-free survival rate. Moreover, the risk of recurrence in the normalized postoperative CEA group of patients was insignificantly different from that of the normalized preoperative CEA patients. Therefore, it is necessary to combine preoperative and postoperative CEA to predict the prognosis of patients with rectal cancer, rather than using it alone.
Introduction
In the past decade, the incidence and mortality rates of colorectal cancer have been surging and proved to be an increasingly heavy health burden in China.Citation1 Notably, early diagnosis and multiple treatments improve the survival rate of colorectal cancer.Citation2 A standard therapy for colorectal cancer, whether in using adjuvant chemotherapy or not after surgery, depends on the stage of the tumor.Citation3,Citation4 Nonetheless, TNM staging does not provide complete prognostic information. Besides, in similar tumor staging of patients, clinical results might exhibit significant differences.Citation5 Therefore, there is a desperate and urgent need for a novel prognostic indicator to assess the risks of rectal cancer recurrence and develop an individualized treatment therapies, including intensive local or systemic therapy for patients with high recovery rates.
Carcinoembryonic antigen (CEA) is the most widely used tumor marker in colorectal cancer.Citation6 The National Comprehensive Cancer Network proposes measuring CEA, a marker of rectal cancer, before the operation of patients with non-metastatic colorectal cancer.Citation3,Citation4,Citation7 Its guidelines recommend the measurement of CEA before the operation of patients diagnosed with colon cancer.Citation3,Citation7 Notably, an increase of CEA before surgery and normalization after the surgery does not mean a poor prognosis for colon cancer. Of note, patients with increased postoperative CEA are under a significantly higher risk of metastasis and recurrence of colon cancer, particularly in the first year post-surgery.Citation5 Thus, carcinoembryonic antigen has been used in the diagnosis of colon cancer before and after surgery as well as a prognostic biomarker. Analysis using the statewide cancer registry shows that the preoperative CEA level predicts the survival and prognosis of patients with colorectal cancer, which is unrelated to the stage of the tumor at diagnosis.Citation8 In contrast with the pathological staging of the specimen, CEA measurement before surgery is a useful prognostic tool for colorectal cancer.Citation9 An analysis of 145 patients with colorectal cancer was performed to investigate the correlation between plasma CEA levels before surgery with the times and sites of disease recurrence. Notably, CEA is a significant factor for stratification after Dukes/Kirklin C resection.Citation10 For colorectal cancer, CEA measurement before surgery is recommended if it helps in staging and surgical planning. It should be measured at intervals of every 3 months for at least 3 years if the patient exhibits a higher risk of recurrence and metastasis after surgery. CEA is by far the preferred tumor marker for monitoring systemic response to metastatic disease.Citation7 After a standard adjuvant therapy, low-level postoperative CEA suggested better survival outcomes for stage II colon cancer patients.Citation11 Regardless of the baseline in CEA secretion status, Pet-ct scanning is a valuable method for detecting the recurrence of CRC patients with elevated CEA levels during follow-up. The possibility of recurrence is proportional to the value of increased CEA levels.Citation12 Abnormally elevated postoperative and preoperative CEA indicators were independent predictors of survival and recurrence, respectively. They help in postoperative monitoring or cancer prognosis in patients diagnosed with colorectal cancer (CRC).Citation13
Therefore, the impact of preoperative and postoperative CEA on the clinical outcome remains unclear in rectal cancer. As such, we analyzed the correlation between the combination of preoperative and postoperative CEA as well as the recurrence and metastasis in a large, multi-centered, stage I–III rectal cancer patients cohorts in China. Moreover, a long-term follow-up of the patients was conducted.
Methods
This study was reviewed and approved by the Institutional Review Board of Yunnan Cancer Hospital and the Sixth Hospital of Sun Yat-Sen University. Due to the retrospective nature of this research, there was no requirement for informed consent from the patients. The data of the patients in the survey was kept confidential. This research was complied with the Declaration of Helsinki.
Patients
Between January 2010 and July 2016, a retrospective analysis of 1,022 patients with stage I to III rectal cancer pathologically diagnosed was conducted in Yunnan Cancer Hospital and the Sixth Affiliated Hospital of Sun Yat-Sen University. Among them, 73 cases of postoperative CEA were missing. shows the experimental design flow chart of this study, as well as the inclusion and exclusion criteria of patients.
