Abstract
Purpose
The present study investigated the expression and function of the long noncoding RNA (lncRNA) actin filament associated protein 1 antisense RNA1 (AFAP1-AS1) related to gastric cancer (GC), based on previous results from a microarray analysis.
Methods
Real-time quantitative polymerase chain reaction (qPCR) was used to verify the expression of AFAP1-AS1 in 97 fresh GC tissues and paired non-GC tissues, as well as in six different GC cell lines (BGC-823, SGC-7901, MGC-803, AGS, MKN-45, and MKN-28). The expression levels were subsequently correlated with the clinicopathological features of patients. siRNA against AFAP1-AS1 was transfected into GC cell lines, and cell proliferation, migration, and invasion were detected before and after silencing of AFAP1-AS1 expression. Luciferase reporter gene analysis was used to confirm the target gene of microRNA-205-5p (miR-205-5p) in 293T cells. The potential mechanism was subsequently investigated.
Results
qPCR results showed that AFAP1-AS1 was significantly overexpressed in GC tumor tissues and also GC cell lines, comparing to their paired non-GC tissues. Furthermore, statistical analysis revealed that the overexpression of AFAP1-AS1 was significantly correlated with tumor size (p=0.018) and grade of differentiation (p=0.042). Subsequently, artificially decreasing the expression of AFAP1-AS1 with its specific siRNA dramatically inhibited the proliferation, migration and invasion of GC cell lines (SGC-7901 and BGC-823 cells). Mechanical analysis suggested that AFAP1-AS1 is involved in regulation of its maternal gene, AFAP1, at both mRNA level and protein level. Luciferase reporter gene assay indicated that lncRNA AFAP1-AS1, as a ceRNA, is able to sponge miR-205-5p. Moreover, miR-205-5p has been well demonstrated to participate in the regulation of AFAP1 expression and the phenotypes of GC cells, including proliferation, migration and invasion.
Conclusion
AFAP1-AS1, as a novel biomarker of GC, promotes the proliferation migration and invasion of GC cells and function as ceRNA to target AFAP1 by sponging miR-205-5p.
Introduction
Despite the rapid advances in medicine, the incidence of cancer has persistently increased over the past decades. Gastric cancer (GC) remains one of the major and most common types of cancer in humans worldwide. Citation1 China is one of the countries with the highest incidence of GC. It is reported that China had 405,000 new cases of GC, accounting for 42.5% of the newly diagnosed cases worldwide.
Gastric adenocarcinomas is a predominant GC subtype accounting for 95% of GG and causing around 700 thousands persons dying from GC. Citation2 Although, the great progress has been obtained in diagnosis and therapy of GC, the prognosis of GC patients remains dissatisfied. In clinical practice, most of GC patients were diagnosed at middle or advantaged stage, who. Therefore, it is still necessary to discover novel potential biomarker of GC and further explore the underlying mechanism of GC progression.
Recently, many lines of evidence have demonstrated that long noncoding RNAs (lncRNAs) are closely related to the development of human cancer. Moreover, altered expression of lncRNAs is frequently detected in several types of cancer, including GC. Citation3–5 The lncRNAs, defined as noncoding RNAs with >200 nucleotides, do not encode proteins. Citation6 Several studies have shown that lncRNAs regulate crucial biological processes, thereby participating in the regulation of oncogenesis and cancer development. Citation7
Actin filament associated protein 1 antisense RNA1 (AFAP1-AS1) is a lncRNA (6810 nucleotides); in humans, the gene is localized on chromosome 4. Several studies have investigated the roles of AFAP1-AS1 in numerous types of cancer, including lung cancer, Citation8,Citation9 pancreatic ductal adenocarcinoma, Citation10 esophageal adenocarcinoma (EA), Citation11 esophageal squamous cell carcinoma, Citation12 nasopharyngeal carcinoma, Citation13 and hepatocellular carcinoma. Citation14,Citation15 In a previous analysis of the lncRNA expression profile using a microarray assay, we found that AFAP1-AS1 was upregulated in six GC tissues compared with paired non-cancerous tissues. To investigate the relationship between AFAP1-AS1 and GC, the expression of AFAP1-AS1 was further verified in a study involving a larger sample size composed of tissues and six different GC cell lines. In addition, the potential functions of AFAP1-AS1 on the proliferation, migration, and invasion of GC cells was also investigated in this study.
Materials and Methods
Tissue Samples and RNA Extraction
In this investigation, 97 fresh frozen GC and their paired non-cancerous tissues were collected at Fuzhou General Hospital, which were used to assess the expression of AFAP1-AS1. The collection and preservation of fresh GC tissue samples were carried out as previously described. Citation16 Briefly, GC tissues and paired non-cancerous tissues were chopped immediately after surgical resection and preserved in RNAlater at −80°C until further use. The RNA from these fresh tissue samples was extracted using TRIzol (Invitrogen, CA, USA) according to the instructions provided by the manufacturer.
