Abstract
Animal models refers to the animal experimental objects and related materials that can simulate human body established in medical research. As the second-largest disease in terms of morbidity and mortality after cardiovascular disease, cancer has always been the focus of human attention all over the world, which makes it a research hotspot in the medical field. At the same time, more and more animal models have been constructed and used in cancer research. With the deepening of research, the construction methods of cancer animal models are becoming more and more diverse, including chemical induction, xenotransplantation, gene programming, and so on. In recent years, patient-derived xenotransplantation (PDX) model has become a research hotspot because it can retain the microenvironment of the primary tumor and the basic characteristics of cells. Animal models can be used not only to study the biochemical and physiological processes of the occurrence and development of cancer in objects but also for the screening of cancer drugs and the exploration of gene therapy. In this paper, several main tumor animal models and the application progress of animal models in tumor research are systematically reviewed. Finally, combined with the latest progress and development trend in this field, the future research of tumor animal model was prospected.
Introduction
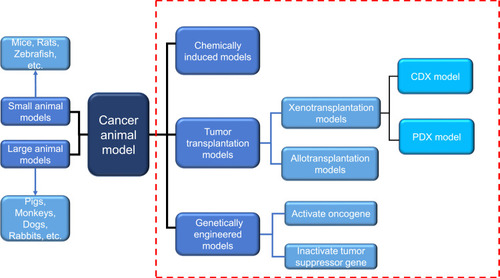
With the effective control of severe infectious diseases and the extension of human life expectancy, cancer has become one of the major diseases that seriously endanger human health. According to 2015 estimates by the World Health Organization (WHO), cancer is the first or second leading cause of death among people under the age of 70 in 91 of these countries.Citation1 Under the combined influence of population aging and population growth, the number of new cancer cases each year is expected to rise from 18.1 million in 2018 to 29.4 million in 2040.Citation2 Due to the late diagnosis of most cancers and inadequate prevention measures, cancer is becoming a heavy burden on residents in low-and middle-income countries. The development and research of new diagnostic methods and innovative treatment tools are essential to reduce the global incidence of cancer.The animal experiment is an important bridge between cell experiment and clinical experiment. Under certain conditions, the occurrence and development of animal diseases are similar to that of human beings, and animals have similar anatomy, physiology and heredity to human beings. Therefore, animal models are often used to study human diseases. In cancer research, the use of animal models can help us understand the genetic basis of cancer and the role of specific genes and gene mutations in the occurrence and development of cancer, which also facilitates the development and testing of antineoplastic drugs.Citation3 With the continuous development of precision medicine and personalized medicine, researchers are looking for standardized and personalized tumor models that are more similar to human tumors.Citation4 There are many animal types and construction methods used to construct cancer animal models, and the progress of each animal model in tumor research has its own characteristics, which will be described below ().
Figure 1 Two commonly used classification methods of cancer animal models. Dashed red box represents the classification according to different modeling methods. Another classification is carried out according to different species. Blue arrows indicate the species of animals included in this classification.
Mouse Model
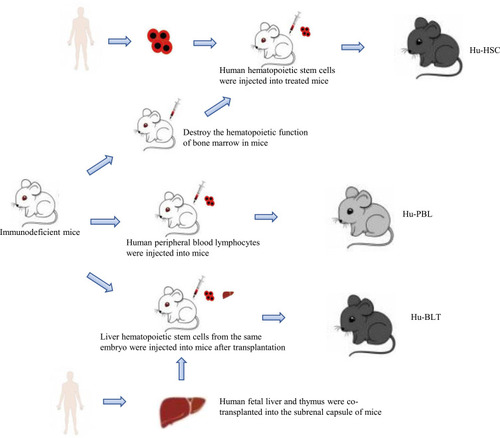
The mouse genome is highly homologous to the human genome, which can simulate a series of biological characteristics such as the occurrence, development and metastasis of human cancer cells in vivo,Citation5 and has the advantages of convenient feeding, low price and easy gene modification. It provides a good tool for cancer research and a valuable platform for drug discovery and verification. At present, there are four commonly used methods to construct mouse cancer model: chemically induced model, cell line-derived xenograft (CDX) model, patient-derived xenograft (PDX) model and genetically engineered mouse model (GEMM).Citation6 The chemical induction model refers to the model of experimental tumor induced by chemical carcinogens, which has the advantage of imitating the occurrence of human cancer from the beginning of the carcinogenic process.Citation7 But the main disadvantage of this method is that it takes 30–50 weeks to form a tumor after using carcinogens.Citation8 The cell line-derived xenograft (CDX) model refers to the xenotransplantation model produced by subcutaneous injection of cancer cell lines into immunodeficient mice.Citation9 The establishment of this model is simple and takes a short time to form a tumor, but after long-term culture in vitro, the biological behavior and tumor heterogeneity of human tumor cell lines are quite different from those of the original tumor tissue.Citation10 The patient-derived xenograft (PDX) model is an animal model established by directly implanting tumor tissue samples from tumor patients into mice, which well maintains the characteristics of tumor histopathology and genetics.Citation11 The genetically engineered mouse model (GEMM) is to induce tumorigenesis by promoting the expression of oncogenes (such as BRAF V600E in melanoma)Citation12 or the deletion of tumor suppressor genes (such as PTEN in prostate cancer)Citation13 by genetic engineering. Compared with the above two transplantation models, GEMM formed an orthotopic tumor in an innate immune maturation microenvironment (natural immune-proficient microenvironment), simulating the process of tumorigenesis.Citation14 However, due to the species differences of the immune system among mammals, these existing models cannot accurately predict the interaction between the human immune system and tumor. Many antineoplastic drugs with a good therapeutic effect in preclinical animal models cannot play a corresponding role in tumor patients. Therefore, it is necessary to establish an animal model that cannot only replicate the tumor microenvironment, but also have a “humanized” immune system at the same time. The humanized mouse model of the human immune system is a mouse model that reconstructs the human immune system by implanting human hematopoietic cells, lymphocytes or tissues into immunodeficient mice.Citation15 On this basis, the implantation of human tumor cells or tumor tissue can be used to study tumor growth in the environment of the human immune system and evaluate anti-tumor therapy, especially the effect of immunotherapy and related mechanism. At present, a variety of human tumor cell lines have been successfully established in humanized mice, such as lymphoma, glioma, breast cancer, colorectal cancer, kidney cancer and prostate cancer cell line.Citation16–Citation19 According to the method of human immune system reconstruction, the humanized mouse models of the immune system are divided into three categories: Hu-BLT (human bone marrow, liver and thymus) model, Hu-HSCs (human hematopoietic stem cell) model and Hu-PBL (human peripheral blood lymphocyte) model ().
Figure 2 Construction method of humanized mice of human immune system. The construction of humanized mice needs to use immunodeficient mice as a tool. By transplanting different human immune organs or cells into immunodeficient mice, three different humanized mice can be constructed. Among them, the Hu-HSC model also needs to destroy the hematopoietic function of bone marrow in mice.
Hu-BLT Model
The model is established by co-transplanting human embryonic liver and thymus into the renal capsule of immunodeficient mice and injecting liver hematopoietic stem cells from the same embryo into mice.Citation20 It can reconstruct the human immune system most perfectly, and detect many kinds of human immune cells, such as T cells, B cells, macrophages and so on, in mice, thus producing human adaptive immune response.Citation21,Citation22 Therefore, the hu-BLT model is considered to be an important model for the study of cancer in the human immune system. For example, Vatakis et alCitation23 used hu-BLT mice to build melanoma models and treated them with T-cell-based immunotherapy. Kaur et al implanted pancreatic tumor cells into hu-BLT mice and found that NK cells could block tumor growth through differentiation and cleavage.Citation24 However, due to the complex and elaborate surgical procedures required in the establishment process and the limited sources of fetal liver and thymus, the application of the hu-BLT model is also limited.Citation25
Hu-HSC Model
The construction of this model requires the destruction of bone marrow hematopoiesis in newborn or adult immunodeficient mice, and then the injection of human hematopoietic stem cell (HSC) into the body. Multi-line hu-HSC developed into immune cells, including B cells, T cells, NK cells and myeloid cells.Citation26 These immune cells interact with transplanted tumor cells and can simulate the tumor microenvironment. Meraz et alCitation27 constructed a hu-HSC model using fresh umbilical cord blood CD34+ HSCs to evaluate the immune response of lung cancer. Hu-HSC model can establish human innate immune system and lymphocytes, but it also has some limitations. For example, a small number of low-active human T cells could not be detected in the peripheral blood until 12 weeks after HSC was implanted into mice.Citation28–Citation30
Hu-PBL Model
Hu-PBL model was established by injecting human peripheral blood lymphocytes into immunodeficient mice. It is the simplest and most economical humanized mouse model at present. At present, the hu-PBL model has been used in many types of cancer research, such as lung cancer, thyroid cancer, cervical cancer, breast cancer, nasopharyngeal carcinoma and so on.Citation31–Citation35 Compared with the hu-HSC model, it can reconstruct high levels of T cells and is an ideal model for the study of mature effector T cells. However, due to the rejection of human T cells and mouse immune cells, this model is prone to graft-versus-host disease (GVHD), which shortens the life span of mice, which limits the research time.
