Abstract
Introduction
Lung cancer is the leading cause of cancer-related mortality. In patients with nonsquamous non-small-cell lung cancer (NSCLC) stage IIIB/IV treatment with chemotherapy plus bevacizumab led to significant improvements in progression-free and median overall survival (OS).
Aim
To report the experience of five Portuguese centers in treating patients with nonsquamous NSCLC in stage IIIB or IV with bevacizumab and chemotherapy regarding survival and toxicity outcomes.
Materials and methods
This was a retrospective, multicenter study on patients with nonsquamous stage IIIB/IV NSCLC treated with bevacizumab and chemotherapy from November 2007 to August 2010 through special use permits. We reviewed the medical records, registry of demographic characteristics, treatments provided, treatment responses, adverse events, and dates of death. Statistical analysis was performed with SPSS statistics software. Median OS and event-free survival (EFS) were calculated using the Kaplan–Meier method.
Results
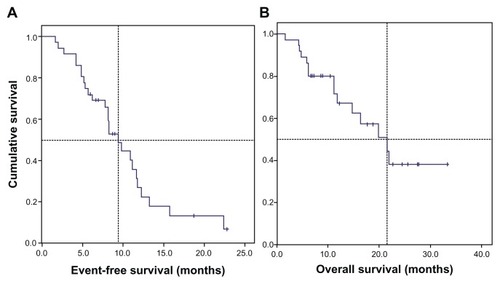
From an eligible population of 41 patients, 37 participants were registered. Study participants were predominantly male (78.4%) with a median age of 53 years (29–75 years). In total, 83.8% patients had stage IV disease (TNM, 6th Ed.). The OS was 21.5 months (95% confidence interval [CI]: 12.6–30.5] and median EFS was 9.4 months (95% CI9: 7.1–11.7). Hematologic toxicity grade 3/4 occurred in 35.1% of patients, and nonhematologic toxicity in 24.3% patients. One fatal thromboembolic event was recorded (2.7%).
Conclusions
The results of chemotherapy plus bevacizumab treatment for nonsquamous NSCLC obtained from the daily clinical practice of the centers involved in this study were similar to those of published clinical trials. Collaboration between the different Portuguese centers is crucial for this kind of study.
Introduction
Lung cancer is the most frequent cancer worldwide, with an estimated 1.61 million new cases in 2008 accounting for 12.7% of all new cancer patients.Citation1 Moreover, lung cancer is the main cause of mortality by malignancy, resulting in 1.38 million deaths, that is, 18.2% of total cancer mortality.Citation1 Globally, the 5-year-survival rate is 15.6%.Citation2
In Portugal, lung cancer is the third most common cancer in men (34.9/100,000), and has the fifth highest incidence when considering both sexes (20.1/100,000).Citation3
At the time of diagnosis, three in every four patients (76.8%) have advanced or metastatic disease (46.9% in stage IV; TNM Classification of Malignant Tumors [TNM] 6th Edition). Around 87.5% of cases are non-small-cell lung cancers (NSCLC), with a predominance of non-squamous types (57.0%).Citation4 In patients diagnosed with stage IIIB/IV NSCLC without epidermal growth factor receptor (EGFR) mutations predictive of response to tyrosine kinase inhibitors, standard treatment relies on platinum-doublet chemotherapy (CT).
Over the last 20 years, survival in lung cancer patients has remained relatively constant,Citation5 highlighting the need for new drugs to improve the prognosis of this disease. Bevacizumab is a novel drug reported to promote improvements in response and survival rates.Citation6–Citation9
Bevacizumab is a recombinant humanized monoclonal IgG1 antibody. The drug acts via selective binding and neutralization of vascular endothelial growth factor (VEGF), thus inhibiting angiogenesis.Citation10,Citation11 Two phase III clinical trials, E4599 of the Eastern Cooperative Oncology Group (ECOG) and AVAiL (Avastin in Lung, also called BO17704), focused on the efficacy and safety of bevacizumab plus CT in the treatment of advanced (stage IIIB/IV) or recurring NSCLC nonsquamous type.Citation6–Citation9 Both studies concluded that the administration of bevacizumab plus CT enhanced response rates (RR) and progression-free survival (PFS) with statistical significance. The E4599 clinical trial additionally showed a significant increase in overall survival (OS; 12.3 m vs 10.3 m (hazard ratio [HR] 0.79; P = 0.003)) in patients treated with bevacizumab plus CT versus CT alone.Citation6–Citation9 Another phase III trial, AVAPERL (MO22089), investigating bevacizumab maintenance was presented at the 2011 European Multidisciplinary Cancer Congress.Citation12 In the study, patients were treated with four cycles of cisplatin plus pemetrexed plus bevacizumab. Patients showing no progression after four treatment cycles were randomized to receive further treatment with bevacizumab alone or bevacizumab plus pemetrexed.Citation12 The preliminary results of AVAPERL disclosed a significant improvement in PFS for patients subjected to maintenance treatment with pemetrexed plus bevacizumab (10.2 m; P < 0.001) versus bevacizumab alone (6.6 m).Citation12
In 2006, the Food and Drug Administration (FDA) granted approval for bevacizumab administration in combination with carboplatin and paclitaxel for the treatment of unresectable, locally advanced, recurrent, or metastatic nonsquamous NSCLC.Citation13 This treatment was implemented in some centers of the National Health System in Portugal through the implementation of special use permits (autorização de utilização especial [AUE]), until its refusal by the National Authority of Medicine and Health Products (INFARMED, Autoridade Nacional do Medicamento e Produtos de Saúde I.P.) in April 2010.Citation14
In the current study, we evaluated the experience of five Portuguese centers that treated patients diagnosed with nonsquamous NSCLC stages IIIB and IV with chemotherapy plus bevacizumab under special use permits, and reported outcomes in terms of survival and toxicity.
