Abstract
Triple-negative breast cancer (TNBC) is a special subtype of breast cancer, accounting for 10–20% of breast cancers with high intrinsic heterogeneity. Its unique immune microenvironment, including high expression of vascular endothelial growth factors, tumor infiltrating lymphocytes (TILs), tumor-associated macrophages (TAMs), and other molecules that promote the growth and migration of tumor cells, has been shown to play a dual role in the occurrence, growth, and metastasis of TNBC. Understanding the TNBC microenvironment is of great significance for the prognosis and treatment of TNBC. In this article, we describe the composition and function of immune cells in the TNBC microenvironment and summarize the major cytokine growth factors and chemokines in the TNBC microenvironment. Finally, we discuss the progress of TNBC, cytokine-induced killer cell therapy, and immune checkpoint therapy.
Introduction
Breast cancer is currently the world’s highest incidence and mortality rate of female diseases. It is a serious threat to women’s life and health and has attracted attention in various fields. The 2018 Global Cancer Survey data show that breast cancer has the highest incidence of malignant tumors among women worldwide, accounting for 15% of all malignant tumor deaths, ranking 6th in the mortality rate of female malignant tumors in China, and shows a rapid growth trend.Citation1–Citation4
Triple-negative breast cancer (TNBC) is a special subtype of breast cancer, accounting for 10–20% of breast cancers. It is an estrogen-like, progesterone-like, and human skin growth factors 2 (Her2) negative invasive breast cancer with internal heterogeneity.Citation5,Citation6 TNBC is usually composed of bio-invasive and histologically high-level tumors with poor prognosis (five-year survival rate is only about 60%), a high recurrence rate (recurrence within 3 years after diagnosis), short survival, and development with clinical, pathological, and genetic factors. The vast majority of TNBCs are highly invasive catheter cancers with higher distant metastasis rates and worse prognoses, accounting for 10% to 24% of all breast cancers.Citation7–Citation9 TNBC is divided into substrate cell sample 1, substrate cell type 2, immunomodulation type, interstate type, interstate stem cell type, and tube cavity androgen-like body type. The immunomodulation type is most common. This subtype is closely related to the tumor immune response, has a high level of immune immersion, is the most immunogenic subtype in breast cancer, and is expected to develop immunotherapy.
Tumor immunotherapy refers to stimulating or regulating the body’s immune system, improving tumor micro-environmental immune status, breaking tumor immunosuppression, and enhancing the killing of tumor cells. The tumor microenvironment refers to the environment in which the tumor occurs, grows, and metastasizes, including the tissue environment in which the tumor is located and the internal environment of the tumor cell itself. The tumor microenvironment consists of non-immune cells such as fibroblasts, endotheliocytes, vascular smooth muscle cells, and immune cells such as T lymphocytes, macrophages, natural killer cells (NK) cells, degenerate cells, and cytokines. As the site of tumor cell immune surveillance and immune escape, the tumor immune microenvironment is closely related to cancer occurrence, development, and prognostication.Citation8,Citation10,Citation11 Its unique immune microenvironment, including high expression of vascular endothelial growth factors, tumor infiltrating lymphocytes (TILs), tumor-associated macrophages (TAMs), and other molecules that promote the growth and migration of tumor cells, has been shown to play a dual role in the occurrence, growth, and metastasis of TNBC.Citation12–Citation19 The study found that the TNBC immune microenvironment plays a role in the occurrence, growth, and metastasis of TNBC. The increase in tumor antigen expression provides the premise for immune system recognition and tumor removal and tumor infiltrating lymphocytes play a role in tumor cell killing and removal, but the high expression of immune checkpoints inhibits immune cell function and promotes tumor immune escape.Citation10,Citation14,Citation16,Citation17,Citation19 Furthermore, there is a lack of information regarding the TNBC immuno-micro-environment. Therefore, it is important to understand the TNBC immune microenvironment for the prognostication and treatment of TNBC.
This paper mainly introduces the immune cells and related factors in the TNBC microenvironment, discusses the current situation of TNBC treatment, and summarizes the cytological characteristics of the TNBC microenvironment and its role in prognosis, in order to: (I) understand the components and functional characteristics of immune cells in the TNBC microenvironment; (II) summarize the main cytokines, growth factors, and chemokines in the TNBC microenvironment; and (III) introduce the progress of TNBC and cytokine-induced killer cell therapy and immune checkpoint therapy.