Definition and Grouping of Preoperative and Postoperative Serum Carcinoembryonic Antigen
The standard definition of preoperative CEA is the baseline CEA value of the most recent test before surgery, while postoperative CEA is the first detection value closest to the time of operation or the final CEA value before starting adjuvant chemotherapy. If the CEA test value for more than 12 weeks is missing, the study will not be conducted. Based on the status of CEA, patients were subdivided into the following categories; (1) patients with preoperative normal CEA (preoperative normal group); (2) patients with elevated CEA before surgery but normalized postoperative group of CEA (Normalized postoperative CEA); (3) patients with elevated CEA levels of preoperative and postoperative (elevated postoperative group). All CEA measurements were made by use of a chemiluminescence immunoassay using the COBAS 800 e602 immunoassay analyzer (Roche Diagnostics, Tokyo, Japan) at Yunnan Cancer Hospital, and Alinity i immunoassay analyzer (Abbott Diagnostics, Chicago, USA) at the Sixth Affiliated Hospital of Sun Yat-sen University, following World Health Organization standard methods (code 73/601).Citation14 The reference range of CEA was between 0.0 and 5.0 ng/mL. The value higher than 5.0 ng/mL was considered an increase in CEA value, whereas the value less than 5.0 ng/mL was considered normal.
Adjuvant Chemotherapy Protocol
Partial patient with stage II–III CRC received the adjuvant chemotherapy according to the National Comprehensive Cancer Network (NCCN) clinical practice guidelines in the CRC.Citation3,Citation4 Adjuvant chemotherapy protocol included FOLFOX, CapeOX, Capecitabine, or 5-FU/leucovorin.Citation15
Surveillance Protocol
The clinical evaluation of the patient included serum CEA detection level, physical examination, imaging examination (CT/MRI), and colonoscopy biopsy. The CEA level was measured at intervals of 3 to 6 months in 3 years. Imaging examinations were performed at least every 12 months and at least 3 years, including the chest of the patient, abdomen, and pelvis plain scan as well as contrast enhancement. Colonoscopy was conducted once a year after the operation and repeated at intervals of every 3 years. A shorter monitoring time for late adenoma recurrence was revealed. All cases of recurrence and metastasis were confirmed by colonoscopy, pathological tissue examination, or imaging examination.
Outcomes
This study combined the levels of preoperative and postoperative CEA to predict and evaluate the possibility and value of disease recurrence in rectal cancer patients subjected to radical surgery. Notably, the recurrence-free survival time refers to the duration between surgery and recurrence, metastasis, as well as the death of the patient. If the patient is lost to follow-up, the recurrence-free survival time will be calculated based on the last follow-up date. Each enrolled patient was completely followed up for 3 years, while those less than 3 years were not enrolled in the study. In total, 1,022 patients were followed up for more than 3 years and met the enrolment requirements, out of which 224 had recurrence and metastasis, with a recurrence rate of 21.92%. The median follow-up was 50.15 months.
Statistical Analysis
SPSS23.0 software was applied for all statistical analyses. Mann Whitney U-test was used to compare continuous variables, while the χ2 test was used to compare categorical variables. Moreover, the Kaplan-Meier method was used to analyze the three-year recurrence-free survival (RFS) of patients for survival analysis over time. The difference in RFS was evaluated by the Log rank test (Log rank test) single-factor analysis. Cox equal proportional hazard regression model was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) of different CEA groups. In univariate analysis, variables with P values less than 0.05 were included in the final Cox multivariate model. Based on CEA levels status, the patients were divided into 3 groups, and in the reference range of 99% confidence interval (CI), rather than 95% CI for the repeat analyses.Citation5
COX proportional hazard regression model evaluated the relationship of CEA levels with a 3 year RFS rates and calculated the HR and 95% CI. According to the recommendation of the STROBE statement,Citation14 3 models were built including, (1) unadjusted; (2) adjusted for demographics; and (3) adjusted for demographics plus clinicopathological characteristics. Furthermore, to test the robustness of these results, the HR changes were compared among the 3 models based on the unadjusted model.Citation15 Through univariate and multivariate Cox regression analysis, the relationship of all variables was evaluated with RFS to compare CEA levels with the performance of predicting clinicopathological parameters of rectal cancer RFS.Citation16
Results
The Relationship Between Preoperative and Postoperative Serum CEA Grouping and Clinical Pathological Characteristics of Patients
This study retrospectively collected 1,487 stage I–III rectal cancer patients pathologically diagnosed and excluded distant metastases from two research institutions (Yunnan Cancer Hospital and the Sixth Affiliated Hospital of Sun Yat-sen University) between 2010 and 2016. Every patient was subjected to radical resection of rectal cancer. After excluding 465 patients, 1022 patients treated by radical surgical resection were eventually enrolled (820 in Yunnan Cancer Hospital and 202 in the Sixth Affiliated Hospital of Sun Yat-Sen University) including, (572 [66.0%] male; median [IQR] age, 55 [21–89] years) with stage I to III rectal cancer. Out of 1,022, 395 had elevated preoperative CEA while 627 had normal preoperative CEA. Among the 395 patients with preoperative CEA elevation, 73 cases had missing postoperative CEA data, 255 cases had normal postoperative CEA, while 67 cases had postoperative CEA elevation. ().