Materials and methods
We conducted a multicenter retrospective study using available lung cancer treatment clinical registry data from five Portuguese centers, including the Portuguese Institute of Oncology of Oporto, Hospital S. João of Oporto, Vila Nova de Gaia – Espinho Medical Center, Faro Hospital, and the Hospital of Santa Maria – Lisbon North Hospitalar Center.
The eligible population was composed of adult patients diagnosed with nonsquamous NSCLC (histological or cytological), stage IIIB/IV, treated with chemotherapy plus bevacizumab under special use permits issued by INFARMED between November 2007 and August 2010. As a criterion for exclusion, we considered the impossibility of obtaining clinical information for individual patients.
The study included the following variables: (1) demographic characteristics of the population, (2) treatment, (3) effectiveness, and (4) safety.
Demographic characteristics included diagnostic data (date of biopsy on the pathology report), pathological diagnosis, comorbidities, ECOG functional state and disease stage (TNM).
Treatment included information about the therapeutic protocol, surgery and/or radiotherapy realization, and other treatments applied for second-line therapy and afterwards.
Effectiveness variables included response after three cycles, and dates of progression and death.
Safety involved a toxicity assessment according to the Common Terminology Criteria for Adverse Events (CTCAE; version 4.02) of the National Cancer Institute (NCI).
Statistical analysis was performed with the SPSS statistics program (v. 19; SPSS Inc, Chicago, IL). Absolute and relative frequencies were calculated for categorical variables. Continuous variables were assessed as follows: (1) for age, the median was calculated, (2) for event-free survival (EFS) and OS, analysis was performed using the Kaplan–Meier method considering the date of (a) first treatment, (b) event, defined as progression of disease confirmed with imaging tests or death if no prior confirmation of progression, and (c) death.
Results
The eligible population consisted of 41 patients, four of whom were excluded owing to lack of clinical data. The participant population (n = 37) was mostly composed of male patients (78.4%) with a median age of 53 years (29–75 years). Eight patients were 65 years or older (21.6%) (). The majority of patients had adenocarcinoma (94.6%) and stage IV disease (83.8%). The ECOG performance status was 0 in 12 patients (32.4%) and 1 in 24 (64.9%) patients. Smoking (62.1%) and hypertension (16.2%) were the most frequent comorbidities. Three patients had a history of previous neoplasm, and two had hematologic diseases (polycythemia vera, idiopathic myelofibrosis).
Table 1 Summary of key demographic characteristics
Treatment at the Portuguese participant centers included the administration of bevacizumab (under special use permits issued by INFARMED) in combination with carboplatin and gemcitabine (59.5%) (). Bevacizumab was additionally administered with a platinum and paclitaxel (24.3%) or platinum and pemetrexed (8.1%) regimen. Only one patient was treated with 15 mg/kg of bevacizumab. The majority of patients were subjected to six cycles of CT (75.7%). During the time of data collection, 10 patients (27.0%) were still receiving first-line treatment. One of the patients was subjected to 26 administrations of bevacizumab, with good tolerance. Twenty patients received second-line CT treatment, the majority with pemetrexed (n = 16). At the time of article preparation, fifteen deaths were recorded (40.5%).
Table 2 Treatment characteristics and patient status
The median OS was 21.5 months (95% confidence interval [CI]: 12.6–30.5) and median event-free survival was 9.4 months (95% CI: 7.1–11.7) ().
Figure 1 Kaplan–Meier estimates of survival of 37 patients with nonsquamous NSCLC, stage IIIB/IV, treated with bevacizumab plus chemotherapy. (A) The median event-free survival was 9.4 months (95% CI: 7.1–11.7) and (B) median overall survival was 21.5 months (95% CI: 12.6–30.5).
Abbreviations: CI, confidence interval; NSCLC, non-small-cell lung cancer.