Zebrafish Model
The zebrafish cancer model is a vertebrate model rising in recent years, and it is one of the most promising models at present. The genomes of zebrafish are homologous and conservative to humans, which provides a good basis for the study of the development of various cancers.Citation36,Citation37 Compared with the most commonly used mouse models, the zebrafish model has some unique advantages in cancer research:Citation38 (1) small size, low cost and fast reproduction; (2) transparent embryos, it is convenient to observe and track the proliferation, spread and metastasis of cancer cells in real time; (3) transgenic zebrafishCitation39 and immunodeficient zebrafishCitation40 can remain transparent after adulthood. (3) because zebrafish is fertilized in vitro, the gene operation is relatively easy, and the transgenic animal model can be established quickly. At present, a variety of zebrafish cancer models have been established by means of transgenic, genome editing, xenotransplantation, drug-induced toxic damage and so on ().
Table 1 Zebrafish Model
Zebrafish Melanoma Model
Melanoma is a highly malignant tumor derived from melanocytes, which is the most difficult to cure in skin cancer.Citation41,Citation42 With the deepening of the understanding of the molecular mechanism of melanoma invasion, proliferation and metastasis, remarkable achievements have been made in the treatment of melanin. Melanoma cells achieve the purpose of rapid growth and metastasis by interacting with the microenvironment.Citation43 Therefore, the establishment of the zebrafish melanoma model is very important for the study of melanoma pathogenesis and anti-melanoma drugs.
Dovey et alCitation44 constructed a zebrafish model expressing NRAS Q61K by transgenic technology, which proved that the loss of p53 and the expression of NRAS promote the occurrence of melanoma. After Fornabaio et alCitation45 injected melanoma cells into zebrafish embryos, the interaction between cancer cells and the outer surface of zebrafish blood vessels was monitored, and the first zebrafish model of melanoma angiogenesis and extravascular migration was established. Gomez-Abenza et alCitation46 in order to study the role of Spint1a in melanoma, zebrafish models were constructed by transgenic technology. The results show that the absence of Spint1a promotes the development of melanoma, which also provides a new direction for the treatment of melanoma. Gabellini et alCitation47 demonstrated that the high expression of bcl-xL and pro-inflammatory chemokine interleukin-8 (CXCL8) in patients with melanoma led to a poor prognosis using a zebrafish model.
Zebrafish Leukemia Model
The similarity between zebrafish and human hematopoietic systems has led to the increasing use of zebrafish to simulate leukemia.Citation48 The establishment of the zebrafish leukemia model plays an important role in understanding the occurrence, development and drug research of human leukemia. Langenau et alCitation49 injected rag2, encoding a lymphocyte-specific promoter into zebrafish to drive the expression of the mouse-derived c-Myc gene. It was found that the fluorescence-labeled leukemia cells in zebrafish were implanted into the immunodeficient thymus, suggesting that the proto-oncogene c-Myc is involved in the formation of zebrafish tumors. Gutierrez et alCitation50 found that 4-hydroxytamoxifen (4HT) can activate Myc, to induce acute T-lymphoblastic leukemia in zebrafish by constructing MYC-ER transgenic zebrafish. Corkery et alCitation51 successfully established a leukemia zebrafish casper model by implanting K562 and NB-4 human leukemia cell lines into zebrafish casper embryos, and conducted targeted inhibitor intervention experiments on this model, which laid a good foundation for tumor research in this whole animal. Since the construction of the first leukemic zebrafish model in 2003, zebrafish has made a great contribution to the study of leukemia. Through these studies, we not only have a deeper understanding of the pathogenesis of leukemia, but also proved a variety of anti-leukemia drugs, including Nimesulide, Lenaldekar and Perphenazine.Citation52–Citation54
Zebrafish Digestive Tract Tumor Model
Due to the concealment of the disease, the difficulty of early diagnosis and the lack of effective treatment, the incidence and mortality of digestive tract tumors are at a high level. Understanding the molecular mechanism of digestive tract tumorigenesis and looking for new drug therapy targets is the focus of the current research. Zebrafish do not have stomach and genes that express specific gastric function, but the gastric cancer cells in the gastric cancer xenotransplantation model have high similarity with humans.Citation55 Tsering et alCitation56 induced zebrafish to establish a gastric cancer xenotransplantation model and found that Triphala could inhibit the growth and metastasis of transplanted gastric cancer cells. Zebrafish liver cancer gene is highly conservative with humans,Citation57 which makes the zebrafish model widely used in liver cancer research. Yan et alCitation58 found an increase in the level of fibrinogen in the zebrafish hepatocellular carcinoma model induced by krasV12. They also found that fibrinogen may bind to Integrin α v β 5 on HSC to activate hepatic stellate cells. Jung et alCitation59 introduced the hIL6 gene into zebrafish to study the relationship between hIL6 expression and liver cancer. The results showed that the transgenic zebrafish liver developed into typical liver cancer, which indicated that the high level of hIL6 caused the occurrence of hepatocellular carcinoma. At present, zebrafish intestinal tumor models are mostly xenotransplantation models. With the development of genetic engineering technology, the application of transgenic models is increasing. Topi et alCitation60 studied the effect of estrogen receptor activator ERB-041 on colon cancer cells in a zebrafish xenotransplantation model. Compared with the control group, it was found that distant metastasis of cancer cells decreased after ERB-041 treatment. Lu et alCitation61 established a krasV12 transgenic zebrafish model induced by mifepristone, which provides a good in vivo model for the study of krasV12-induced colorectal cancer. Although zebrafish do not have a discrete pancreas, it has exocrine acinar cells and intestines similar to the functional and histological characteristics of mammalian pancreas.Citation62 Guo et alCitation63 established a model of pancreatic cancer xenotransplantation by injecting human pancreatic cancer cells into zebrafish and found that a small molecule U0126 can inhibit the proliferation and metastasis of human pancreatic cancer cells in zebrafish by inhibiting Ras/Raf/MEK/ERK pathway. This also shows the feasibility of the zebrafish model for screening and identifying new therapeutic drugs for pancreatic cancer.
Other Zebrafish Cancer Models
Breast cancer is the most common cancer among women,Citation64 and the incidence is increasing and getting younger worldwide. The mouse is a traditional tool to study breast cancer. It has many disadvantages, such as long cycle, high cost, complex operation and so on.Citation65,Citation66 With the development of the zebrafish model, more and more breast cancer studies take zebrafish as the experimental object. Ren et alCitation67 constructed xenograft zebrafish breast cancer (co-) injection models to study the role of BMP antagonists GREMLIN-1 (GREM1) in the infiltration and exudation of breast cancer cells. In this model, they found that GREM1 promotes fibrosis in breast cancer-associated fibroblast (CAF) and promotes intravasation and extravasation in breast cancer. Zhu et alCitation68 established a breast cancer xenotransplantation model in zebrafish and used Furanodiene and 5-FU (5-Fluorouracil) to treat zebrafish. Compared with the results of furadiene alone, the results showed that the two drugs had an obvious synergistic anticancer effect. Lung cancer is a kind of cancer with the highest morbidity and mortality in the world. In order to improve the diagnosis and treatment of lung cancer, it is necessary to further understand the pathogenesis and related molecular mechanism of lung cancer. Shen et alCitation69 established a xenotransplantation model after LINC00152 knockout lung cancer cells were implanted into zebrafish. The comparison between the control group and stereoscopic microscope showed that the silencing of LINC00152 could inhibit the proliferation and metastasis of lung cancer cells. The zebrafish model can also be used to evaluate the efficacy and safety of anti-lung cancer drugs to find a more suitable treatment. In order to evaluate the effect of DFIQ (a Novel Quinoline Derivative) on non-small-cell lung cancer (NSCLC) in vivo, Huang et alCitation70 used the zebrafish lung cancer model to receive DFIQ treatment. By monitoring cell growth, migration and apoptosis, it was found that DFIQ could inhibit cancer cells to a certain extent. From the current research, with the in-depth study of the zebrafish tumor model, it will open a new way for the molecular research mechanism of various cancers.
Patient-Derived Tumor Xenograft (PDX) Model
The traditional xenotransplantation model is to establish a stable cell line by screening human tumor cells in vitro, subculturing them, and then injecting them into immunodeficient mice to establish model.Citation71 This model is called the cell line-derived xenograft (CDX) model, which has the advantages of easy to obtain tumor cell lines and easy to repeat experiments. However, with the continuous passage of tumor cells in order to adapt to the external petri dish environment, the tumor microenvironment has changed, resulting in the formation of tumors in mice cannot accurately reflect the characteristics of the original tumor. PDX model is a tumor model established by transplanting fresh tumor tissue from patients into animals by surgery.Citation72,Citation73 At present, the animals used are mainly immunodeficient mice. With the exploration of researchers, zebrafish and other animals provide a new tool for the establishment of PDX models. Compared with the CDX model, the most important advantage of the PDX model is that it completely retains the tumor microenvironment, avoids the effect of repeated passage on tumor heterogeneity, and can better simulate the tumor growth process in patients.Citation74 The researchers carried out various biological tests on the transplanted tumors in liver cancer, colorectal cancer, melanoma, esophageal cancer and borderline cancer. It was confirmed that the PDX model maintained the biological characteristics of primary tumors and provided an accurate animal model for the study of oncology.Citation75–Citation77 Because the tumor tissue comes from different patients and uses one model to correspond to the pattern of one patient, the PDX model can reflect the genetic diversity of patients.Citation72 These advantages make the PDX model widely used in all kinds of cancer research (). With the development of tumor research, the shortcomings of the traditional PDX model are also exposed. The traditional PDX model generally uses immunodeficient mice, but the immune system of mice is different from that of humans, so it can reflect the interaction between immunity and tumor.Citation78 At the same time, the life span of immunodeficient mice is short, At the same time, the life span of immunodeficient mice is short, and there is the possibility of spontaneous tumor.Citation79 The success rate of PDX modeling was also different among different tumor types, and the success rate of colorectal cancer and lung cancer was significantly higher than that of prostate cancer.Citation80 These shortcomings promote the emergence of some improved PDX models.