Serum samples from the same patients were also routinely collected before the operation to assess the levels of the digestive tract and epithelial tumor markers (carcinoembryonic antigen, carbohydrate antigen 19–9 [CA19-9], alpha fetoprotein, and CA125). Previously described assay methods and normal reference values were utilized. Citation16 TNM staging and histological typing were routinely performed by pathologists in compliance with Chinese and international standards. Data regarding the clinicopathological features of patients were collected from their medical records.
Ethics Statement
This study was approved by the Ethics Committee of 900 Hospital of the Joint Logistics Team Hospital (Fuzhou General Hospital), and informed consent was provided by all patients. This study was conducted in accordance with the Declaration of Helsinki.
Microarray for the Expression Profile of lncRNA
The expression profile of lncRNAs was conducted by Kangchen Biotech (Shanghai, China) in our previous study. Citation17
Cell Lines and siRNA Against AFAP1-AS1
The human gastric epithelial cells line (GES-1) and GC cell lines (BGC-823, SGC-7901, MGC-803, MKN-45, MKN-28, and AGS) were purchased from Beijing Cancer Institute (Beijing, China), the Cell Bank of Shanghai Institute of Biochemistry and Cell Biology, and the Chinese Academy of Sciences in Shanghai, China. The cells were cultured in Dulbecco’s modified Eagle’s medium (GES-1), RPMI 1640 (BGC-823, SGC-7901, MGC-803, MKN-45, and MKN-28), and F12 medium (AGS) supplemented with 10% fetal bovine serum, in an incubator with 5% CO2 at 37°C. To silence the expression of AFAP1-AS1, cells were seeded overnight and transfected with either 5 µL of 20 μM siRNA (final concentration: 50 nM) against AFAP1-AS1 (AFAP1-AS1 si1 and AFAP1-AS1 si3) or control siRNA (non-target scramble siRNA, NC) (Rubio, Guangzhou, China) () with Lipofectamine 2000 (Invitrogen) in Opti-MEM (Invitrogen) according to the instructions provided by the manufacturer.
Table 1 The Sequences of siRNA Against AFAP1-AS1
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA from the cultured cells was extracted as previously described. Citation16 RT-qPCR was performed using a SYBR Green Mix Kit (Promega, Madison, WI, USA) in a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA), to determine the relative expression levels of AFAP1-AS1. The primers used for RT-qPCR are listed in . The levels of 18S were used as internal reference to normalize the relative expression of AFAP1-AS1 in both tissue and cell samples. Each measurement was performed in triplicate, and the average ΔCt value was calculated.
Table 2 The Primer Sequences for qPCR
Migration and Invasion Assays
Both cell migration and invasion assays were conducted using transwell chambers (diameter: 8 μm; Millipore). In the migration assay, the chambers did not contain Matrigel. In contrast, in the invasion assay, they were uniformly coated with 50 µL of Matrigel (1:3 dilution with culture medium; BD Biosciences). Cells (5×104) were suspended in 100 µL of serum-free RPMI 1640 medium containing 2% bovine serum albumin and seeded in the upper chamber. Subsequently, the set-up was placed in a 24-well plate containing 600 µL of RPMI 1640 with 10% fetal bovine serum. After incubation at 37°C for 24 h and 36 h for the migration and invasion assay, respectively, the cells in the chambers were fixed with methanol and stained with 0.1% crystal violet. Subsequently, the cells in the upper chamber were removed using cotton swabs, and the number of migrated and invasive tumor cells in five randomly selected fields at 200× magnification were counted for each experiment to determine the average.
Cell Proliferation Assay
After transfection with siRNA for 48 h, the cells were seeded and cultured in a 96-well plate at a density of 2500 cells/well (day 0). A Cell Counting Kit-8 (CCK-8; GenVIEW) was utilized for the cell proliferation assay on days 0, 1, 2, and 4 for SGC-7901 and BGC-823 cells according to the instructions provided by the manufacturer. CCK-8 reagent (10 µL) was added to each well and the cells were incubated at 37°C for 1 h. The absorbance was measured at 450 nm using a microplate reader (SpectraMax 190; Molecular Devices), and growth curves for cells were constructed. The experiments were conducted in six replicate wells and performed twice.
Colony Formation Assay
A single-cell suspension was achieved by trypsinizing the cultured cells. The cells were seeded at a density of 500/well in a six-well plate and cultured for 14 days to allow colony formation. The clones, defined as >50 cells/per colony, were counted using a grid. Three independent experiments were performed.
5-Ethynyl-2ʹ-Deoxyuridine (EdU) Incorporation Assay
The EdU incorporation assay was conducted using a Cell-Light EdU DNA Cell Proliferation Kit (RiboBio, Shanghai, China). Cells (1×104/well) were seeded in 96-well plates. EdU (50 mM) was added, and the cells were incubated for 2 h, fixed with 4% paraformaldehyde, and stained with Apollo Dye Solution. The nucleic acid within the cells was traced by staining with Hoechst 33,342. Images were examined with an Olympus FSX100 microscope (Olympus), and the number of EdU-positive cells was determined in five randomly selected fields. The results exhibited the average number of positive cells per field.