Thirteen patients (35.1%) displayed hematologic toxicity grade 3 or 4, with neutropenia as the most frequent adverse event (27.0%) (). Thrombocytosis was diagnosed in one patient (2.7%) with a previous history of splenectomy. Nonhematological toxicity of grade 3 or higher was reported for nine patients (24.3%), with a predominance of hypertension (10.8%), followed by hemoptysis (5.4%). One fatal thromboembolic event (2.7%) was recorded. It should be noted that at the time of data collection, a patient with polycythemia vera displayed good tolerance to 14 administrations of bevacizumab.
Table 3 Adverse events reported during treatment with chemotherapy plus bevacizumab (grade 3 or higher) according to the Common Terminology Criteria for Adverse Events (version 4.02) of the National Cancer Institute
Discussion
In the present study, the OS and EFS were 21.5 months (95% CI: 12.6–30.5) and 9.4 months (95% CI: 7.1–11.7), respectively. Overall, the treatment was well tolerated. There was a predominance of hematologic toxicity (grade 3 or higher) (35.1%) over nonhematological toxicity (24.3%).
Despite the limitations regarding the design (a retrospective study with no control group and different treatment combinations), the results were consistent with those obtained in earlier phase III clinical trials with evidence level 1b (proposed by Oxford Centre for Evidence-Based Medicine).Citation15 For statistical analysis, EFS was defined as progression of disease confirmed with imaging tests or death in case of no prior validation of progression, and used roughly as the equivalent of PFS. We propose that EFS is a better parameter definition for daily clinical practice and is almost equivalent to PFS for clinical trials, since the various centers do not have the image evaluation defined on time as in clinical trials.
In the randomized E4599 study involving two groups (CT using carboplatin and paclitaxel [n = 444] or CT plus bevacizumab [n = 434]), increases in RR, PFS and OS were observed in the group treated with bevacizumab. RR was 35% vs 15% (P < 0.001), PFS was 6.2 m vs 4.5 m (HR 0.66; 95% CI: 0.57–0.77; P < 0.001) and OS was 12.3 m vs 10.3 m (HR 0.79; 95% CI: 0.67–0.92; P = 0.003).Citation6 Patients analyzed in this study had nonsquamous type NSCLC stage IIIB with pleural effusion, stage IV or recurrent disease, without previous administration of chemotherapy. Our study included four patients with stage IIIB disease without pleural effusion (N3 disease and no indication for radiotherapy), and therefore, longer survival times were expected. In the E4599 study, 15 treatment-related deaths in the chemotherapy plus bevacizumab group were reported, including five from pulmonary hemorrhage. There were no records of grade 5 hemorrhage in our analysis, which may be attributable to the sample size or patient selection.
The AVAiL study included patients with stage IV and IIIB NSCLC with and without pleural effusion (supraclavicular lymph node metastasis) or with recurrent disease. This was a randomized study with three treatment groups, including: (1) CT with cisplatin and gemcitabine (n = 347), (2) CT plus low-dose bevacizumab −7.5 mg/kg (n = 345); (3) CT plus high-dose bevacizumab −15 mg/kg (n = 351).Citation8,Citation9 The study had two major limitations: the primary goal was changed to PFS after implementation of the study, and the study did not provide statistical power for comparing the two doses of bevacizumab. The AVAiL study revealed a statistically significant increase in PFS in the two groups of patients treated with low and high doses of bevacizumab (HR 0.75; 6.7 m vs 6.1 m; P = 0.003), and (HR 0.82; 6.5 m vs 6.1 m; P = 0.03), respectively. A statistically significant increase in OS was observed. This may be due to the presence of confounding factors or inclusion of patients with characteristics predictive of better response to subsequent treatment lines. The median number of bevacizumab administrations was 5 and 6, respectively for high- and low-dose treatments. In our study, the median number of bevacizumab administrations was higher (nine). This may be due to the study design (as discussed above), presence of confounding factors in both studies, as well as different periodicities of imaging in clinical trials (every 8 or 9 weeks) and clinical practice in the community setting (usually after the third and sixth cycles of chemotherapy and 8 to 12 weeks thereafter). In the AVAiL study, the incidence of adverse effects (grade 3 or higher) was similar among all groups.
SAiL (Safety of Avastin in Lung; MO19390), a phase IV study involving 2212 patients,Citation16 was performed with the main objective of assessing the safety of bevacizumab in everyday clinical practice. Severe toxicity, possibly related to bevacizumab administration, was observed in 13% of the patients (n = 288). Adverse events, grade 3 or higher, of specific interest included: thromboembolic events in 172 patients (8%), hemorrhage in 4%, and gastrointestinal perforation in 1% patients (n = 27).