Immune Cells in TNBC Microenvironment
Tumor Infiltrating Lymphocytes (TILs)
A large number of TILs infiltrate the TNBC microenvironment. TILs have intrinsic immunogenicity in TNBC.Citation20,Citation21 An endogenous antitumor immune response was used to induce favorable tumor microenvironment (TME), inhibit tumor progression, and improve the recurrence-free survival rate of TNBC patients.Citation5,Citation22–Citation28 It has been found that malignant tumors with high immunogenicity (high level of TILs) may grow rapidly and have a high rate of tumor necrosis.Citation5 I In another study, it was found that the high TIL group had a more negative threshold level of Ki-67 (<14%), which verified that TNBC patients with high TIL levels may have lower KI-67 levels, less proliferation of malignant tumor cells, and a positive response to treatment.Citation29 A meta-analysis of the prognostic value of TILs in TNBC showed that a high TIL level was significantly associated with a better survival outcome in TNBC, and the authors concluded that TIL status should be considered a strong prognostic factor for this subtype of breast cancer.Citation30 According to a 2014 meta-analysis, TIL levels were positively correlated with the prognosis of patients with TNBC. The more matrix TIL TNBC patients had, the better the prognosis was, and the higher the TIL level, the higher the pathologic complete response rate (pCR) and survival rate.Citation29–Citation34 Other studies have shown that low TIL levels and poor long-term survival outcomes (10%) are independent and intrinsic prognostic factors for early TNBC patients who have not received systematic treatment, which strongly predicts the risk of digital radiography (DR) and poor long-term survival outcomes. Studies have compared the treatment rate of TNBC patients with different TIL levels, and that of TNBC patients with high TIL levels has a higher treatment rate (OR 2.14, 95%; CI 1.43–3.19), indicating that high levels of TILs are a positive prognostic factor for TNBC patients.Citation23 In addition, a study on the application of magnetic resonance imaging (MRI) in TNBC confirmed that TNBC with high TIL levels had a higher average tumor roundness score, which means that TIL levels were positively correlated with the roundness of TNBC tumors, which is often used as a descriptive term for benign lesions.Citation29,Citation35 In conclusion, TNBC patients with higher TIL levels have a better prognosis.
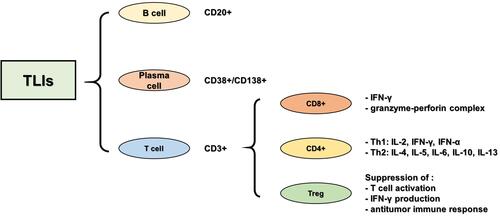
TILs include CD3+T and CD20+B cells, and CD38+/CD138+ plasma cells, among which infiltration of CD3+T cells is relatively common. CD3+T cells are co-differentiated antigens of mature T cells and are expressed on the surface of all mature T cells, representing total T lymphocytes in the tissue. The CD3+T cell population was further divided into CD8+ T lymphocytes, CD4+ helper T lymphocytes, and CD4+ regulatory T cells (Tregs) (). CD8+ T lymphocytes are abundant in the TNBC microenvironment, and many studies have reported the relationship between TILs and the prognosis of TNBC, suggesting that the high expression of CD8+ T lymphocytes is related to a better clinical prognosis of TNBC. CD4+ helper T lymphocytes can be divided into Th1 and Th2 subtypes, according to the secreted cytokines. Th1 cells are mainly involved in cellular immunity, while Th2 cells are involved in humoral immunity. CD4+ helper T lymphocytes assist CD8+ T lymphocyte-mediated cell killing to play an active role in the tumor immune response.Citation36–Citation38 Tregs, which account for 10% of all CD4+ T lymphocytes in the peripheral blood of normal people, rise to between 30 and 50% in tumor lesions, inhibit the activation of CD8+T and CD4+T lymphocytes, and play an important role in immune suppression and angiogenesis.Citation39,Citation40