Fluorescence in situ Hybridization (FISH) Assay
The subcellular localization of AFAP1-AS1 in GC cells was determined using a FISH assay. According to the instructions of the manufacturer of Ribo™ lncRNA FISH Probe Mix (Red) (RiboBio Co., Ltd., Guangzhou, China), the assay was performed as follows: the coverslips were put into 24-well plates, and the cells at the logarithmic growth phase were inoculated onto the coverslips per well. When the cell confluence reached approximately 80% after 1 day of culture, the coverslips were washed with phosphate-buffered saline (PBS) and the cells were fixed with 4% paraformaldehyde at room temperature. The cells were subsequently prepared for prehybridization, and 250 μL of prehybridization solution was added to the cells. Next, the cells were incubated at 42°C for 1 h. Subsequently, the prehybridization solution was removed, and 250 μL of hybridization solution containing 300 ng/mL probe was added onto the cells, which were further incubated at 42°C overnight. The following day, the cells were washed with PBS with Tween-20 (PBST), and nucleus staining was performed by addition of 4′, 6-diamidino-2-phenylindole staining solution (diluted with PBST at a ratio of 1:800) for 5 min. Subsequently, the coverslips were washed with PBST and sealed with an anti-fluorescence quencher. Finally, five different fields were randomly observed with a fluorescence microscope (Olympus) and photographed accordingly.
Luciferase Reporter Assay
HEK-293T cells (5×103) were seeded into 96-well plates and co-transfected with a mixture of 50 ng of AFAP1-AS1 wild type or mutant firefly luciferase reporter, 5 ng of pRL-CMV Renilla luciferase reporter, and miRNA mimics. After 48 h of transfection, the firefly and Renilla luciferase activities were quantified with a dual-luciferase reporter assay (Promega).
Western Blotting Assay and Antibodies
Cells protein lysates separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis were transferred to 0.45 μm polyvinylidene difluoride membranes (Millipore) and incubated with specific antibodies. The enhanced chemiluminescence chromogenic substrate (GENVIEW) was used for quantification by densitometry (Quantity One software; Bio-Rad). The β-actin antibody (control) and AFAP1 antibody (1:1000 dilution) were purchased from Bioss Antibodies.
Statistical Analysis
Statistical analysis was conducted using the SPSS software, version 19.0 (IBM Corporation, Armonk, NY, USA). The statistically significant differences between any two groups and multiple groups were assessed using Student’s t-test and one-way analysis of variance (ANOVA), respectively. The Kaplan–Meier method was used to calculate the overall survival (OS), and p<0.05 values determined by the Log rank test denoted statistical significance. All data were expressed as means ± standard deviation.
Results
AFAP1-AS1 Was Overexpressed in GC Tissues
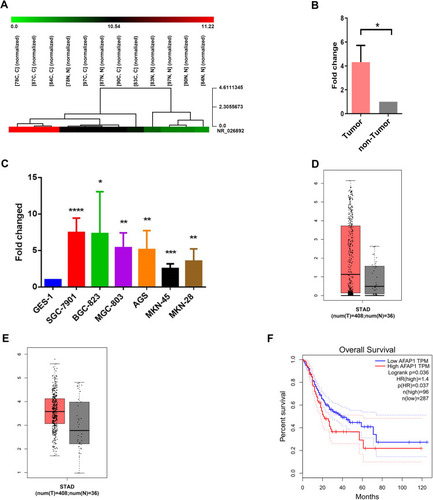
The results of the microarray assay showed that AFAP1-AS1 was overexpressed in GC tissues with a mean increase of 4.319-fold change (p=0.018) (), suggesting that this lncRNA may be associated with GC.
Figure 1 LncRNA microarray assay exhibited that AFAP1-AS1 was overexpressed in gastric cancer tissues. (A) Numbers (78, 87, 84, 83, 90, 97) indicate the tissue samples used for the microarray assay. (B) The relative expression of AFAP1-AS1 in tumorous and adjacent non-tumorous tissues was measured using RT-qPCR. (C) The relative expression of AFAP1-AS1 was measured in various cell lines, including one immortalized gastric epithelial cell line (GES-1) and six GC cell lines (AGS, SGC-7901, BGC-823, MGC-803, MKN-45, and MKN-28). (D) Data collected from the GEPIA database showed the relative expression of AFAP1-AS1 in GC tissues (n=408) and their corresponding non-tumorous tissues (n=36). (E) Data collected from the GEPIA database showed the relative expression of AFAP1 in GC tissues (n=408) and their corresponding non-tumorous tissues (n=36). (F) Kaplan–Meier overall survival curves according to the levels of AFAP1 expression. Error bars indicate mean±standard errors of the mean. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
AFAP1-AS1 Was Overexpressed in GC Tissues and Cell Lines
The results of the RT-qPCR analysis showed that AFAP1-AS1 was overexpressed in 53/97 fresh GC tissues (54.6%) compared with their paired non-cancerous tissues (p<0.05; ).