Table S1 shows the clinical and demographic pathological characteristics of the 3 groups of patients with normal preoperative, elevated preoperative CEA or normal postoperative CEA, including preoperative CEA, postoperative CEA, tumor differentiation, T stage, N stage, AJCC 7th edition staging, lymphovascular invasion, perineural invasion, tumor deposit, and adjuvant chemotherapy. A total of 224 patients experienced tumor recurrence or metastasis. No relationship was observed between the site of initial recurrence and preoperative and postoperative serum CEA except other sites included peritoneum, lymph node, ovary, bone, and kidney (P=0.002) (Table S2).
Kaplan–Meier Analysis of Different Preoperative and Postoperative Serum CEA Groups
The 3-year RFS rate of 627 normal preoperative CEA patients was 84.9% (95% CI, 82.2%-87.8%). On the other hand, the 3-year RFS rate of 395 elevated preoperative CEA patients was 72.5% (95% CI, 68.2%-77.1%). The difference of the 2 groups was statistically significant (HR, 1.98; 95% CI, 1.50–2.61; P <0.0001) ().
Figure 2 Recurrence-free survival by preoperative and postoperative CEA levels. (A) Patients with normal vs elevated preoperative CEA. (B) Patients with normal vs elevated postoperative CEA. (C) Patients with normal preoperative, normalized postoperative, or elevated postoperative CEA.
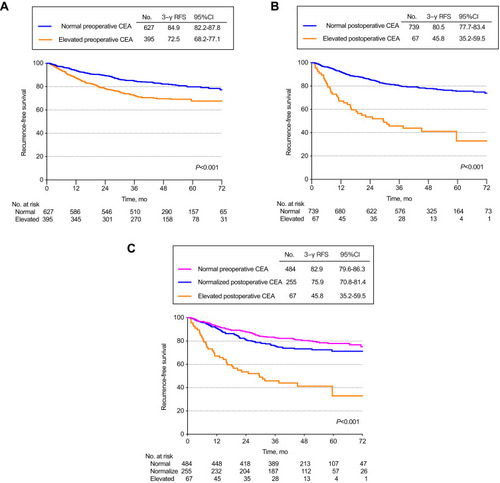
The 3-year RFS was 45.8% (95%CI, 35.2%-59.5%) for the 67 patients whose CEA levels remained elevated after surgery compared to 82.3% (95%CI, 79.8%-84.9%) for the 882 patients with either normal preoperative CEA (n = 627) or normalized postoperative CEA (n = 255) (HR, 4.23; 95% CI, 2.94–6.09; P<0.0001) ().
On the contrary, the 3-year RFS rate of the 67 patients with persistently elevated CEA after surgery was 45.8% (95%CI, 35.2%-59.5%), which was significantly lower compared to that of the other 2 groups. The 3-year RFS for the 255 patients with normalized postoperative CEA was 75.9% (95%CI, 70.8% −81.4%), which was statistically indistinguishable from the 3-year RFS in the 627 patients with normal preoperative CEA levels 84.9% (95%CI, 82.2%-87.8%). (Elevated postoperative CEA Vs normalized postoperative CEA: HR, 1.68; 95% CI, 1.22–2.33; P=0.0016 and normalized postoperative CEA vs normal preoperative CEA: HR, 5.04; 95% CI, 3.43–7.42; P<0.0001) (Overall log-rank P <0.001) (). Similar results can be obtained with the CEA cut-off value of 10.0 ng/mL (eFigure 1).