Table 2 PDX Model
Humanized Patient-Derived Xenograft (Hu-PDX) Model
Firstly, the immune system of severe immunodeficiency mice (NOG or NSG, etc.) was rebuilt into a state consistent with that of normal people or clinical patients, and then human tumor tissue blocks were orthotopically transplanted into immune system humanized mice. This model is called the hu-PDX model. This model can provide a growth environment more similar to that of the human body for tumors, and has important application value in tumor treatment and the study of tumor occurrence, development and metastasis, especially in tumor immunotherapy.Citation81–Citation83 Hu-PDX model has been applied to many types of tumor research. Lin et alCitation28 established human immunodeficient mice by implanting peripheral blood lymphocytes and proved that this PBMCs-derived PDX model is an effective tool for studying PD-L1/PD-1 targeted cancer immunotherapy. Rosato et alCitation84 demonstrated the availability of anti-programmed cell death-1 (PD-1) immunotherapy in the TNBC study after constructing a PDX model derived from triple-negative breast cancer patients. Sanmamed et alCitation16 injected lymphocytes from gastric cancer patients into immunodeficient mice, then transplanted gastric cancer tissue from the same patient into mice and finally injected mice with nivolumab (a PD-1 inhibitor) and urelumab (an anti-CD137 agonist). The results show that the use of the above drugs can induce the attack of self-T cells and slow down the growth of tumors. However, there are some problems in the current Hu-PDX model, such as low success rate of modeling, short existence time of humanized immune system, incomplete immune function and so on. Future research should focus on improving the modeling technology of humanized mice and improving the efficiency and duration of immune system implantation.Citation85
Patient-Derived Orthotropic Xenograft (PDOX) Model
Most of the transplantation sites of the PDX model are subcutaneous or renal capsule, lack of in situ environment for tumor growth. It has been found that orthotopic transplantation of tumor tissue into animal organs corresponding to the primary site can provide an in vivo environment suitable for tumor growth.Citation86 Therefore, the PDOX model is established on the basis of the PDX model. Compared with the traditional PDX model, this model can simulate the evolution of human tumors in vivo more objectively and accurately. Hiroshima et alCitation87 established 10 cases of subcutaneous injection of PDX model and 8 cases of PDOX model using cervical cancer tissue. The results showed that tumor metastasis was found in half of the PDOX model, but not in the PDX model. The results show that the PDOX model is more likely to show the biological characteristics of malignant tumor invasion and metastasis than the PDX model. And a number of studies have shown that swelling. The organ microenvironment in which the tumor grows can directly affect the biological characteristics of the tumor. PDOX model can also accurately predict the prognosis of cancer patients and select the most suitable individual treatment for patients. Hiroshima once again established the PDOX model and subcutaneous PDX model of human cervical cancer again and treated the two models with entinostat (a benzamide histone deacetylase inhibitor).Citation88 Finally, it was found that only the tumor growth was inhibited in the PDOX model. Because most of the tumors in the PDOX model are located in vivo, it is difficult to observe the growth of tumors by traditional detection methods, and it is more difficult to find the location of metastatic focus.Citation89 Making a PDOX model that is easy to measure has become a problem that needs to be solved in the future.
Mini Patient Derived Xenograft (Mini-PDX) Model
Mini-PDX model is a drug sensitivity test model established after human tumor tissue was transplanted into immunodeficient mice by a special method.Citation90 This special method is to first inject the digested cell suspension of the patient’s tumor tissue into the microcapsule and then transplant the capsule into the mouse.Citation91 Zhan et alCitation92 established a Mini-PDX model using tumor tissues of patients with gallbladder cancer to detect the sensitivity of the five most commonly used chemotherapeutic drugs, (gemcitabine, oxaliplatin, 5-fluorouracil and nanoparticle albumin-bound (nab)-paclitaxel, and irinotecan). The results showed that the proliferation rate of gallbladder cancer cells in the model was relatively low after treatment with irinotecan and gemcitabine. The advantages of this model for drug sensitivity testing are short time, low cost, and high consistency with the results of the traditional PDX model. Zhang et alCitation90 constructed Mini-PDX models of lung cancer, gastric cancer and pancreatic cancer, and used the PDX model as a reference to test the drug sensitivity of the Mini-PDX model. The results show that the consistency of the results of drug sensitivity testing using the Mini-PDX model and the traditional PDX model is 92%, but the time required to use Mini-PDX model is significantly shorter than that of the PDX model. This shows that this model can be used as a good substitute for the PDX model in evaluating cancer treatment. Because of these advantages, the Mini-PDX model is expected to become a tool to help cancer patients with personalized treatment.
Other Animal Models
With the deepening of cancer research, more and more animals are used to build animal models. At present, most of the animal models commonly used in cancer research are small animal models, such as mice, rats, zebrafish, fruit flies and so on. Among them, mice and zebrafish are the most widely used. Small animal models have many advantages, such as strong reproductive ability, low cost, easy maintenance and so on. However, because of its small size and limited blood supply, it is difficult to carry out interventions such as surgery and radiography on small animals.Citation93 The emergence of some large animal cancer models has provided new directions for researchers, including dogs, non-human primates, tree shrews and pigs.
Compared with rodents, canine genomes are more similar to human genomes.Citation94 Canines can spontaneously form some cancers, which are similar to human cancers in clinical, molecular and histological features.Citation95 Uva et alCitation96 analyzed gene expression in human and dog breast cancer and normal breast samples and found that uncontrolled genes in human breast cancer could also be observed in canine breast cancer samples. Ressel et alCitation97 found that there is also a loss of expression of the PTEN gene in canine breast cancer, and the same phenomenon can be observed in human breast cancer. These findings support the possibility of canine cancer models as a study of human cancer. Non-human primates are closely related to human beings and highly similar to human beings in physiology, metabolism, immunity, heredity and many other aspects, so it is an excellent cancer research model. Puente et alCitation98 found that almost all human cancer genes are highly conserved between chimpanzees and humans. However, due to the high cost of breeding and feeding, complex experimental techniques and ethical problems, the use of non-human primates has been limited.Citation99 The tree shrew is a new experimental animal in recent years, its whole genome is very similar to primates, and its physiology and biochemistry, tissue anatomy and immunology are similar to humans.Citation100 Tu et alCitation101 constructed the pancreatic cancer model of tree shrew using lentivirus and analyzed the gene expression profile by RNA sequencing. The results showed that the molecular mechanism of the tree shrew pancreatic cancer model was more similar to that of the human pancreatic cancer model than that of the mouse pancreatic cancer model. Compared with non-human primates, it also has the advantages of small size, short reproductive cycle, easy feeding and so on. It also allows tree shrews to replace primates for cancer research. Ge et alCitation102 successfully constructed a tree shrew breast cancer model with lentivirus expressing PyMT gene and found that chemotherapy drugs commonly used in human breast cancer (cisplatin and Ebramycin) can significantly inhibit tree shrew breast tumor. The pig genome has highly conserved epigenetic regulation and has high homology with the human genome.Citation103,Citation104 The anatomical, physiological and genetic characteristics of pigs are very similar to those of humans, and they are ideal animal models for cancer research. Mitchell et alCitation105 induced hepatocellular carcinoma in pigs using diethylnitrosamine (DEN) and found that partial hepatic embolism could promote the construction of the model. And the emergence of gene editing pigs provides a new tool for the study of cancer-related genes. Wang et alCitation106 established gene editing pigs expressing Cas9 under the induction of Cre enzyme, and established a pig model of lung cancer after activating one oncogene (KRAS) and five tumor suppressor genes (TP53, PTEN, APC, BRCA1 and BRCA2) at the same time. Importantly, studies have shown that young pigs can predict pharmacokinetics in children,Citation107 which provides a basis for the use of piglet models to develop anticancer drugs.
Summary and Prospect
As an excellent tool to study the pathogenesis of human diseases and explore the principles of disease prevention and treatment, animal models are constantly providing new ideas for cancer research. However, there are some differences in physiology, heredity and immunity between animal models and human beings. Therefore, it is an urgent need for the current biomedical development to develop animal models that can better reflect human biological characteristics and replace human beings to carry out preclinical research. Because of the strong heterogeneity of cancer, the tumor tissue constructed by cell line-derived xenograft (CDX) model and traditional in vitro culture is different from humans, which cannot accurately reflect the biological process of human tumor tissue and reduce the efficiency of cancer research.Citation108 The use of the patient-derived xenograft (PDX) model can better simulate the tumor growth process in patients, and the consistent rate of drug response is high.Citation74 On this basis, in order to further simulate the physiological or pathological state of human beings, the humanized animal model came into being. In recent years, with the continuous development and improvement of gene-editing technology, it is possible to make a variety of cancer animal models, which has greatly promoted the related research of cancer. Researchers are working on a model that is more similar to the human genome.Citation109 In the future, we can combine various construction methods with the help of gene-editing technology to construct a model that is more suitable for cancer research.
Disclosure
The authors report no conflicts of interest for this work.