The results also revealed that AFAP1-AS1 was significantly overexpressed in SGC-7901, BGC-823, SGC-7901, MGC-803, AGS, MKN-45, and MKN-28 cells compared with the non-cancer GES-1 cells (p<0.0001, p<0.05, p<0.01, p<0.01, p<0.001, and p<0.01, respectively) (). These data demonstrated that AFAP1-AS1 is highly expressed in GC cells.
AFAP1 and AFAP1-AS1 Were Overexpressed in GC Tissues and High AFAP1 Was Associated with Poor OS
The analysis of 444 human GC and normal tissues using the bioinformatics tool “GEPIA (Gene Expression Profiling Interactive Analysis)” revealed that AFAP1-AS1 and AFAP1 were markedly overexpressed in GC tissues compared with adjacent normal tissues ().
The OS curve analysis and the Kaplan–Meier method showed that patients expressing higher levels of AFAP1 were associated with poorer OS (p=0.01) (). These results indicated that the expression of AFAP1 is a valuable indicator of prognosis and disease progression in GC.
AFAP1-AS1 Expression Correlates with the Tumor Diameter and Differentiation Grade
The AFAP1-AS1 expression was significantly related to the tumor diameter (cm) (p=0.018) and differentiation grade (p=0.042) (). In contrast, it was not related to age, sex, tumor location, venous invasion, invasive depth, lymphatic metastasis, perineural invasion, serum carcinoembryonic antigen, CA19-9, alpha fetoprotein, or CA125 (p>0.05 for all).
Table 3 Association of AFAP1-AS1 Expression (Δct) with the Clinicopathological Features of Patients
Silenced AFAP1-AS1 Expression Inhibited GC Cell Proliferation
The results of RT-qPCR, in SGC-7901 and BGC-823 cells showed that two different siRNAs (AFAP1-AS1 si1 and AFAP1-AS1 si3) effectively decreased the levels of AFAP1-AS1 in both cell lines ().
Figure 2 AFAP1-AS1 promotes GC cell proliferation in vitro. (A) RT-qPCR analysis of AFAP1-AS1 expression in BGC-823 cells transfected with control (scrambled), si-AFAP1-AS1 si1, and AFAP1-AS1 si3. (B) RT-qPCR analysis of AFAP1-AS1 expression in SGC-7901 cells transfected with control (scrambled), si-AFAP1-AS1 1 si1, and si-AFAP1-AS1 si3. (C and D) Colony formation assays were used to detect the proliferation of si-AFAP1-AS1-transfected GC cells. Colonies were counted and photographed. (E and F) CCK-8 assay was performed to determine the viability of GC cells treated with si-AFAP1-AS1. (G) Proliferous GC cells were determined using the EdU immunostaining assay. Values are presented as the mean±standard deviation of three independent experiments. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
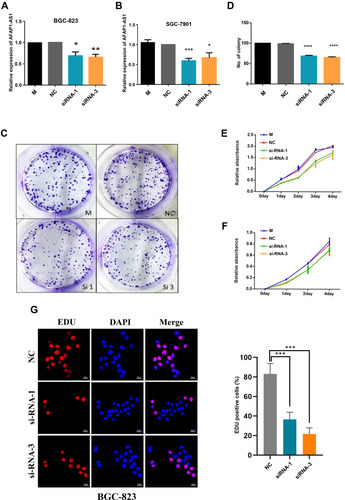
SGC-7901 and BGC-823 cells transfected with siRNA against AFAP1-AS1 exhibited a significantly reduced colony formation ratio (), decreased proliferation (), and inhibited DNA synthesis as determined by the EdU assay () versus those transfected with scrambled siRNA (control siRNA) (p<0.05 after day 2 and at day 14 for the CCK-8 assay and colony formation assay, respectively). The knockdown of AFAP1-AS1 significantly reduced the colony formation ability compared with scrambled siRNA. The ratio of colony formation in specific siRNA-transfected SGC-7901 cells was decreased by approximately 30%. These data suggested that the lncRNA AFAP1-AS1 may be involved in the regulation of GC cell proliferation. We observed that AFAP1-AS1 silencing significantly inhibited the cell proliferation rate as indicated by the effect on DNA synthesis determined through the EdU assay ().
Silencing of AFAP1-AS1 Inhibited GC Cell Migration and Invasion
The siRNA treatment led to a significantly decreased migratory potential in both SGC-7901 and BGC-823 cells versus those transfected with scrambled siRNA (p<0.05; ). Similarly, the Matrigel invasion assays exhibited that the invasive capability was also significantly decreased in AFAP1-AS1-silenced SGC-7901 and BGC-823 cells (p<0.05; ).
Figure 3 Cell migration and invasion in gastric cancer cell lines after silencing of AFAP1-AS1 expression. (A) Representative migration and invasion of BGC-823 cells before and after silencing of AFAP1-AS1 expression. (B) The results for the migration and invasion of BGC-823 cells are illustrated by histograms. (C) Representative migration and invasion of SGC-7901 cells before and after silencing of AFAP1-AS1 expression. (D) The results for the migration and invasion of SGC-7901 cells are illustrated by histograms. Data are representative of three independent experiments. **p<0.01, ***p<0.001.