In the present investigation, we observed one thromboembolic event of grade 3 or higher (2.7%; grade 5) and hemoptysis in two patients (5.4%). In the SAiL study, survival analysis was a secondary objective. The PFS was 7.8 m (95% CI: 7.5–8.1) and median OS was 14.6 m (95% CI: 13.8–15.3). About 69% of patients (n = 525) showed disease progression, but no information was available about further treatments. In the current study, 20 patients (54.1%) received second-line treatment, mainly chemotherapy with pemetrexed (n = 16).
The AVAPERL study (MO22089) is an open-label phase III trial including 362 patients with advanced, metastatic or recurrent NSCLC.Citation12 The preliminary data, presented at the 2011 European Multidisciplinary Cancer Congress, revealed a significant improvement in PFS for patients subjected to maintenance treatment with pemetrexed plus bevacizumab (10.2 m; P < 0.001) versus bevacizumab only (6.6 m). EFS in our study was 9.4 months (95% CI: 7.1–11.7),Citation12 which may be explained by the study design or selection of patients, as mentioned previously.
Conclusions
This study describes the experience of five centers in Portugal with chemotherapy plus bevacizumab treatment administered under Special Use Permits issued by INFARMED to patients with nonsquamous NSCLC, which appears similar to that described earlier in published phase III and IV studies.
In the study population obtained from our daily clinical practice, the addition of bevacizumab to standard chemotherapy did not increase or induce unexpected toxicity and improved survival, which may be attributed to the rigorous selection of patients.
In our opinion, it is important to publish the Portuguese experience with novel drugs for lung cancer, and collaboration between different centers is crucial to achieve this aim.
Future studies should focus on the importance of predictive factors of response, evaluation of the quality of life, and pharmacoeconomic studies (cost minimization analysis: complete economic assessment with cost-benefit analysis, cost-effectiveness and cost-utility) in this group of patients.
Acknowledgments
The authors acknowledge the elements of the services included, without which treatment and follow-up of this group of patients would not have been possible.
Disclosures
António Araújo has an advisory and consultant role at F. Hoffmann-La Roche Lda.; Eli Lilly and Company; AstraZeneca. The other authors report no potential conflicts of interest.
References
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- HowladerNNooneAMKrapchoMSEER Cancer Statistics Review 1975–2008National Cancer InstituteBethesda, MD2011 [updated 2010 November, posted 2011]. Available from: http://seer.cancer.gov/csr/1975_2008/Accessed August 8, 2011
- PontesLSilvaMAMatosoFRegisto Oncológico Nacional 2005 [National Cancer Registry 2005]PortugalRegisto Oncológico Regional Centro2009
- ParenteBQueirogaHTeixeiraEEstudo epidemiológico do cancro do pulmão em Portugal nos anos de 2000/2002 [Epidemiologic study of lung cancer in Portugal from 2000 to 2002]Rev Port Pneumol200713225526517571453
- Sotto-MayorRMarcos históricos no estudo do cancro do pulmãoRev Port Pneumol200612440144616969572
- SandlerAGrayRPerryMPaclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancerN Engl J Med2006355242542255017167137
- SandlerABevacizumab in non-small cell lung cancerClin Cancer Res200713154613s4616s
- ReckMvon PawelJZatloukalPPhase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAiLJ Clin Oncol20092781227123419188680
- ReckMvon PawelJZatloukalPOverall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL)Ann Oncol20102191804180920150572
- FolkmanJMerlerEAbernathyCGretchenWIsolation of a tumor factor responsible for angiogenesisJ Exp Med197113322752884332371
- FerraraNHillanKGerberHNovotnyWDiscovery and development of bevacizumab, an anti-VEGF antibody for treating cancerNat Rev Drug Discov20043539140015136787
- BarlesiFde CastroJDvornichenkoVAVAPERL (MO22089): final efficacy outcomes for patients (pts) with advanced non-squamous non-small cell lung cancer (nsNSCLC) randomised to continuation maintenance (mtc) with bevacizumab (bev) or bev+pemetrexed (pem) after first-line (1L) bev-cisplatin (cis)-pem treatment (Tx)Paper presented at: 2011 European Multidisciplinary Cancer CongressSeptember 23–27, 2011Stockholm, Sweden.
- CohenMGootenbergJKeeganPPazdurRFDA Drug approval summary: bevacizumab (avastin) plus carboplatin and paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancerOncologist200712671371817602060
- InfarmedRelatório de avaliação prévia de medicamento para uso em meio hospitalar [Preliminary report on drug use in hospital environment]Lisbon, PortugalAutoridade Nacional do Medicamento e Produtos de Saúde I.P2010
- PhillipsBSackettDBadenochDOxford Centre for Evidence-based Medicine – Levels of Evidence (March 2009) Available from: http://www.cebm.net/index.aspx?o=1025Acessed August 30, 2011
- CrinòLDansinEGarridoPSafety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 studyLancet Oncol201011873374020650686