Acknowledgment
The following authors contributed equally to this work and are all first authors: Zhitao Li, Wubin Zheng, and Hanjin Wang.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.2149230207593
- Wild CP. The global cancer burden: necessity is the mother of prevention. Nat Rev Cancer. 2019;19(3):123–124. doi:10.1038/s41568-019-0110-330683893
- Schachtschneider KM, Schwind RM, Newson J, et al. The oncopig cancer model: an innovative large animal translational oncology platform. Front Oncol. 2017;7:190. doi:10.3389/fonc.2017.0019028879168
- Xu C, Wu S, Schook LB, Schachtschneider KM. Translating human cancer sequences into personalized porcine cancer models. Front Oncol. 2019;9:105. doi:10.3389/fonc.2019.0010530873383
- Mural RJ, Adams MD, Myers EW, et al. A comparison of whole-genome shotgun-derived mouse chromosome 16 and the human genome. Science. 2002;296(5573):1661–1671. doi:10.1126/science.106919312040188
- Mendes N, Dias Carvalho P, Martins F, et al. Animal models to study cancer and its microenvironment. Adv Exp Med Biol. 2020;1219:389–401.32130710
- Liu Y, Yin T, Feng Y, et al. Mammalian models of chemically induced primary malignancies exploitable for imaging-based preclinical theragnostic research. Quant Imaging Med Surg. 2015;5(5):708–729. doi:10.3978/j.issn.2223-4292.2015.06.0126682141
- De Minicis S, Kisseleva T, Francis H, et al. Liver carcinogenesis: rodent models of hepatocarcinoma and cholangiocarcinoma. Dig Liver Dis. 2013;45(6):450–459. doi:10.1016/j.dld.2012.10.00823177172
- Brennecke P, Arlt MJ, Campanile C, et al. CXCR4 antibody treatment suppresses metastatic spread to the lung of intratibial human osteosarcoma xenografts in mice. Clin Exp Metastasis. 2014;31(3):339–349. doi:10.1007/s10585-013-9632-324390633
- Ye F, Chen C, Qin J, Liu J, Zheng C. Genetic profiling reveals an alarming rate of cross-contamination among human cell lines used in China. FASEB J. 2015;29(10):4268–4272. doi:10.1096/fj.14-26671826116706
- Hidalgo M, Amant F, Biankin AV, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. doi:10.1158/2159-8290.CD-14-000125185190
- Hooijkaas AI, Gadiot J, van der Valk M, Mooi WJ, Blank CU. Targeting BRAFV600E in an inducible murine model of melanoma. Am J Pathol. 2012;181(3):785–794. doi:10.1016/j.ajpath.2012.06.00222796458
- Chen Z, Trotman LC, Shaffer D, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436(7051):725–730. doi:10.1038/nature0391816079851
- Kersten K, de Visser KE, van Miltenburg MH, Jonkers J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol Med. 2017;9(2):137–153. doi:10.15252/emmm.20160685728028012
- Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12(11):786–798. doi:10.1038/nri331123059428
- Sanmamed MF, Rodriguez I, Schalper KA, et al. Nivolumab and urelumab enhance antitumor activity of human T lymphocytes engrafted in Rag2-/-IL2Rgammanull immunodeficient mice. Cancer Res. 2015;75(17):3466–3478. doi:10.1158/0008-5472.CAN-14-351026113085
- Ashizawa T, Iizuka A, Nonomura C, et al. Antitumor effect of programmed death-1 (PD-1) blockade in humanized the NOG-MHC double knockout mouse. Clin Cancer Res. 2017;23(1):149–158. doi:10.1158/1078-0432.CCR-16-012227458246
- Roth MD, Harui A. Human tumor infiltrating lymphocytes cooperatively regulate prostate tumor growth in a humanized mouse model. J Immunother Cancer. 2015;3:12. doi:10.1186/s40425-015-0056-225901284
- Wang L, Wen W, Yuan J, et al. Immunotherapy for human renal cell carcinoma by adoptive transfer of autologous transforming growth factor beta-insensitive CD8+ T cells. Clin Cancer Res. 2010;16(1):164–173. doi:10.1158/1078-0432.CCR-09-175820028741
- Melkus MW, Estes JD, Padgett-Thomas A, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12(11):1316–1322. doi:10.1038/nm143117057712
- Wege AK, Melkus MW, Denton PW, Estes JD, Garcia JV. Functional and phenotypic characterization of the humanized BLT mouse model. Curr Top Microbiol Immunol. 2008;324:149–165. doi:10.1007/978-3-540-75647-7_1018481459
- Shimizu S, Hong P, Arumugam B, et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood. 2010;115(8):1534–1544. doi:10.1182/blood-2009-04-21585520018916
- Vatakis DN, Koya RC, Nixon CC, et al. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc Natl Acad Sci U S A. 2011;108(51):E1408–E1416. doi:10.1073/pnas.111505010822123951
- Kaur K, Kozlowska AK, Topchyan P, et al. Probiotic-treated super-charged NK cells efficiently clear poorly differentiated pancreatic tumors in Hu-BLT mice. Cancers (Basel). 2019;12(1):63. doi:10.3390/cancers12010063
- Reardon S. Trump administration halts fetal-tissue research by government scientists. Nature. 2019;570(7760):148. doi:10.1038/d41586-019-01783-6
- Rongvaux A, Takizawa H, Strowig T, et al. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol. 2013;31:635–674. doi:10.1146/annurev-immunol-032712-09592123330956
- Meraz IM, Majidi M, Meng F, et al. An improved patient-derived xenograft humanized mouse model for evaluation of lung cancer immune responses. Cancer Immunol Res. 2019;7(8):1267–1279. doi:10.1158/2326-6066.CIR-18-087431186248
- Lin S, Huang G, Cheng L, et al. Establishment of peripheral blood mononuclear cell-derived humanized lung cancer mouse models for studying efficacy of PD-L1/PD-1 targeted immunotherapy. MAbs. 2018;10(8):1301–1311. doi:10.1080/19420862.2018.151894830204048
- Liu G, Fan X, Cai Y, et al. Efficacy of dendritic cell-based immunotherapy produced from cord blood in vitro and in a humanized NSG mouse cancer model. Immunotherapy. 2019;11(7):599–616. doi:10.2217/imt-2018-010330943862
- Legrand N, Weijer K, Spits H. Experimental models to study development and function of the human immune system in vivo. J Immunol. 2006;176(4):2053–2058. doi:10.4049/jimmunol.176.4.205316455958
- Pyo KH, Kim JH, Lee JM, et al. Promising preclinical platform for evaluation of immuno-oncology drugs using Hu-PBL-NSG lung cancer models. Lung Cancer. 2019;127:112–121. doi:10.1016/j.lungcan.2018.11.03530642538
- Gyory F, Mezosi E, Szakall S, et al. Establishment of the hu-PBL-SCID mouse model for the investigation of thyroid cancer. Exp Clin Endocrinol Diabetes. 2005;113(7):359–364. doi:10.1055/s-2005-86574016025395
- Liang ZX, Cheng Q, Chen HZ, Xie X, Ye DF. [Development of HPV16 positive cervical cancer model in the hu-PBL-SCID mouse and its immunological features]. Zhonghua Yi Xue Za Zhi. 2004;84(17):1465–1469. Chinese.15500747
- Chung MA, Luo Y, O’Donnell M, et al. Development and preclinical evaluation of a Bacillus Calmette-Guerin-MUC1-based novel breast cancer vaccine. Cancer Res. 2003;63(6):1280–1287.12649188
- Wang JJ, Liu YH, Li GC. Induction of protective and therapeutic anti-cancer immunity by using bispecific anti-idiotype antibody G22-I50 for nasopharyngeal carcinoma. Int Immunopharmacol. 2015;28(2):1026–1033. doi:10.1016/j.intimp.2015.07.02626303768
- Teittinen KJ, Gronroos T, Parikka M, Ramet M, Lohi O. The zebrafish as a tool in leukemia research. Leuk Res. 2012;36(9):1082–1088. doi:10.1016/j.leukres.2012.06.00122749067
- Konantz M, Balci TB, Hartwig UF, et al. Zebrafish xenografts as a tool for in vivo studies on human cancer. Ann N Y Acad Sci. 2012;1266:124–137. doi:10.1111/j.1749-6632.2012.06575.x22901264
- Veinotte CJ, Dellaire G, Berman JN. Hooking the big one: the potential of zebrafish xenotransplantation to reform cancer drug screening in the genomic era. Dis Model Mech. 2014;7(7):745–754. doi:10.1242/dmm.01578424973744
- White RM, Sessa A, Burke C, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2(2):183–189. doi:10.1016/j.stem.2007.11.00218371439
- Tang Q, Moore JC, Ignatius MS, et al. Imaging tumour cell heterogeneity following cell transplantation into optically clear immune-deficient zebrafish. Nat Commun. 2016;7:10358. doi:10.1038/ncomms1035826790525
- Tsao H, Fukunaga-Kalabis M, Herlyn M. Recent advances in melanoma and melanocyte biology. J Invest Dermatol. 2017;137(3):557–560. doi:10.1016/j.jid.2016.11.00428089201
- Gru AA, Becker N, Dehner LP, Pfeifer JD. Mucosal melanoma: correlation of clinicopathologic, prognostic, and molecular features. Melanoma Res. 2014;24(4):360–370. doi:10.1097/CMR.000000000000008224870295