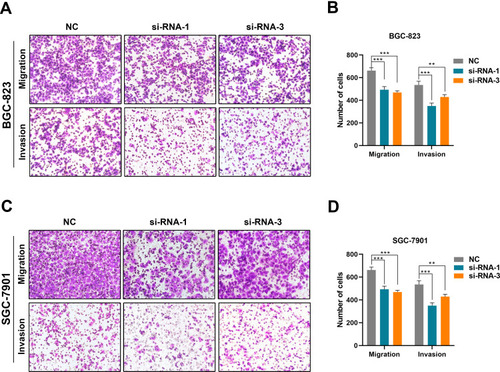
These data suggested that the lncRNA AFAP1-AS1 may also be involved in the regulation of migration and invasion in GC.
Subcellular Distribution of AFAP1-AS1 in GC Cells
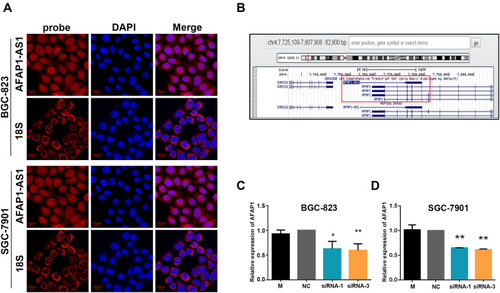
FISH analysis () revealed that, although AFAP1-AS1 appeared in both the cytoplasm and nucleus of BGC-823 and SGC-7901 cells, it was mainly located in the cytoplasm.
Figure 4 Location of AFAP1-AS1 expression in GC cells and the correlation between AFAP1-AS1 and AFAP1 expression. (A) RNA FISH for the location of AFAP1-AS1 in GC cells. (B) The schematic drawing of the genomics loci of AFAP1-AS1. (C and D) The results of RT-qPCR showed that silencing of AFAP1-AS1 expression significantly decreased the expression of AFAP1 in BGC-823 and SGC-7901 cells. Data are presented as the mean±standard deviation, n = 3. *p<0.05, **p<0.01.
Silencing of AFAP1-AS1 Decreased AFAP1 mRNA
AFAP1-AS1 was localized at the antisense chain of AFAP1. Furthermore, complementary and overlapping regions were identified among the exon 2 and exons 14, 15, and 16 of AFAP1 (). In terms of the association between AFAP1-AS1 and AFAP1 mRNA expression, the results from the RT-qPCR analysis exhibited that the silencing of AFAP1-AS1 expression in both BGC-823 and SGC-7901 cells significantly decreased the AFAP1 mRNA expression compared with that noted in scrambled siRNA-transfected cells (; p<0.05). These data indicated that AFAP1 may be linked to AFAP1-AS1 mRNA in GC, and functional alterations in AFAP1-AS1-regulated GC cells may be involved in the modulation of AFAP1 transcription.
AFAP1-AS1 Was Validated as a Target Gene of miR-205-5p
Analysis using Targetscan ( http://www.targetscan.org/vert_72/ ) showed that AFAP1-AS1 had the greatest potential to be the downstream target of miR-205-5p (). 293T cells co-transfected with a plasmid containing 3ʹ-untranslated region-wild type regions of AFAP1-AS1 and miR-205-5p mimics had significantly less relative luciferase activity than control cells co-transfected with miRNA NC mimics. Notably, mutation of the potential miR-205-5p binding sites in the AFAP1-AS1 3ʹ-untranslated region abolished this effect (). These results suggested that AFAP1-AS1 may be a putative target of miR-205-5p.
Figure 5 AFAP1-AS1 acts as a miRNA sponge for miR-205-5p. (A) A schematic drawing shows the putative binding sites of miR-205-5p with AFAP1-AS1. (B) A luciferase reporter assay was used to measure the luciferase activity of wild type or mutant LUC-AFAP1-AS1 in HEK-293T cell co-transfected with miRNA mimics. (C) AFAP1 protein expression was inhibited by silencing of AFAP1-AS1 in GC cells. (D) The results regarding AFAP1 protein expression are depicted by histograms. (E) Cell proliferation was inhibited and increased by overexpression and silencing of miR-205-5p in GC cells, respectively. The results regarding cell proliferation are depicted by histograms. (F) Cell invasion was inhibited and increased by overexpression and silencing of miR-205-5p in GC cells, respectively. The results regarding cell invasion are depicted by histograms. **p<0.01, ***p<0.001, ****p<0.0001.
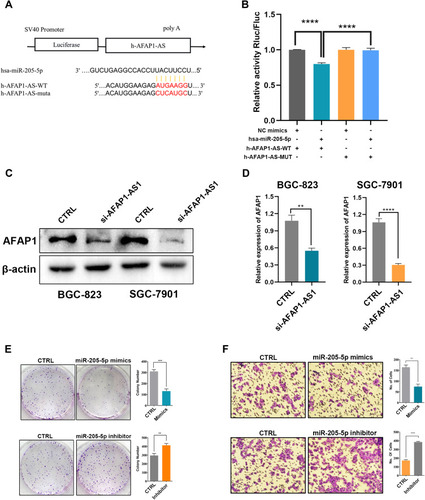
Low AFAP1-AS1 and High miR-205-5p Inhibited AFAP1 Expression, as Well as Cell Proliferation and Invasion
Western blotting demonstrated that silencing of AFAP1-AS1 considerably downregulated the expression of AFAP1 in GC cells ( and D). Following transfection with miR-205-5p mimics, reduced GC cell proliferation and invasion were detected ( ).