Association of Rectal Cancer Recurrence with the Overall Population
Table S3 shows the association of the CEA level of preoperative and postoperative serum and 3year RFS.
Association of Rectal Cancer Recurrence with Subpopulations
eFigure 2 shows the subgroup analysis results.
eFigure 2 shows the results of the study of CEA in the N-stage subpopulation. Patients included 3 cohorts (normal preoperative, normalized postoperative, and elevated postoperative CEA) in phase N0 (eFigure 2A) or N1 (eFigure 2B in Supplementary information) or N2 (eFigure 2C) There were significant differences in RFS. Among N stage rectal cancer patients, the RFS of the normalized postoperative CEA group was significantly higher compared to that of the elevated postoperative CEA group, but the prognosis was similar to that of the normal preoperative CEA group (overall log-rank P<0.05).
Multivariate Analyses of All Variables
shows the univariate and multivariate analysis of influencing factors related to RFS. A univariate analysis of 3-year RFS concluded that the preoperative increase in CEA and the postoperative normal group were correlated with 3-year RFS (P value=0.002). After adjusting the confounding factors, multiple COX models were included. There was no correlation between the normal group and 3-year RFS after operation (HR, 1.38; 95% CI, 1.00–1.92; P=0.05)
Table 1 Univariate and Multivariate Analyses of 3-Year Recurrence-Free Survival
Discussion
Our research findings confirmed that postoperative carcinoembryonic antigen (CEA) elevation is an independent risk prognostic factor for rectal cancer patients which exhibits an equivalent prognostic value to the classical tumor stage for a 3-year recurrence. The risk of recurrence and metastasis in the normalized postoperative CEA group of patients had no significant difference from that of the normalized preoperative CEA patients. Notably, a large-scale cohort study that provides evidence for the prognostic value of preoperative and postoperative CEA in rectal cancer patients is lacking. Our findings indicate that preoperative and postoperative serum CEA constitutes the prognostic biomarker for rectal cancer as described in previous studies.Citation5–Citation13 In the existing guidelines, no evidence shows CEA as a predictor of adjuvant chemotherapy.Citation3,Citation4,Citation7 This work determined that whether the CEA cut-off value is 5.0 ng/mL or 10.0 ng/mL is the CEA cut-off value, so it can be judged that CEA is increasing or decreasing. We found that the normal preoperative CEA group had the best prognosis, followed by the normalized postoperative CEA group, while the worst group was the elevated postoperative CEA group. Of note, there were statistical differences between the 3 groups. P<0.001. After adjusting the confounding factors, multiple COX models were included. There was no correlation between the normalized postoperative and 3-year RFS (HR, 1.38; 95% CI, 1.00–1.92; P=0.05), which means there may be other factors lead to differences in the survival curves of these three groups of patients. As such, the detection of CEA in rectal cancer patients after surgery is of fundamental significance.
Further, we systematically reviewed articles published between 2005 and July 2019, and have carried on the database query, and focused on the clinical outcomes of CEA in CRC. Precisely, it was found that most of these studies only assessed the effects of preoperative or postoperative CEA on the prognosis, whereas only one study narrowed on the trend of preoperative and postoperative CEA in patients with colon cancer.Citation5 Nevertheless, whether this result exists in patients with rectal cancer warrants further investigation. Thus, this study was designed to improve the preoperative and postoperative monitoring approaches of patients as well as provide novel prevention and treatment strategies for colorectal cancer. Additionally, we confirm that rectal cancer patients with rising postoperative CEA are at higher risk of recurrence. These findings have fundamental clinical implications. Firstly, our research design enabled the subdivision of all patients into 3 categories, unlike previous study designs where preoperative and postoperative CEA in CRC was only divided into 2 groups.Citation11,Citation13 Of note, the postoperative CEA clearance rate was a useful prognostic determinant. After surgery, a randomized pattern of CEA clearance patients should be considered to exhibit a continuing source of CEA and necessitates consideration on strengthening follow-up or adjuvant therapy.Citation19 COX multivariate proportional hazard model analysis demonstrated that postoperative pathological