- Olsen CM, Lane SW, Green AC. Increased risk of melanoma in patients with chronic lymphocytic leukaemia: systematic review and meta-analysis of cohort studies. Melanoma Res. 2016;26(2):188–194. doi:10.1097/CMR.000000000000021926630660
- Dovey M, White RM, Zon LI. Oncogenic NRAS cooperates with p53 loss to generate melanoma in zebrafish. Zebrafish. 2009;6(4):397–404. doi:10.1089/zeb.2009.060619954345
- Fornabaio G, Barnhill RL, Lugassy C, et al. Angiotropism and extravascular migratory metastasis in cutaneous and uveal melanoma progression in a zebrafish model. Sci Rep. 2018;8(1):10448. doi:10.1038/s41598-018-28515-629992995
- Gomez-Abenza E, Ibanez-Molero S, Garcia-Moreno D, et al. Zebrafish modeling reveals that SPINT1 regulates the aggressiveness of skin cutaneous melanoma and its crosstalk with tumor immune microenvironment. J Exp Clin Cancer Res. 2019;38(1):405. doi:10.1186/s13046-019-1389-331519199
- Gabellini C, Gomez-Abenza E, Ibanez-Molero S, et al. Interleukin 8 mediates bcl-xL-induced enhancement of human melanoma cell dissemination and angiogenesis in a zebrafish xenograft model. Int J Cancer. 2018;142(3):584–596. doi:10.1002/ijc.3107528949016
- Jing L, Zon LI. Zebrafish as a model for normal and malignant hematopoiesis. Dis Model Mech. 2011;4(4):433–438. doi:10.1242/dmm.00679121708900
- Langenau DM, Traver D, Ferrando AA, et al. Myc-induced T cell leukemia in transgenic zebrafish. Science. 2003;299(5608):887–890. doi:10.1126/science.108028012574629
- Gutierrez A, Grebliunaite R, Feng H, et al. Pten mediates Myc oncogene dependence in a conditional zebrafish model of T cell acute lymphoblastic leukemia. J Exp Med. 2011;208(8):1595–1603. doi:10.1084/jem.2010169121727187
- Corkery DP, Dellaire G, Berman JN. Leukaemia xenotransplantation in zebrafish–chemotherapy response assay in vivo. Br J Haematol. 2011;153(6):786–789. doi:10.1111/j.1365-2141.2011.08661.x21517816
- Yeh JR, Munson KM, Elagib KE, Goldfarb AN, Sweetser DA, Peterson RT. Discovering chemical modifiers of oncogene-regulated hematopoietic differentiation. Nat Chem Biol. 2009;5(4):236–243. doi:10.1038/nchembio.14719172146
- Gutierrez A, Pan L, Groen RW, et al. Phenothiazines induce PP2A-mediated apoptosis in T cell acute lymphoblastic leukemia. J Clin Invest. 2014;124(2):644–655. doi:10.1172/JCI6509324401270
- Ridges S, Heaton WL, Joshi D, et al. Zebrafish screen identifies novel compound with selective toxicity against leukemia. Blood. 2012;119(24):5621–5631. doi:10.1182/blood-2011-12-39881822490804
- Wallace KN, Pack M. Unique and conserved aspects of gut development in zebrafish. Dev Biol. 2003;255(1):12–29. doi:10.1016/S0012-1606(02)00034-912618131
- Tsering J, Hu X. Triphala suppresses growth and migration of human gastric carcinoma cells in vitro and in a zebrafish xenograft model. Biomed Res Int. 2018;2018:7046927. doi:10.1155/2018/704692730643816
- Lam SH, Wu YL, Vega VB, et al. Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat Biotechnol. 2006;24(1):73–75. doi:10.1038/nbt116916327811
- Yan C, Yang Q, Gong Z. Activation of hepatic stellate cells during liver carcinogenesis requires fibrinogen/integrin alphavbeta5 in zebrafish. Neoplasia. 2018;20(5):533–542. doi:10.1016/j.neo.2018.02.00229649779
- Jung IH, Choi JH, Chung YY, Lim GL, Park YN, Park SW. Predominant activation of JAK/STAT3 pathway by interleukin-6 is implicated in hepatocarcinogenesis. Neoplasia. 2015;17(7):586–597. doi:10.1016/j.neo.2015.07.00526297436
- Topi G, Satapathy SR, Dash P, et al. Tumour-suppressive effect of oestrogen receptor beta in colorectal cancer patients, colon cancer cells, and a zebrafish model. J Pathol. 2020;251(3):297–309. doi:10.1002/path.545332333795
- Lu JW, Raghuram D, Fong PA, Gong Z. Inducible intestine-specific expression of kras(V12) triggers intestinal tumorigenesis in transgenic zebrafish. Neoplasia. 2018;20(12):1187–1197. doi:10.1016/j.neo.2018.10.00230390498
- Menke AL, Spitsbergen JM, Wolterbeek AP, Woutersen RA. Normal anatomy and histology of the adult zebrafish. Toxicol Pathol. 2011;39(5):759–775. doi:10.1177/019262331140959721636695
- Guo M, Wei H, Hu J, Sun S, Long J, Wang X. U0126 inhibits pancreatic cancer progression via the KRAS signaling pathway in a zebrafish xenotransplantation model. Oncol Rep. 2015;34(2):699–706. doi:10.3892/or.2015.401926035715
- Fahad Ullah M. Breast cancer: current perspectives on the disease status. Adv Exp Med Biol. 2019;1152:51–64.31456179
- McCarthy A, Savage K, Gabriel A, Naceur C, Reis-Filho JS, Ashworth A. A mouse model of basal-like breast carcinoma with metaplastic elements. J Pathol. 2007;211(4):389–398.17212342
- Fantozzi A, Christofori G. Mouse models of breast cancer metastasis. Breast Cancer Res. 2006;8(4):212. doi:10.1186/bcr153016887003
- Ren J, Smid M, Iaria J, et al. Cancer-associated fibroblast-derived Gremlin 1 promotes breast cancer progression. Breast Cancer Res. 2019;21(1):109. doi:10.1186/s13058-019-1194-031533776
- Zhu XY, Guo DW, Lao QC, et al. Sensitization and synergistic anti-cancer effects of Furanodiene identified in zebrafish models. Sci Rep. 2019;9(1):4541. doi:10.1038/s41598-019-40866-230872660
- Shen W, Pu J, Sun J, et al. Zebrafish xenograft model of human lung cancer for studying the function of LINC00152 in cell proliferation and invasion. Cancer Cell Int. 2020;20:376. doi:10.1186/s12935-020-01460-z32774169
- Huang HW, Bow YD, Wang CY, et al. DFIQ, a novel quinoline derivative, shows anticancer potential by inducing apoptosis and autophagy in NSCLC cell and in vivo zebrafish xenograft models. Cancers (Basel). 2020;12(5):1348. doi:10.3390/cancers12051348
- Keysar SB, Astling DP, Anderson RT, et al. A patient tumor transplant model of squamous cell cancer identifies PI3K inhibitors as candidate therapeutics in defined molecular bins. Mol Oncol. 2013;7(4):776–790. doi:10.1016/j.molonc.2013.03.00423607916
- Morton CL, Houghton PJ. Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc. 2007;2(2):247–250. doi:10.1038/nprot.2007.2517406581
- Wang Y, Sudilovsky D, Zhang B, et al. A human prostatic epithelial model of hormonal carcinogenesis. Cancer Res. 2001;61(16):6064–6072.11507055
- Owonikoko TK, Zhang G, Kim HS, et al. Patient-derived xenografts faithfully replicated clinical outcome in a Phase II co-clinical trial of arsenic trioxide in relapsed small cell lung cancer. J Transl Med. 2016;14(1):111. doi:10.1186/s12967-016-0861-527142472
- Marangoni E, Vincent-Salomon A, Auger N, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res. 2007;13(13):3989–3998. doi:10.1158/1078-0432.CCR-07-007817606733
- Dodbiba L, Teichman J, Fleet A, et al. Primary esophageal and gastro-esophageal junction cancer xenograft models: clinicopathological features and engraftment. Lab Invest. 2013;93(4):397–407. doi:10.1038/labinvest.2013.823399854
- El-Rifai W, Harper JC, Cummings OW, et al. Consistent genetic alterations in xenografts of proximal stomach and gastro-esophageal junction adenocarcinomas. Cancer Res. 1998;58(1):34–37.9426053
- Tanaskovic O, Verga Falzacappa MV, Pelicci PG, Kim CH. Human cord blood (hCB)-CD34+ humanized mice fail to reject human acute myeloid leukemia cells. PLoS One. 2019;14(9):e0217345. doi:10.1371/journal.pone.021734531536492
- Moyer AM, Yu J, Sinnwell JP, et al. Spontaneous murine tumors in the development of patient-derived xenografts: a potential pitfall. Oncotarget. 2019;10(39):3924–3930. doi:10.18632/oncotarget.2700131231469
- Giraud J, Bouriez D, Seeneevassen L, et al. Orthotopic patient-derived xenografts of gastric cancer to decipher drugs effects on cancer stem cells and metastatic dissemination. Cancers (Basel). 2019;11(4):560. doi:10.3390/cancers11040560
- Morton JJ, Bird G, Keysar SB, et al. XactMice: humanizing mouse bone marrow enables microenvironment reconstitution in a patient-derived xenograft model of head and neck cancer. Oncogene. 2016;35(3):290–300. doi:10.1038/onc.2015.9425893296
- Rongvaux A, Willinger T, Martinek J, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014;32(4):364–372. doi:10.1038/nbt.285824633240
- Wang X, Qi Z, Wei H, Tian Z, Sun R. Characterization of human B cells in umbilical cord blood-transplanted NOD/SCID mice. Transpl Immunol. 2012;26(2–3):156–162. doi:10.1016/j.trim.2011.12.00322198522