Discussion
Emerging findings have shown that the abnormal expression of lncRNAs is closely related to carcinogenesis and progression of cancer in humans. Citation2–9 In this study, by combining our previously determined lncRNA expression profile through microarray analysis Citation17 with documented literature, Citation2–9 we selected the lncRNA AFAP1-AS1 for further investigation. According to the microarray analysis, this lncRNA was upregulated in six GC tissues compared with six paired non-cancerous tissues. Its aberrant expression and functional involvement in different types of cancer have been identified in several previous studies and investigated in GC. Citation18–30 However, the relationship between AFAP1-AS1 and AFAP1 in GC has not been analyzed. Thus, the expression of ASAP1-AS1 in both GC tissues and GC cell lines was further investigated using RT-qPCR. The results confirmed that this lncRNA was overexpressed in fresh GC tissues as well as in all six GC cell lines examined in this study. Furthermore, the expression of AFAP1-AS1 was linked to the tumor diameter and differentiation grade. Tumors with a diameter <5 cm were associated with higher AFAP1-AS1 expression than those with a diameter ≥5 cm, indicating that AFAP1-AS1 may be a biomarker of early GC. Higher AFAP1-AS1 expression was also linked to a poor differentiation grade.
By utilizing the siRNA knockdown technology combined with in vitro experiments, we found that silencing of AFAP1-AS1 expression significantly decreased the proliferation, growth, migration, and invasion of SGC-7901 and BGC-823 cells. These results indicated that AFAP1-AS1 may play a role in the development of GC through the regulation of tumor cell proliferation, growth, migration, and invasion. This expression pattern and functions were similar to those of other cancers. Citation8–15
AFAP1-AS1 is an antisense lncRNA. Previous findings have shown that the genomic location of lncRNA could affect its activity. Antisense lncRNAs are located on the opposite strand with respect to the gene encoding the protein and commonly regulate that specific gene. Citation31–34 The antisense RNA is usually able to regulate its cognate sense gene expression; nevertheless, whether AFAP1-AS1 can regulate the expression of AFAP1 in GC remains unclear. In this study, by silencing AFAP1-AS1 using siRNA and assessing the mRNA and protein levels of AFAP1 through RT-qPCR and Western blotting, respectively, we found that AFAP1-AS1 knockdown significantly inhibited the mRNA and protein expression of AFAP1. Therefore, a reverse regulatory relationship between the expression levels of AFAP1-AS1 and AFAP1 may ensue.
AFAP1 is an adapter molecule that links to other proteins, including protein kinases such as protein kinase C (PKC). Furthermore, AFAP1 partakes in the regulation of actin filament integrity and lamellipodia formation. Citation35,Citation36 Regarding the roles of the AFAP1 gene in oncogenesis, studies of breast and prostate cancer have demonstrated its involvement in the regulation of breast cancer cell pathophysiology through cell adhesion and the formation of actin stress fiber. Citation37 AFAP1 is upregulated in prostate cancer and mediates contacts with focal cells to regulate oncogenesis. Citation38 Since silencing of AFAP1-AS1 decreased AFAP1 expression, we might speculate that AFAP1-AS1 mediates the development of GC via AFAP1 mRNA and protein expression.
Further exploration of the molecular mechanism revealed that AFAP1-AS1 may be the target gene of miR-205-5p, through which it regulated the mRNA and protein expression of AFAP1, as well as the proliferation, migration, and invasion of GC cells.
AFAP1-AS1 was initially found to be overexpressed and hypomethylated in primary EA tissues, which was related to increased proliferation, migration, and invasion of EA cells. Citation11 Ye et al demonstrated that increased expression of AFAP1-AS1 in pancreatic ductal adenocarcinoma was associated with worse survival and poor clinical outcomes, Citation10 which was in agreement with our results. These findings suggested a critical role of AFAP1-AS1 in oncogenesis.
Conclusions
The present study demonstrated that AFAP1-AS1 was upregulated in GC and may play a pivotal role in the occurrence of GC. Overexpression of AFAP1-AS1 was related to increased proliferation, migration, and invasion of GC cells; thus, AFAP1-AS1 may also serve as a treatment target for GC. These findings reveal a new mechanistic connection between AFAP1-AS1 and AFAP1 in regulating the progression of GC and may provide new insights and therapeutic strategies for the prevention and treatment of GC.
Abbreviations
lncRNAs, long noncoding RNAs; GC, gastric cancer; AFAP1-AS1, actin filament associated protein 1 antisense RNA1; AFAP1, actin filament associated protein 1; RT-qPCR, Real-time quantitative reverse transcription PCR; GES-1, gastric epithelial cells line; FBS, fetal bovine serum.