In immune subsets, CD8+ lymphocytes are the main effectors of the anti-tumor immune response. Activated by major histocompatibility complex (MHC) molecules expressed on tumor cells, CD8+ T lymphocytes differentiate into cytotoxic CD8+T lymphocytes, which release interferon-γ (IFN-γ) to kill tumor cells and play an anti-tumor role. Cytotoxic CD8+ T lymphocytes are active CD8+ T lymphocytes with anti-tumor immunity that play a key role in adaptive immune defense against exogenous factors and tumor cells. In the case of TNBC, an increased number of CD8+ T lymphocytes were found to have infiltrated the TNBC microenvironment. CD8+ T lymphocytes have been reported to be associated with improved clinical outcomes in TNBC and a better response to systemic treatment of TNBC. In addition, CD8+T lymphocyte infiltration may be related to the increased survival time of patients with TNBC. Studies have shown that the more CD8+T lymphocyte infiltration, the better the prognosis of TNBC. However, this relationship does not exist in other types of breast cancer, therefore it can be used as an independent prognostic factor for TNBC.Citation41,Citation42 In TNBC, the effect of CD8+ T lymphocytes on tumor progression depends not only on their number and distribution, but also on their activity. Studies have shown that CD8+ T lymphocytes are more active in hormone receptor-negative breast cancer.Citation43
The role of CD4+ T lymphocytes in tumor regression during breast cancer chemotherapy is poorly understood in current studies regarding the dynamic function of CD4+T cell subsets during breast cancer progression, in which CD4+T cell subsets are decisive factors for breast cancer clinical outcomes. Therefore, a better understanding of the nature and function of CD4+T lymphocyte subsets in tumor immunity is very important for TNBC immunotherapy. CD4+ T lymphocytes are mainly divided into CD4+ helper T lymphocytes and CD4+ regulatory T lymphocytes. CD4+ helper T lymphocytes are rare in breast cancers. In TNBC, CD4+ helper T lymphocytes are activated by MHC molecules, differentiated into Th1, Th2, and other subtypes based on the level of cytokines in the tumor microenvironment, and play a regulatory role in the immune system by regulating B cell CD8+ T lymphocytes and macrophages.Citation44 With increased efforts, the specific mechanism and role of CD4+ helper T lymphocytes in the development and treatment of tumors will be further revealed, which is helpful to better judge the prognosis. CD4+ Tregs are subsets of CD4+ T lymphocytes with an immunophenotype of CD4+ CD25+ FOXP3+. In addition, FOXP3+ is an important marker of Tregs. In a normal immune environment, Tregs regulate and suppress the immune response to prevent an autoimmune response. In TNBC, Tregs induce an immunosuppressive microenvironment, inhibit the activation of CD8+ and CD4+T lymphocytes, and prevent the body’s anti-tumor immune response.Citation45 The immunosuppressive Treg subset accumulated in the TNBC microenvironment, and FOXP3+ cell infiltration was the highest in the microenvironment compared to surrounding areas. Studies have shown that FOXP3+ Tregs can reduce autoantigen immune responses, inhibit anti-tumor immunity, and can predict poor prognosis.Citation46–Citation48 At present, the infiltration of Tregs is a prognostic indicator of TNBC and the monitoring and treatment of infiltrated Tregs in TNBC lesions can provide greater benefits to patients.