- Rosato RR, Davila-Gonzalez D, Choi DS, et al. Evaluation of anti-PD-1-based therapy against triple-negative breast cancer patient-derived xenograft tumors engrafted in humanized mouse models. Breast Cancer Res. 2018;20(1):108. doi:10.1186/s13058-018-1037-430185216
- Danisch S, Slabik C, Cornelius A, et al. Spatiotemporally skewed activation of programmed cell death receptor 1-positive T cells after epstein-barr virus infection and tumor development in long-term fully humanized mice. Am J Pathol. 2019;189(3):521–539. doi:10.1016/j.ajpath.2018.11.01430593822
- Sicklick JK, Leonard SY, Babicky ML, et al. Generation of orthotopic patient-derived xenografts from gastrointestinal stromal tumor. J Transl Med. 2014;12:41. doi:10.1186/1479-5876-12-4124507750
- Hiroshima Y, Zhang Y, Zhang N, et al. Establishment of a patient-derived orthotopic Xenograft (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLoS One. 2015;10(2):e0117417. doi:10.1371/journal.pone.011741725689852
- Hiroshima Y, Maawy A, Zhang Y, et al. Patient-derived mouse models of cancer need to be orthotopic in order to evaluate targeted anti-metastatic therapy. Oncotarget. 2016;7(44):71696–71702. doi:10.18632/oncotarget.1232227765934
- Igarashi K, Kawaguchi K, Kiyuna T, et al. Patient-derived orthotopic xenograft (PDOX) mouse model of adult rhabdomyosarcoma invades and recurs after resection in contrast to the subcutaneous ectopic model. Cell Cycle. 2017;16(1):91–94. doi:10.1080/15384101.2016.125288527830986
- Zhang F, Wang W, Long Y, et al. Characterization of drug responses of mini patient-derived xenografts in mice for predicting cancer patient clinical therapeutic response. Cancer Commun (Lond). 2018;38(1):60. doi:10.1186/s40880-018-0329-530257718
- Frank ND, Jones ME, Vang B, et al. Evaluation of reagents used to coat the hollow-fiber bioreactor membrane of the Quantum(R) Cell Expansion System for the culture of human mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2019;96:77–85. doi:10.1016/j.msec.2018.10.08130606590
- Zhan M, Yang RM, Wang H, et al. Guided chemotherapy based on patient-derived mini-xenograft models improves survival of gallbladder carcinoma patients. Cancer Commun (Lond). 2018;38(1):48. doi:10.1186/s40880-018-0318-830016995
- Mei LJ, Yang XJ, Tang L, Hassan AH, Yonemura Y, Li Y. Establishment and identification of a rabbit model of peritoneal carcinomatosis from gastric cancer. BMC Cancer. 2010;10:124. doi:10.1186/1471-2407-10-12420359350
- Pinho SS, Carvalho S, Cabral J, Reis CA, Gartner F. Canine tumors: a spontaneous animal model of human carcinogenesis. Transl Res. 2012;159(3):165–172. doi:10.1016/j.trsl.2011.11.00522340765
- Gardner HL, Fenger JM, London CA. Dogs as a model for cancer. Annu Rev Anim Biosci. 2016;4:199–222. doi:10.1146/annurev-animal-022114-11091126566160
- Uva P, Aurisicchio L, Watters J, et al. Comparative expression pathway analysis of human and canine mammary tumors. BMC Genomics. 2009;10:135. doi:10.1186/1471-2164-10-13519327144
- Ressel L, Millanta F, Caleri E, Innocenti VM, Poli A. Reduced PTEN protein expression and its prognostic implications in canine and feline mammary tumors. Vet Pathol. 2009;46(5):860–868. doi:10.1354/vp.08-VP-0273-P-FL19429983
- Puente XS, Velasco G, Gutierrez-Fernandez A, Bertranpetit J, King MC, Lopez-Otin C. Comparative analysis of cancer genes in the human and chimpanzee genomes. BMC Genomics. 2006;7:15. doi:10.1186/1471-2164-7-1516438707
- Pouladi MA, Morton AJ, Hayden MR. Choosing an animal model for the study of Huntington’s disease. Nat Rev Neurosci. 2013;14(10):708–721. doi:10.1038/nrn357024052178
- Fan Y, Huang ZY, Cao CC, et al. Genome of the Chinese tree shrew. Nat Commun. 2013;4:1426. doi:10.1038/ncomms241623385571
- Tu Q, Yang D, Zhang X, et al. A novel pancreatic cancer model originated from transformation of acinar cells in adult tree shrew, a primate-like animal. Dis Model Mech. 2019;12(4):dmm038703. doi:10.1242/dmm.03870330910991
- Ge GZ, Xia HJ, He BL, et al. Generation and characterization of a breast carcinoma model by PyMT overexpression in mammary epithelial cells of tree shrew, an animal close to primates in evolution. Int J Cancer. 2016;138(3):642–651. doi:10.1002/ijc.2981426296387
- Schachtschneider KM, Madsen O, Park C, Rund LA, Groenen MA, Schook LB. Adult porcine genome-wide DNA methylation patterns support pigs as a biomedical model. BMC Genomics. 2015;16:743. doi:10.1186/s12864-015-1938-x26438392
- Groenen MA, Archibald AL, Uenishi H, et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature. 2012;491(7424):393–398. doi:10.1038/nature1162223151582
- Mitchell J, Tinkey PT, Avritscher R, et al. Validation of a preclinical model of diethylnitrosamine-induced hepatic neoplasia in yucatan miniature pigs. Oncology. 2016;91(2):90–100. doi:10.1159/00044607427305144
- Wang K, Jin Q, Ruan D, et al. Cre-dependent Cas9-expressing pigs enable efficient in vivo genome editing. Genome Res. 2017;27(12):2061–2071. doi:10.1101/gr.222521.11729146772
- Roth WJ, Kissinger CB, McCain RR, et al. Assessment of juvenile pigs to serve as human pediatric surrogates for preclinical formulation pharmacokinetic testing. AAPS J. 2013;15(3):763–774. doi:10.1208/s12248-013-9482-623595360
- Kucharavy A, Rubinstein B, Zhu J, Li R, Mogilner A. Robustness and evolvability of heterogeneous cell populations. Mol Biol Cell. 2018;29(11):1400–1409. doi:10.1091/mbc.E18-01-007029851566
- Leidy-Davis T, Cheng K, Goodwin LO, et al. Viable mice with extensive gene humanization (25-kbp) created using embryonic stem cell/blastocyst and CRISPR/zygote injection approaches. Sci Rep. 2018;8(1):15028. doi:10.1038/s41598-018-33408-930301924
- Borga C, Park G, Foster C, et al. Simultaneous B and T cell acute lymphoblastic leukemias in zebrafish driven by transgenic MYC: implications for oncogenesis and lymphopoiesis. Leukemia. 2019;33(2):333–347. doi:10.1038/s41375-018-0226-630111845
- Lu JW, Hsieh MS, Hou HA, Chen CY, Tien HF, Lin LI. Overexpression of SOX4 correlates with poor prognosis of acute myeloid leukemia and is leukemogenic in zebrafish. Blood Cancer J. 2017;7(8):e593. doi:10.1038/bcj.2017.7428841206
- He S, Lamers GE, Beenakker JW, et al. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J Pathol. 2012;227(4):431–445. doi:10.1002/path.401322374800
- Paul CD, Bishop K, Devine A, et al. Tissue architectural cues drive organ targeting of tumor cells in zebrafish. Cell Syst. 2019;9(2):187–206 e116. doi:10.1016/j.cels.2019.07.00531445892
- Fenizia C, Bottino C, Corbetta S, et al. SMYD3 promotes the epithelial-mesenchymal transition in breast cancer. Nucleic Acids Res. 2019;47(3):1278–1293. doi:10.1093/nar/gky122130544196
- Nadar RA, Asokan N, Degli Esposti L, et al. Preclinical evaluation of platinum-loaded hydroxyapatite nanoparticles in an embryonic zebrafish xenograft model. Nanoscale. 2020;12(25):13582–13594. doi:10.1039/D0NR04064A32555916
- Basti A, Fior R, Yalin M, et al. The core-clock gene NR1D1 impacts cell motility in vitro and invasiveness in a zebrafish xenograft colon cancer model. Cancers (Basel). 2020;12(4):853. doi:10.3390/cancers12040853
- Paauwe M, Schoonderwoerd MJA, Helderman R, et al. Endoglin expression on cancer-associated fibroblasts regulates invasion and stimulates colorectal cancer metastasis. Clin Cancer Res. 2018;24(24):6331–6344. doi:10.1158/1078-0432.CCR-18-032929945992
- Jiang X, Liu G, Hu Z, Chen G, Chen J, Lv Z. cGAMP inhibits tumor growth in colorectal cancer metastasis through the STING/STAT3 axis in a zebrafish xenograft model. Fish Shellfish Immunol. 2019;95:220–226. doi:10.1016/j.fsi.2019.09.07531586458
- Fior R, Povoa V, Mendes RV, et al. Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts. Proc Natl Acad Sci U S A. 2017;114(39):E8234–E8243. doi:10.1073/pnas.161838911428835536
- Petrovic J, Glamoclija J, Ilic-Tomic T, et al. Lectin from Laetiporus sulphureus effectively inhibits angiogenesis and tumor development in the zebrafish xenograft models of colorectal carcinoma and melanoma. Int J Biol Macromol. 2020;148:129–139. doi:10.1016/j.ijbiomac.2020.01.03331935408
- Roel M, Rubiolo JA, Guerra-Varela J, et al. Marine guanidine alkaloids crambescidins inhibit tumor growth and activate intrinsic apoptotic signaling inducing tumor regression in a colorectal carcinoma zebrafish xenograft model. Oncotarget. 2016;7(50):83071–83087. doi:10.18632/oncotarget.1306827825113