Data Sharing Statement
All data generated or analysed during this study are included in this published article.
Disclosure
The authors declare that they have no competing interests.
References
- Ferro A , Peleteiro B , Malvezzi M , et al. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer . 2014;50(7):1330–1344. doi:10.1016/j.ejca.2014.01.029 24650579
- Ferlay J , Soerjomataram I , Ervik M , et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11.
- Gao Y , Chen G , Zeng Y , et al. Invasion and metastasis-related long noncoding RNA expression profiles in hepatocellular carcinoma. Tumour Biol . 2015;36(10):7409–7422. doi:10.1007/s13277-015-3408-0 25900874
- Wang Y , Feng X , Jia R , et al. Microarray expression profile analysis of long non-coding RNAs of advanced stage human gastric cardia adenocarcinoma. Mol Genet Genomics . 2014;289(3):291–302. doi:10.1007/s00438-013-0810-4 24414129
- Song H , Sun W , Ye G , et al. Long non-coding RNA expression profile in human gastric cancer and its clinical significances. J Transl Med . 2013;11(1):225. doi:10.1186/1479-5876-11-225 24063685
- Wapinski O , Chang HY . Long noncoding RNAs and human disease. Trends Cell Biol . 2011;21(6):354–361. doi:10.1016/j.tcb.2011.04.001 21550244
- Fang XY , Pan HF , Leng RX , et al. Long noncoding RNAs: novel insights into gastric cancer. Cancer Lett . 2015;356(2):357–366. doi:10.1016/j.canlet.2014.11.005 25444905
- Deng J , Liang Y , Liu C , et al. The up-regulation of long non-coding RNA AFAP1-AS1 is associated with the poor prognosis of NSCLC patients. Biomed Pharmacother . 2015;75:8–11. doi:10.1016/j.biopha.2015.07.003 26463625
- Zeng Z , Bo H , Gong Z , et al. AFAP1-AS1, a long noncoding RNA upregulated in lung cancer and promotes invasion and metastasis. Tumour Biol . 2016;37(1):729–737. doi:10.1007/s13277-015-3860-x 26245991
- Ye Y , Chen J , Zhou Y , et al. High expression of AFAP1-AS1 is associated with poor survival and short-term recurrence in pancreatic ductal adenocarcinoma. J Transl Med . 2015;13(1):137.25925763
- Wu W , Bhagat TD , Yang X , et al. Hypomethylation of noncoding DNA regions and overexpression of the long noncoding RNA, AFAP1-AS1, in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterology . 2013;144(5):956–966.23333711
- Zhou XL , Wang WW , Zhu WG , et al. High expression of long non-coding RNA AFAP1-AS1 predicts chemoradioresistance and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Mol Carcinog . 2016;55(12):2095–2105. doi:10.1002/mc.22454 26756568
- Bo H , Gong Z , Zhang W , et al. Upregulated long non-coding RNA AFAP1-AS1 expression is associated with progression and poor prognosis of nasopharyngeal carcinoma. Oncotarget . 2015;6(24):20404–20418. doi:10.18632/oncotarget.4057 26246469
- Zhang JY , Weng MZ , Song FB , et al. Long noncoding RNA AFAP1-AS1 indicates a poor prognosis of hepatocellular carcinoma and promotes cell proliferation and invasion via upregulation of the RhoA/Rac2 signaling. Int J Oncol . 2016;48(4):1590–1598. doi:10.3892/ijo.2016.3385 26892468
- Lu X , Zhou C , Li R , et al. Critical role for the long non-coding RNA AFAP1-AS1 in the proliferation and metastasis of hepatocellular carcinoma. Tumour Biol . 2016;37(7):9699–9707. doi:10.1007/s13277-016-4858-8 26803513
- Dang Y , Lan F , Ouyang X , et al. Expression and clinical significance of long non-coding RNA HNF1A-AS1 in human gastric cancer. World J Surg Oncol . 2015;13(1):302. doi:10.1186/s12957-015-0706-3 26472090
- Dang Y , Lin L , Ouyang X , et al. LncRNA expression profiling in advanced resected gastric adenocarcinoma tissues. Clin Lab . 2019;65(12). doi:10.7754/Clin.Lab.2019.190432
- Li Z , Ding Z , Rong D , Tang W , Cao H . Overexpression of lncRNA AFAP1-AS1 promotes cell proliferation and invasion in gastric cancer. Oncol Lett . 2019;18(3):3211–3217. doi:10.3892/ol.2019.10640 31452798
- Feng Y , Zhang Q , Wang J , Liu P . Increased lncRNA AFAP1-AS1 expression predicts poor prognosis and promotes malignant phenotypes in gastric cancer. Eur Rev Med Pharmacol Sci . 2017;21(17):3842–3849.28975981