examinations showed positive lymph nodes and higher serum CEA levels (≥5ng/mL) were independent risk factors for rectal cancer after radical surgery. Therefore, for high preoperative CEA levels CRC patients, postoperative CEA levels can be an independent risk factor in predicting the prognosis. It is thus necessary to strengthen the follow-up and adjuvant therapy, even after therapeutic resection.Citation20 The serum CEA level before surgery of 2,093 patients with CRC was measured. In previous studies, there was no significant correlation between preoperative serum CEA elevation and local recurrence. Nevertheless, a significant correlation was found between preoperative CEA elevation and systemic recurrence. The five-year disease-free survival rate of normal preoperative CEA levels patients was distinctly higher compared to that of elevated preoperative CEA levels patients (P<0.01).Citation21
In a study of colorectal cancer, the patients were divided into 4 groups based on their CEA levels. The study concluded that the normalized postoperative CEA group exhibited the lowest recurrence rate of 22.8%. Besides, fluctuating levels of CEA influenced postoperative outcomes.Citation22 Previous studies revealed no significant difference in the recurrence rate of colorectal cancer after tumor resection or overall survival rate (imaging HR). This was among patients treated in high-intensity and low-intensity imaging examinations or CEA test monitoring facilities, respectively. Monitoring the intensity of patients treated by stage I, II, and III CRC treatment exhibits no significant relationship with the detection of recurrence.Citation23 For stage II and III resectable colorectal cancer patients, postoperative CEA monitoring, combined with CT scan and colonoscopy for every 3 to 6 months must be performed to monitor recurrence. Nevertheless, CEA is an unsuitable screening test for colon cancer due to its low specificity. The higher the CEA level before and after colon cancer resection, the worse the prognosis. Patients after radical resection of colon cancer with CEA levels above 5 ng/mL or continuous detection levels were associated with cancer recurrence.Citation24 For patients with negative preoperative CEA, when the postoperative cut-off value was 5ng/mL, the diagnostic accuracy of recurrence was 89.1%.Citation25
However, for elevated preoperative CEA patients, when the postoperative cutoff value was 5ng/mL, the accuracy of a judgment was 58.4%, while when the cutoff value was adjusted to 8ng/mL, it increased to 75.6%. Among patients with elevated preoperative CEA, patients with CEA ≥ 8 ng/mL had a distinctly higher recurrence survival rate compared to patients with CEA <8 ng/mL. The practicability and accuracy of serum CEA markers in the diagnosis of recurrence can be improved by adjusting the preoperative CEA cut-off value.Citation26
Our study had two significant limitations. First, the CEA time collected by our research data was detected within 3 months after operation or before postoperative adjuvant chemotherapy, thus we did not analyze the specific time of postoperative measurement. Nonetheless, one study confirmed a one-week half-life of CEA. Therefore, CEA should be timely detected to avoid changes after surgery. Secondly, it was common to find false elevations of carcinoembryonic antigen levels in patients whose recurrence of colorectal cancer was monitored, specifically in a few benign lesions. They were not considered here because the methodologies for their identification were unavailable in the hospital before 2016.
In conclusion, our findings provide valuable insights into the prognostic value of the preoperative and postoperative CEA trends for rectal cancer patients and its relationship with clinical outcomes.
Disclosure
The authors report no conflicts of interest for this work.
Acknowledgments
This study was funded by research grants from the National Natural Science Foundation of China [82001986], the Applied Basic Research Projects of Yunnan Province, China [2019FE001-083 and 2018FE001-065], Yunnan digitalization, development and application of biotic resource [202002AA100007].
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.2149230207593
- Boland PM, Ma WW. Immunotherapy for Colorectal Cancer. Cancers (Basel). 2017;9:5. doi:10.3390/cancers9050050
- Network NCC. NCCN Clinical Practice. Guidelines in Oncology (NCCN Guidelines): Colon Cancer. (Version 2.2018). Fort Washington, PA: National Comprehensive Cancer Network; 2018.
- Network NCC. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Rectal Cancer. (Version 3.2018). Fort Washington, PA: National Comprehensive Cancer Network; 2018.