Other studies analyzed the changes in the ratio of CD8+T lymphocytes, FOXP3+ Tregs, and CD8+ /FOXP3+ in residual tumors of more than 100 TNBC patients after chemotherapy. The results showed that an increased number of CD8+ T lymphocytes, along with an increased CD8+/FOXP3+ ratio were significantly correlated with improved clinical efficacy, and that a high number of CD8+ T lymphocytes and CD8+/FOXP3+ ratio predicted good survival in TNBC patients without pCR. This study showed that the CD8+/FOXP3+ ratio, measured after chemotherapy, was associated with TNBC prognosis.Citation49
Tumor-Associated Macrophages (TAMs)
Macrophages are an important part of the host defense system. They are divided into specific functional subsets according to the polarized phenotype and play an important role in the early days and adaptive immune response. TAMs originate from circulating blood monocytes and differentiate into macrophages after exosmosis, which are important components of the tumor microenvironment. Tumor cells secrete cytokines and chemokines and recruit macrophages from the peripheral blood and tissue into solid tumor tissue to form a tumor microenvironment. TAMs account for up to 50 and 80% of mesenchymal cells and are closely related to the occurrence and development of tumors.Citation50–Citation52 Studies have shown that TAMs can reduce the immune effect of TILs and promote the anti-tumor immune activity of Tregs.Citation53–Citation55
TAMs are composed of two distinct subtypes: M1-type macrophages (classical macrophages) and M2-type macrophages (replacement-activated macrophages). M1-type macrophages are associated with an inflammatory response and induce a Th1 immune response by releasing proinflammatory cytokines. M2-type macrophages are associated with tumor progression, and their secretion of interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) inhibit the Th1 immune response and promote tumor invasion and metastasis. Furthermore, M2-type macrophages can also secrete angiogenic factors such as VEGF, promote tumor angiogenesis, and provide nutrition and metastasis pathways for tumor growth (). Breast cancer cells secrete cytokines to promote M1-type macrophage polarization into M2-type states including IL-2, IL-10, TGF-β, and macrophage colony stimulating factor (M-CSF). On this basis, TNBC secretes more granulocyte colony stimulating factor (G-CSF), which promotes the transformation of M1-type macrophages into the M2-type state to a greater extent.Citation14,Citation19,Citation56,Citation57
Studies have found that TAMs directly and indirectly promote tumorigenesis and development. The specific mechanisms include: (I) Promoting tumor cell division. TAMs secrete growth factors such as basic fibroblast growth factor-2 (BFGF-2), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and TGF-β, which directly promote tumor cell division. (II) Promoting the generation of tumor blood vessels. On the one hand, TAMs release angiogenic factors and growth factors, such as VEGF, TNF-β, IL-8, and cellulose growth factor family, etc. On the other hand, TAMs express enzymes that regulate angiogenesis, such as matrix metalloproteinases MMP-2, MMP-7, MMP-9, MMP-12, and cox-2 (COX-2), which promote angiogenesis. (III) Induction of tumor invasion. TAMs can produce a variety of enzymes that can degrade the extracellular matrix, such as MMP-2, MMP-9, and urokinase-type plasminogen activator, promote ECM degradation, and facilitate tumor cell invasion and metastasis. (IV) Participate in immunosuppression and escape. TAMs secrete cytokines, such as TGF-β and IL-13, which inhibit the proliferation and differentiation of lymphocytes, including lymphocytes activating killer (LAK) cells, NK cells, and cytotoxic T lymphocytes (CTL).Citation58–Citation63 Other studies have shown that TAMs play an immunosuppressive role through the following mechanisms: (I) secreting inhibitory cytokines, (II) reducing the anti-tumor immune effect of TILs, (III) promoting Treg infiltration, and (IV) generation of reactive oxygen species.Citation14
Studies have shown that highly invasive TAMs are significantly correlated with tumor size, lymph node metastasis, and the degree of differentiation. TAMs co-cultured with MDA-MB-231 cells have greater invasiveness and morphological changes. TAMs undergo polarized activation and have the ability to induce tumors and promote tumor progression and metastasis. In TNBC, TAMs promote the occurrence and development of tumors, and directly and indirectly regulate the expression of programmed death ligands (PD-1/PD-L1) in the tumor environment, which is closely related to the poor prognosis of tumor patients.Citation14,Citation64