- Banasavadi-Siddegowda YK, Welker AM, An M, et al. PRMT5 as a druggable target for glioblastoma therapy. Neuro Oncol. 2018;20(6):753–763. doi:10.1093/neuonc/nox20629106602
- Nguyen AT, Emelyanov A, Koh CH, Spitsbergen JM, Parinov S, Gong Z. An inducible kras(V12) transgenic zebrafish model for liver tumorigenesis and chemical drug screening. Dis Model Mech. 2012;5(1):63–72. doi:10.1242/dmm.00836721903676
- Li Z, Zheng W, Wang Z, et al. A transgenic zebrafish liver tumor model with inducible Myc expression reveals conserved Myc signatures with mammalian liver tumors. Dis Model Mech. 2013;6(2):414–423. doi:10.1242/dmm.01046223038063
- Mizgireuv IV, Majorova IG, Gorodinskaya VM, Khudoley VV, Revskoy SY. Carcinogenic effect of N-nitrosodimethylamine on diploid and triploid zebrafish (Danio rerio). Toxicol Pathol. 2004;32(5):514–518. doi:10.1080/0192623049049631115603536
- Shwartz A, Goessling W, Yin C. Macrophages in zebrafish models of liver diseases. Front Immunol. 2019;10:2840. doi:10.3389/fimmu.2019.0284031867007
- Yang Q, Yan C, Gong Z. Activation of liver stromal cells is associated with male-biased liver tumor initiation in xmrk and Myc transgenic zebrafish. Sci Rep. 2017;7(1):10315. doi:10.1038/s41598-017-10529-128871112
- Li Z, Huang X, Zhan H, et al. Inducible and repressable oncogene-addicted hepatocellular carcinoma in Tet-on xmrk transgenic zebrafish. J Hepatol. 2012;56(2):419–425. doi:10.1016/j.jhep.2011.07.02521888874
- Li Z, Luo H, Li C, et al. Transcriptomic analysis of a transgenic zebrafish hepatocellular carcinoma model reveals a prominent role of immune responses in tumour progression and regression. Int J Cancer. 2014;135(7):1564–1573. doi:10.1002/ijc.2879424550086
- Evason KJ, Francisco MT, Juric V, et al. Identification of chemical inhibitors of beta-catenin-driven liver tumorigenesis in zebrafish. PLoS Genet. 2015;11(7):e1005305. doi:10.1371/journal.pgen.100530526134322
- Yan C, Huo X, Wang S, Feng Y, Gong Z. Stimulation of hepatocarcinogenesis by neutrophils upon induction of oncogenic kras expression in transgenic zebrafish. J Hepatol. 2015;63(2):420–428. doi:10.1016/j.jhep.2015.03.02425828472
- Pruvot B, Jacquel A, Droin N, et al. Leukemic cell xenograft in zebrafish embryo for investigating drug efficacy. Haematologica. 2011;96(4):612–616. doi:10.3324/haematol.2010.03140121228037
- Zhang B, Shimada Y, Kuroyanagi J, Umemoto N, Nishimura Y, Tanaka T. Quantitative phenotyping-based in vivo chemical screening in a zebrafish model of leukemia stem cell xenotransplantation. PLoS One. 2014;9(1):e85439. doi:10.1371/journal.pone.008543924454867
- Yang Q, Salim L, Yan C, Gong Z. Rapid analysis of effects of environmental toxicants on tumorigenesis and inflammation using a transgenic zebrafish model for liver cancer. Mar Biotechnol (NY). 2019;21(3):396–405. doi:10.1007/s10126-019-09889-830852708
- Chew TW, Liu XJ, Liu L, Spitsbergen JM, Gong Z, Low BC. Crosstalk of Ras and Rho: activation of RhoA abates Kras-induced liver tumorigenesis in transgenic zebrafish models. Oncogene. 2014;33(21):2717–2727. doi:10.1038/onc.2013.24023812423
- Chiu CC, Chou HL, Chen BH, et al. BPIQ, a novel synthetic quinoline derivative, inhibits growth and induces mitochondrial apoptosis of lung cancer cells in vitro and in zebrafish xenograft model. BMC Cancer. 2015;15:962. doi:10.1186/s12885-015-1970-x26672745
- Jin Y, Wei L, Jiang Q, et al. Comparison of efficacy and toxicity of bevacizumab, endostar and apatinib in transgenic and human lung cancer xenograftzebrafish model. Sci Rep. 2018;8(1):15837. doi:10.1038/s41598-018-34030-530367145
- Topczewska JM, Postovit LM, Margaryan NV, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12(8):925–932. doi:10.1038/nm144816892036
- Patton EE, Widlund HR, Kutok JL, et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15(3):249–254. doi:10.1016/j.cub.2005.01.03115694309
- Antonio N, Bonnelykke-Behrndtz ML, Ward LC, et al. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015;34(17):2219–2236. doi:10.15252/embj.20149014726136213
- Hosono Y, Niknafs YS, Prensner JR, et al. Oncogenic role of THOR, a conserved cancer/testis long non-coding RNA. Cell. 2017;171(7):1559–1572 e1520. doi:10.1016/j.cell.2017.11.04029245011
- Verykiou S, Alexander M, Edwards N, et al. Harnessing autophagy to overcome mitogen-activated protein kinase kinase inhibitor-induced resistance in metastatic melanoma. Br J Dermatol. 2019;180(2):346–356. doi:10.1111/bjd.1733330339727
- Marques IJ, Weiss FU, Vlecken DH, et al. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer. 2009;9:128. doi:10.1186/1471-2407-9-12819400945
- Kendall GC, Watson S, Xu L, et al. PAX3-FOXO1 transgenic zebrafish models identify HES3 as a mediator of rhabdomyosarcoma tumorigenesis. Elife. 2018;7. doi:10.7554/eLife.33800
- Bentley VL, Veinotte CJ, Corkery DP, et al. Focused chemical genomics using zebrafish xenotransplantation as a pre-clinical therapeutic platform for T-cell acute lymphoblastic leukemia. Haematologica. 2015;100(1):70–76. doi:10.3324/haematol.2014.11074225281505
- Anelli V, Villefranc JA, Chhangawala S, et al. Oncogenic BRAF disrupts thyroid morphogenesis and function via twist expression. Elife. 2017;6. doi:10.7554/eLife.20728
- Chen C, Choudhury S, Wangsa D, et al. A multiplex preclinical model for adenoid cystic carcinoma of the salivary gland identifies regorafenib as a potential therapeutic drug. Sci Rep. 2017;7(1):11410. doi:10.1038/s41598-017-11764-228900283
- Alzubi MA, Turner TH, Olex AL, et al. Separation of breast cancer and organ microenvironment transcriptomes in metastases. Breast Cancer Res. 2019;21(1):36. doi:10.1186/s13058-019-1123-230841919
- Mercatali L, La Manna F, Groenewoud A, et al. Development of a patient-derived xenograft (PDX) of breast cancer bone metastasis in a zebrafish model. Int J Mol Sci. 2016;17(8):1375. doi:10.3390/ijms17081375
- Goto H, Shimono Y, Funakoshi Y, et al. Adipose-derived stem cells enhance human breast cancer growth and cancer stem cell-like properties through adipsin. Oncogene. 2019;38(6):767–779. doi:10.1038/s41388-018-0477-830177835
- Evans KW, Yuca E, Akcakanat A, et al. A population of heterogeneous breast cancer patient-derived xenografts demonstrate broad activity of PARP inhibitor in BRCA1/2 wild-type tumors. Clin Cancer Res. 2017;23(21):6468–6477. doi:10.1158/1078-0432.CCR-17-061529093017
- Bieniasz M, Radhakrishnan P, Faham N, De La OJ, Welm AL. Preclinical efficacy of ron kinase inhibitors alone and in combination with PI3K inhibitors for treatment of sfRon-expressing breast cancer patient-derived xenografts. Clin Cancer Res. 2015;21(24):5588–5600. doi:10.1158/1078-0432.CCR-14-328326289070
- Noh JJ, Kim MS, Cho YJ, et al. Anti-cancer activity of As4O6 and its efficacy in a series of patient-derived xenografts for human cervical cancer. Pharmaceutics. 2020;12(10):987. doi:10.3390/pharmaceutics12100987
- Teng R, Zhao J, Zhao Y, et al. Chimeric antigen receptor-modified T cells repressed solid tumors and their relapse in an established patient-derived colon carcinoma xenograft model. J Immunother. 2019;42(2):33–42. doi:10.1097/CJI.000000000000025130586347
- Yamaguchi N, Weinberg EM, Nguyen A, et al. PCK1 and DHODH drive colorectal cancer liver metastatic colonization and hypoxic growth by promoting nucleotide synthesis. Elife. 2019;8. doi:10.7554/eLife.52135
- Yao YM, Donoho GP, Iversen PW, et al. Mouse PDX trial suggests synergy of concurrent inhibition of RAF and EGFR in colorectal cancer with BRAF or KRAS mutations. Clin Cancer Res. 2017;23(18):5547–5560. doi:10.1158/1078-0432.CCR-16-325028611205
- Takeda M, Koseki J, Takahashi H, et al. Disruption of endolysosomal RAB5/7 efficiently eliminates colorectal cancer stem cells. Cancer Res. 2019;79(7):1426–1437. doi:10.1158/0008-5472.CAN-18-219230765602
- Kavuri SM, Jain N, Galimi F, et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015;5(8):832–841. doi:10.1158/2159-8290.CD-14-121126243863
- Mohamed Suhaimi NA, Phyo WM, Yap HY, et al. Metformin inhibits cellular proliferation and bioenergetics in colorectal cancer patient-derived xenografts. Mol Cancer Ther. 2017;16(9):2035–2044. doi:10.1158/1535-7163.MCT-16-079328533437