- Guo JQ , Li SJ , Guo GX . Long noncoding RNA AFAP1-AS1 promotes cell proliferation and apoptosis of gastric cancer cells via PTEN/p-AKT pathway. Dig Dis Sci . 2017;62(8):2004–2010. doi:10.1007/s10620-017-4584-0 28451917
- Gao S , Zhao ZY , Wu R , Zhang Y , Zhang ZY . Prognostic value of long noncoding RNAs in gastric cancer: a meta-analysis. Onco Targets Ther . 2018;11:4877–4891. doi:10.2147/OTT.S169823 30147339
- Ji D , Zhong X , Jiang X , et al. The role of long non-coding RNA AFAP1-AS1 in human malignant tumors. Pathol Res Pract . 2018;214(10):1524–1531. doi:10.1016/j.prp.2018.08.014 30173945
- Ye F , Gong Y , Chen X , et al. Long noncoding AFAP1-antisense RNA 1 is upregulated and promotes tumorigenesis in gastric cancer. Oncol Lett . 2018;15(5):7523–7530. doi:10.3892/ol.2018.8266 29740481
- Esfandi F , Taheri M , Namvar A , Oskooei VK , Ghafouri-Fard S . AFAP1 and its naturally occurring antisense RNA are downregulated in gastric cancer samples. Biomed Rep . 2019;10(5):296–302. doi:10.3892/br.2019.1207 31086663
- Zhao H , Zhang K , Wang T , et al. Long non-coding RNA AFAP1-antisense RNA 1 promotes the proliferation, migration and invasion of gastric cancer cells and is associated with poor patient survival. Oncol Lett . 2018;15(6):8620–8626. doi:10.3892/ol.2018.8389 29805596
- Zhang F , Li J , Xiao H , Zou Y , Liu Y , Huang W . AFAP1-AS1: a novel oncogenic long non-coding RNA in human cancers. Cell Prolif . 2018;51(1):e12397. doi:10.1111/cpr.12397
- Liu W , Li Y , Zhang Y , et al. Circulating long non-coding RNA FEZF1-AS1 and AFAP1-AS1 serve as potential diagnostic biomarkers for gastric cancer. Pathol Res Pract . 2020;216(1):152757. doi:10.1016/j.prp.2019.152757 31785996
- Ma HW , Xi DY , Ma JZ , et al. Long noncoding RNA AFAP1-AS1 promotes cell proliferation and metastasis via the miR-155-5p/FGF7 axis and predicts poor prognosis in gastric cancer. Dis Markers . 2020;2020:8140989. doi:10.1155/2020/8140989 32051698
- Yuan X-H , Li J , Cao Y , Jie Z-G , Zeng Y-F . Long non-coding RNA AFAP1-AS1 promotes proliferation and migration of gastric cancer by downregulating KLF2. Eur Rev Med Pharmacol Sci . 2020;24(2):673–680. doi:10.26355/eurrev_202001_20044 32016968
- Mo X , Wu Y , Chen L , et al. Global expression profiling of metabolic pathway-related lncRNAs in human gastric cancer and the identification of RP11-555H23.1 as a new diagnostic biomarker. J Clin Lab Anal . 2019;33(2):e22692. doi:10.1002/jcla.22692 30320481
- Yuan SX , Tao QF , Wang J , et al. Antisense long non-coding RNA PCNA-AS1 promotes tumor growth by regulating proliferating cell nuclear antigen in hepatocellular carcinoma. Cancer Lett . 2014;349(1):87–94. doi:10.1016/j.canlet.2014.03.029 24704293
- Sehgal L , Mathur R , Braun FK , et al. FAS-antisense 1 lncRNA and production of soluble versus membrane Fas in B-cell lymphoma. Leukemia . 2014;28(12):2376–2387. doi:10.1038/leu.2014.126 24811343
- Wang Y , Pang WJ , Wei N , et al. Identification, stability and expression of Sirt1 antisense long non-coding RNA. Gene . 2014;539:117–124.24480449
- Carrieri C , Cimatti L , Biagioli M , et al. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature . 2012;491(7424):454–457. doi:10.1038/nature11508 23064229
- Baisden JM , Qian Y , Zot HM , et al. The actin filament-associated protein AFAP-110 is an adaptor protein that modulates changes in actin filament integrity. Oncogene . 2001;20(44):6435–6447. doi:10.1038/sj.onc.1204784 11607843
- Qian Y , Baisden JM , Westin EH , et al. Src can regulate carboxy terminal interactions with AFAP-110, which influence self-association, cell localization and actin filament integrity. Oncogene . 1998;16(17):2185–2195. doi:10.1038/sj.onc.1201753 9619827
- Dorfleutner A , Stehlik C , Zhang J , et al. AFAP-110 is required for actin stress fiber formation and cell adhesion in MDA-MB-231 breast cancer cells. J Cell Physiol . 2007;213(3):740–749. doi:10.1002/jcp.21143 17520695
- Zhang J , Park SI , Artime MC , et al. AFAP-110 is overexpressed in prostate cancer and contributes to tumorigenic growth by regulating focal contacts. J Clin Invest . 2007;117(10):2962–2973. doi:10.1172/JCI30710 17885682