- Konishi T, Shimada Y, Hsu M, et al. Association of preoperative and postoperative serum carcinoembryonic antigen and colon cancer outcome. JAMA Oncol. 2018;4(3):309–315. doi:10.1001/jamaoncol.2017.442029270608
- Gold P, Freedman SO. Demonstration of tumor specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965;121:439–462.14270243
- Meyerhardt JA, Mangu PB, Flynn PJ, et al. American Society of Clinical Oncology. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: american Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31(35):4465–4470. doi:10.1200/JCO.2013.50.744224220554
- Sener SF, Imperato JP, Chmiel J, et al. The use of cancer registry data to study preoperative carcinoembryonic antigen level as an indicator of survival in colorectal cancer. CA Cancer J Clin. 1989;39(1):50–57.2492877
- Herrera MA, Chu TM, Holyoke ED, et al. Carcinoembryonic antigen (CEA) as a prognostic and monitoring test in clinically complete resection of colorectal carcinoma. Ann Surg. 1976;183(1):5–9.1247300
- Goslin R, Steele G, Macintyre J, et al. The use of preoperative plasma CEA levels for the Stratification of patients after curative resection of colorectal cancers. Ann Surg. 1980;192(6):747–751.7447529
- Auclin E, André T, Taieb J, et al. Low-level postoperative carcinoembryonic antigen improves survival outcomes stratification in patients with stage II colon cancer treated with standard adjuvant treatments. Eur J Cancer. 2018;97:55–56.29731226
- Vallam KC, Guruchannabasavaiah B, Agrawal A, et al. Carcinoembryonic antigen directed PET-CECT scanning for postoperative surveillance of colorectal cancer. Colorectal Dis. 2017;19(10):907–911.28444968
- Wang JY, Lu CY, Chu KS, et al. Prognostic significance of pre- and postoperative serum carcinoembryonic antigen levels in patients with colorectal cancer. Eur Surg Res. 2007;39(4):245–250.17457032
- Laurence DJ, Turberville C, Anderson SG, Neville AM. First British standard for carcinoembryonic antigen (CEA). Br J Cancer. 1975;32:295–299.822862
- Li Z, Li S, Liang Y, et al. Predictive value of postoperative peripheral CD4+ T cells percentage in Stage I-III colorectal cancer: a retrospective multicenter cohort study of 1028 subjects. Cancer Manag Res. 2020;12:5505–5513.32753965
- Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi:10.1016/s0140-6736(07)61602-x18064739
- Kim JY, Kim NK, Sohn SK, et al. Prognostic value of postoperative CEA clearance in rectal cancer patients with high preoperative CEA levels. Ann Surg Oncol. 2009;16(10):2771–2778.19657698
- Ma CJ, Hsieh JS, Wang WM, et al. Multivariate analysis of prognostic determinants for colorectal cancer patients with high preoperative serum CEA levels: prognostic value of postoperative serum CEA levels. Kaohsiung J Med Sci. 2006;22(12):604–609.17116621
- Ramphal W, Boeding JRE, van Iwaarden M, et al. Serum carcinoembryonic antigen to predict recurrence in the follow-up of patients with colorectal cancer. Int J Biol Markers. 2019;34(1):60–68.30852955
- Khan MS, Khan MA, Akbar SA, et al. Prognostic significance of pre- and post-operative serum carcinoembryonic antigen levels in patients presented with rectal carcinoma; an experience from Shaukat Khanum Memorial Cancer Hospital and Research Center Lahore. J Pak Med Assoc. 2019;69(10):1431–1436.
- Snyder RA, Hu CY, Cuddy A. Alliance for clinical trials in oncology network cancer surveillance optimization working group, et al.association between intensity of posttreatment surveillance testing and detection of recurrence in patients with colorectal cancer. JAMA. 2018;319(20):2104–2115.29800181
- Kim SS, Donahue TR, Girgis MD, et al. Carcinoembryonic antigen for diagnosis of colorectal cancer recurrence. JAMA. 2018;320(3):298–299.30027230
- Saito G, Sadahiro S, Kamata H, et al. Monitoring of serum carcinoembryonic antigen levels after curative resection of colon cancer: cutoff values determined according to preoperative levels enhance the diagnostic accuracy for recurrence. Oncology. 2017;92(5):276–282.28178692
- Huh JW, Kim CH, Lim SW, et al. Factors predicting long-term survival in colorectal cancer patients with a normal preoperative serum level of carcinoembryonic antigen. J Cancer Res Clin Oncol. 2013;139(9):1449–1455.23765330
- Yakabe T, Nakafusa Y, Sumi K, et al. Clinical significance of CEA and CA19-9 in postoperative follow-up of colorectal cancer. Ann Surg Oncol. 2010;17(9):2349–2356.20217258
- Barton MK, et al. False elevations of carcinoembryonic antigen levels are common in patients under surveillance for colorectal cancer recurrence.CA. Cancer J Clin. 2014;64(6):365–366.