Cancer-Associated Fibroblasts (CAFs)
Cancer-Associated Fibroblasts (CAFs) are an activated form of fibroblast with a high degree of heterogeneity accompanied by dynamic changes in tumor development. The marker commonly used to detect CAFs is α-SMA.Citation65 Sources of CAFs include: (1) CAFs are activated and proliferated by resident fibroblasts, and tumor cells secrete TGF-β and PDGF, thereby activating normal fibroblasts in the tissue to transform into CAFs; (2) Bone marrow-derived stem cells differentiate: tumors recruit bone marrow-derived progenitors or mesenchymal stem cells to differentiate into CAFs; (3) Pericyte transdifferentiation: stellate cell (pericyte) transdifferentiation is the main source of CAFs in liver and pancreas, and plays an important regulatory role in the development of liver cancer and pancreatic cancer.Citation65,Citation66
CAFs reduce anti-tumor immunity, enhance tumor cell proliferation and invasion, promote neoangiogenesis of tumor cells, reshape the extracellular matrix (EMC), and contribute to the formation of an immunosuppressive microenvironment.Citation67–Citation73 Studies have shown that CAFs autophagy can improve the in vitro migration of TNBC cell lines MDA-MD-231 and BT-549 cells.Citation74 Other studies have shown that CAFs may promote the development of TNBC by activating TGF-β.Citation75 Co-culture experiments demonstrated that interactions between basal-like breast cancer cells and fibroblasts induced high levels of interleukin-chemokine expression, including IL-6, IL-8, CXCL1, CXCL3, and TGF-β.Citation76 In addition, CXCL16 expressed by myeloid cells activates CAFs and recruits more myeloid cells and fibroblasts in TNBC.Citation77 There are four CAF subgroups in the microenvironment of human breast cancer, with different accumulation in different subtypes. There are four CAF subgroups in the microenvironment of human breast cancer, with different accumulation in different subtypes. Another fibroblast subgroup, CAF-S4, showed no such activity. TNBC can be divided into two subgroups according to the enrichment degree of CAF-S1 or CAF-S4. Compared with the enrichment of CAF-S4 in TNBC, the enrichment of CAF-S1 in TNBC was more aggressive and the infiltration of CD8+ T cells was lower.Citation13
Tumor Associated Neutrophils (TANs)
Neutrophils account for about 60% of all white blood cells in circulation and are the first line of defense at sites of infection or inflammation. In inflammatory tissues, neutrophils interact with macrophages, dendritic cells, natural killer cells, lymphocytes, and mesenchymal cells in a complex bi-directional manner. Activated neutrophils release a variety of chemokines and peroxidases (MPO), activate and recruit monocytes/macrophages to the inflammatory sites.Citation12,Citation78,Citation79
Tumor associated neutrophils (TANs) are an important component of tumor microenvironment and can be activated under various conditions to directly lyse tumor cells or induce antitumor function through cytotoxicity, acting as immunosuppressive cells.Citation80 TAN is divided into two phenotypes, N1 and N2. The N2-type neutrophils promotes tumor growth, and blocking TGF- or enhancing IFN- converts TANs to the anti-tumor N1-type neutrophils. TGF-β had similar effects on the activation of M2-like TAMs and N2-like TANs, suggesting that TAMs and TANs are closely related in tumor microenvironment.Citation81–Citation83 Studies have shown that TANs in TNBC promotes tumor proliferation, migration, invasion and metastasis and inhibits anti-tumor immunity. In addition, TNBC cells secrete granulocyte-macrophage colony stimulating factor (GM-CSF), which stimulates TANs to release tumor suppressor M, promotes angiogenesis, and improves tumor cell infiltration.Citation84
NK Cell
NK Cell is involved in the innate immune cytotoxic cells, mainly derived from bone marrow hematopoietic stem cells. It is distributed in peripheral lymphatic non-lymphoid and lymphoid organs and tissues. As the first line of defense against tumor and infection, NK Cells are activated by a series of germline encoded surface receptors without specific antigen stimulation, and has a good prospect in the field of immunotherapy.