- Scott AJ, Arcaroli JJ, Bagby SM, et al. Cabozantinib exhibits potent antitumor activity in colorectal cancer patient-derived tumor xenograft models via autophagy and signaling mechanisms. Mol Cancer Ther. 2018;17(10):2112–2122. doi:10.1158/1535-7163.MCT-17-013130026382
- Jin G, Yao K, Guo Z, et al. APIO-EE-9 is a novel Aurora A and B antagonist that suppresses esophageal cancer growth in a PDX mouse model. Oncotarget. 2017;8(32):53387–53404. doi:10.18632/oncotarget.1850828881819
- Wu JQ, Zhai J, Li CY, et al. Patient-derived xenograft in zebrafish embryos: a new platform for translational research in gastric cancer. J Exp Clin Cancer Res. 2017;36(1):160. doi:10.1186/s13046-017-0631-029141689
- Zhang X, Li Z, Xuan Z, et al. Novel role of miR-133a-3p in repressing gastric cancer growth and metastasis via blocking autophagy-mediated glutaminolysis. J Exp Clin Cancer Res. 2018;37(1):320. doi:10.1186/s13046-018-0993-y30572959
- Zhang X, Wang S, Wang H, et al. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol Cancer. 2019;18(1):20. doi:10.1186/s12943-018-0935-530717751
- Li Q, Zhu CC, Ni B, et al. Lysyl oxidase promotes liver metastasis of gastric cancer via facilitating the reciprocal interactions between tumor cells and cancer associated fibroblasts. EBioMedicine. 2019;49:157–171. doi:10.1016/j.ebiom.2019.10.03731678002
- Li S, Zhuang Z, Wu T, et al. Nicotinamide nucleotide transhydrogenase-mediated redox homeostasis promotes tumor growth and metastasis in gastric cancer. Redox Biol. 2018;18:246–255. doi:10.1016/j.redox.2018.07.01730059901
- Huang T, Zhou F, Yuan X, et al. Reactive oxygen species are involved in the development of gastric cancer and gastric cancer-related depression through ABL1-mediated inflammation signaling pathway. Oxid Med Cell Longev. 2019;2019:5813985. doi:10.1155/2019/581398531396300
- Zu LD, Peng XC, Zeng Z, et al. Gastrin inhibits gastric cancer progression through activating the ERK-P65-miR23a/27a/24 axis. J Exp Clin Cancer Res. 2018;37(1):115. doi:10.1186/s13046-018-0782-729866191
- Li C, Deng C, Pan G, et al. Lycorine hydrochloride inhibits cell proliferation and induces apoptosis through promoting FBXW7-MCL1 axis in gastric cancer. J Exp Clin Cancer Res. 2020;39(1):230. doi:10.1186/s13046-020-01743-333126914
- Wang X, Fu R, Hu Y, et al. EGFR gene status predicts response and survival benefit in a preclinical gastric cancer trial treating patientderived xenografts with cetuximab. Oncol Rep. 2017;38(4):2387–2393. doi:10.3892/or.2017.590728849161
- Wang Q, Zhang X, Shen E, et al. The anti-HER3 antibody in combination with trastuzumab exerts synergistic antitumor activity in HER2-positive gastric cancer. Cancer Lett. 2016;380(1):20–30.27317872
- Guo X, Zhou N, Chen Z, et al. Construction of (124)I-trastuzumab for noninvasive PET imaging of HER2 expression: from patient-derived xenograft models to gastric cancer patients. Gastric Cancer. 2020;23(4):614–626. doi:10.1007/s10120-019-01035-631919745
- Wu CX, Wang XQ, Chok SH, et al. Blocking CDK1/PDK1/beta-Catenin signaling by CDK1 inhibitor RO3306 increased the efficacy of sorafenib treatment by targeting cancer stem cells in a preclinical model of hepatocellular carcinoma. Theranostics. 2018;8(14):3737–3750.30083256
- Harrington BS, He Y, Davies CM, et al. Cell line and patient-derived xenograft models reveal elevated CDCP1 as a target in high-grade serous ovarian cancer. Br J Cancer. 2016;114(4):417–426. doi:10.1038/bjc.2015.47126882065
- Zhang S, Zheng C, Zhu W, et al. A novel anti-DR5 antibody-drug conjugate possesses a high-potential therapeutic efficacy for leukemia and solid tumors. Theranostics. 2019;9(18):5412–5423. doi:10.7150/thno.3359831410224
- Katz A, Barash U, Boyango I, et al. Patient derived xenografts (PDX) predict an effective heparanase-based therapy for lung cancer. Oncotarget. 2018;9(27):19294–19306. doi:10.18632/oncotarget.2502229721203
- He D, Zhang J, Wu W, et al. A novel immunodeficient rat model supports human lung cancer xenografts. FASEB J. 2019;33(1):140–150. doi:10.1096/fj.201800102RR29944447
- Weeden CE, Holik AZ, Young RJ, et al. Cisplatin increases sensitivity to FGFR inhibition in patient-derived xenograft models of lung squamous cell carcinoma. Mol Cancer Ther. 2017;16(8):1610–1622. doi:10.1158/1535-7163.MCT-17-017428611104
- Kong Y, Sheng X, Wu X, et al. Frequent genetic aberrations in the CDK4 pathway in acral melanoma indicate the potential for CDK4/6 inhibitors in targeted therapy. Clin Cancer Res. 2017;23(22):6946–6957. doi:10.1158/1078-0432.CCR-17-007028830923
- Liu DS, Read M, Cullinane C, et al. APR-246 potently inhibits tumour growth and overcomes chemoresistance in preclinical models of oesophageal adenocarcinoma. Gut. 2015;64(10):1506–1516. doi:10.1136/gutjnl-2015-30977026187504
- Butler KA, Hou X, Becker MA, et al. Prevention of human lymphoproliferative tumor formation in ovarian cancer patient-derived xenografts. Neoplasia. 2017;19(8):628–636. doi:10.1016/j.neo.2017.04.00728658648
- Ricci F, Guffanti F, Damia G, Broggini M. Combination of paclitaxel, bevacizumab and MEK162 in second line treatment in platinum-relapsing patient derived ovarian cancer xenografts. Mol Cancer. 2017;16(1):97. doi:10.1186/s12943-017-0662-328558767
- Guo S, Gao S, Liu R, et al. Oncological and genetic factors impacting PDX model construction with NSG mice in pancreatic cancer. FASEB J. 2019;33(1):873–884. doi:10.1096/fj.201800617R30091943
- Fang C, Dai CY, Mei Z, et al. microRNA-193a stimulates pancreatic cancer cell repopulation and metastasis through modulating TGF-beta2/TGF-betaRIII signalings. J Exp Clin Cancer Res. 2018;37(1):25. doi:10.1186/s13046-018-0697-329433538
- Yang D, Zhang Q, Ma Y, et al. Augmenting the therapeutic efficacy of adenosine against pancreatic cancer by switching the Akt/p21-dependent senescence to apoptosis. EBioMedicine. 2019;47:114–127. doi:10.1016/j.ebiom.2019.08.06831495718
- Cai W, Ratnayake R, Gerber MH, et al. Development of apratoxin S10 (Apra S10) as an anti-pancreatic cancer agent and its preliminary evaluation in an orthotopic patient-derived xenograft (PDX) model. Invest New Drugs. 2019;37(2):364–374. doi:10.1007/s10637-018-0647-030073464
- Schuller AG, Barry ER, Jones RD, et al. The MET inhibitor AZD6094 (Savolitinib, HMPL-504) induces regression in papillary renal cell carcinoma patient-derived xenograft models. Clin Cancer Res. 2015;21(12):2811–2819. doi:10.1158/1078-0432.CCR-14-268525779944
- Varkaris A, Corn PG, Parikh NU, et al. Integrating murine and clinical trials with cabozantinib to understand roles of MET and VEGFR2 as targets for growth inhibition of prostate cancer. Clin Cancer Res. 2016;22(1):107–121. doi:10.1158/1078-0432.CCR-15-023526272062
- Tsai H, Morais CL, Alshalalfa M, et al. Cyclin D1 loss distinguishes prostatic small-cell carcinoma from most prostatic adenocarcinomas. Clin Cancer Res. 2015;21(24):5619–5629. doi:10.1158/1078-0432.CCR-15-074426246306
- Hatem R, Labiod D, Chateau-Joubert S, et al. Vandetanib as a potential new treatment for estrogen receptor-negative breast cancers. Int J Cancer. 2016;138(10):2510–2521. doi:10.1002/ijc.2997426686064
- Laird JH, Lok BH, Ma J, et al. Talazoparib is a potent radiosensitizer in small cell lung cancer cell lines and xenografts. Clin Cancer Res. 2018;24(20):5143–5152. doi:10.1158/1078-0432.CCR-18-040129945991
- Gonzalez-Gonzalez A, Munoz-Muela E, Marchal JA, et al. Activating transcription factor 4 modulates TGFbeta-induced aggressiveness in triple-negative breast cancer via SMAD2/3/4 and mTORC2 signaling. Clin Cancer Res. 2018;24(22):5697–5709. doi:10.1158/1078-0432.CCR-17-312530012564
- Marangoni E, Laurent C, Coussy F, et al. Capecitabine efficacy is correlated with TYMP and RB1 expression in PDX established from triple-negative breast cancers. Clin Cancer Res. 2018;24(11):2605–2615. doi:10.1158/1078-0432.CCR-17-349029463559
- Arango NP, Yuca E, Zhao M, et al. Selinexor (KPT-330) demonstrates anti-tumor efficacy in preclinical models of triple-negative breast cancer. Breast Cancer Res. 2017;19(1):93. doi:10.1186/s13058-017-0878-628810913