NK cells have the ability to induce tumor cell death in the absence of specific immunity. When activated NK cells come into contact with tumor cells, they release perforin/granzyme, secreting cytokines such as TNF-α and IFN-γ, which play a cytolysis role. NK cells effectively recognize and kill tumor cells through MHC-I down-regulation mechanism, which is a common mechanism for cancer cells to avoid T cell recognition. A recent meta-analysis showed that ArNKG2D and DNAM1 ligands were expressed in NK cells in the microenvironment of breast cancer.Citation57,Citation85 It was also reported that patients with higher expression of NCR3 (NKp30), NCR1 (NKp46), CD96 CRTAM DNAM1 and NKG2D had better survival rate, and the increase of NK cells was positively correlated with better pathological reaction. On the other hand, a significant correlation between BASELINE tumor infiltrating NK cells and pCR was observed in HER2-positive cancers.Citation86,Citation87
Cancer-Associated Adipocytes (CAAs)
Cancer-associated adipocytes (CAAs) are understudied cells from the tumor microenvironment. CAAs is one of the cellular components that constitute the microenvironment of breast cancer. It was initially determined to provide large energy-storage metabolites with high energy, from which tumor cells capture metabolites such as ketones, fatty acids, pyruvate and lactic acid. In breast cancer microenvironment, CAAs secretes chemokine ligand 2 (CCL2), chemokine ligand 5 (CCL5), interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF).Citation88–Citation91 Increased levels of CCL5, CCL2 and inflammatory cytokines IL-6 and TNF-αpromote tumor cell proliferation and invasion and angiogenesis.Citation15 There is evidence that CAAs promotes the migration of breast cancer cells by secreting IL-6 and CCL2, increases the aggressiveness of cancer cells, and drives tumor progression.Citation88,Citation92 Adipocytes also enhanced the invasiveness of MDA-MB-231 cells, and this effect was partly induced by CCL5, which was negatively correlated with OS.Citation90 Studies have shown that breast cancer cells can stimulate adipocytes to release fatty acids, and co-culture with adipocytes can promote the growth and migration of McF-7 and MDA-MB-231 cells.Citation93 Other studies have shown that CAAs metabolic reprogramming drives cancer progression, and that CAAs metabolic reprogramming can be attributed to its potentially high tumor-promoting ability. In addition, the expression of PD-L1 in CAAs prevents the anti-tumor function of CD8+ T cells. Lipoinhibitors selectively reduce the expression of PD-L1 in CAAs and contribute to the immunotherapy of breast cancer.Citation15
Unique Features of Immune Cells in TNBC Microenvironment
TNBC accounts for 10%-20% of breast cancer and is the subtype with the worst prognosis. Various cell components in the TNBC microenvironment can be used as prognostic factors to predict the clinical results of TNBC and guide the treatment of TNBC. The tumor microenvironment consists of tumor cells and a variety of stromal cells, including tumor-related immune cells, fibroblasts and adipocytes. The microenvironment components and functions of different subtypes of breast cancer are different. For example, the cell infiltration rate of TILs and TAMs in the TNBC microenvironment is higher than that of other subtypes of breast cancer. In TNBC, M2-type macrophages are polymorphic in anti-inflammatory and stem cell renewal, and are significantly up-regulated compared with other subtypes of breast cancer. In addition, CAAs in TNBC microenvironment had a more significant effect on tumor cell growth and invasion ().Citation66,Citation94
Table 1 The Main Cell Components in Microenvironment of Different Subtypes of Breast Cancer
Cytokines in the TNBC Microenvironment
One of the important factors of tumor microenvironment (TME) is tumor interstitial fluid (TIF) containing tumor secretory bodies.Citation95 TIF surrounds tumor cells and stromal cells, and contains a variety of cytokines, nutrients, and other factors that determine the outcome of virtually every aspect of tumor angiogenesis, growth, metastasis, and treatment. With the expansion of cancer immunotherapy to TME, TIF and its various cytokines become an important source of new targets for cancer therapy.
Angiogenesis is one of the main features of cancer. Tumor cells and stromal cells secrete cytokines and growth factors that stimulate endothelial cell proliferation and establish blood vessels to promote growth and blood-borne metastasis. Among all known angiogenic factors, VEGF is closely associated with tumor cell invasion and metastasis, and up-regulation under hypoxia conditions.Citation96–Citation99 Preclinical and clinical studies have shown that VEGF ACTS as a target for reducing angiogenesis. VEGF blocking has been shown to promote ovarian, cervical cancer, colorectal, kidney cancer, lung cancer, and breast cancer survival, especially when combined with chemotherapy, to effectively slow tumor growth.Citation95,Citation100,Citation101 The level of VEGF in plasma may be a biomarker for anti-angiogenic therapy in tumors. TNBC expresses high levels of VEGF and molecules that promote tumor cell growth and migration. VEGF is an important pro-angiogenic factor in the TNBC microenvironment, and its expression is highly dysregulated, which is of great significance for the prognosis of TNBC. In TNBC, VEGF binds to endothelial cell surface receptors, which affecting tumor growth. It was found that VEGF can induce the adhesion and migration of MDA-MB-231 cells when co-cultured with endothelial cells. In addition, TNBC can express endothelial markers and form vascular-like channels through the differentiation of endothelial cells in vitro and in vivo, generating blood space surrounded by tumor cells. Research data showed that VEGF level in the TNBC microenvironment was significantly higher than that in non-TNBC tumors, which was 3 times and 1.5 times as high as that in the ER/PR positive group and her-2 positive group.Citation66,Citation102–Citation105
TNBC exists in a variety of cytokines and chemokines, including IFN-γ, TNF-α, TGF-β, IL-6, IL-8, IL-2, IL-15, IL-18 and IL-1β, which influence tumor growth, metastasis and drug resistance, mediated immune suppression and antitumor activity, plays an important role in micro environment.
IFN-γ and its transcriptional regulator STAT1 participate in the immune response, promote M1 macrophage polarization, and activate the body’s anti-tumor response. TAMs can induce PD-L1 expression by secreting IFN-γ and activating JAK/STAT3 pathways. Studies have shown that activation of the JAK/STAT signaling pathway in TNBC can somehow inhibit tumor growth. More recently, IFN-γ associated mRNA profiles have been proposed as predictors of PD-1 blocking clinical responses.Citation14,Citation106,Citation107
TGF-β is a multifunctional cytokine belonging to the TGF superfamily that is involved in the production of Tregs and inhibits the effector function of CTLs (cytotoxic T cells) in the tumor microenvironment of mice model. TGF-β can also increase the inhibitory activity of TAMs and promote tumor escape by promoting M1-to-M2 phenotype polarization and inducing PD-L1 up-regulation.Citation14
IL-6 is a pluripotent cytokine and a classic inflammatory cytokine that interferes with the expression of cell adhesion and surface antigen molecules, regulates a variety of cell functions, including proliferation, differentiation and immune defense, participates in TNBC growth, and is related to TNBC prognosis.Citation14,Citation108,Citation109 IL-6 enhances the cell lysis capacity of NK cells and promotes the proliferation and differentiation of bone marrow-derived cells. Under the condition of IL-6 deficiency, the expression of PD-L1 was enhanced, and the inhibitory activity of anti-PD-L1 antibody in vivo was significantly enhanced. TNBC cells secrete a large amount of IL-6, which interacts with IL-6 receptors in LEC to activate the JAK2-STAT3 signaling pathway, up-regulate CCL5 and VEGF in LEC, and induce the synthesis of CCL5 and VEGF in lung lymphocytes (LEC).Citation110 CCL5 is a widely studied chemokine, which can recruit leukocytes to the inflammatory site and bind with CCR5 to enhance the ability of TNBC metastasis and invasion and promote the TNBC lymph node metastasis.Citation110,Citation111 VEGF contributes to lymph node angiogenesis, promotes TNBC cells to generate blood vessels in LEC to maintain growth, and helps tumor cells to spread to the outside of the lung. Studies have found that inhibition of CCR5 or VEGF can prevent TNBC tumor metastasis to the lungs.Citation110,Citation112
IL-8, IL-2, IL-15, etc. are important indicators of immune response in breast cancer and are differentially expressed in different subgroups of breast cancer. Compared with other subtypes of breast cancer, IL-8 is most expressed in the TNBC stroma. IL-8, an immune-related cytokine and inflammatory mediator, has tumorigenic and angiogenic properties, and is most expressed in TNBC. Studies have found that the expression of IL-8 in the interstitial of cancer is related to the prognosis of TNBC patients, and is negatively correlated with the metastasis and local recurrence of TNBC. IL-8 is secreted by monocytes and endothelial cells. IL-8 secreted by tumor cells, invasive neutrophils and tumor-related macrophages participates in the angiogenesis, proliferation and migration of tumor cells in the tumor microenvironment.Citation110,Citation113 The IL-8 receptors CXCR1 and CXCR2 selectively form dimers. CXCR1 interacts with IL-6 and IL-8, while CXCR2 binds to IL-1, IL-2, IL-3, IL-5, IL-6, IL-7 and IL-8. CXCR1 plays a role in IL-8-induced immune response chemotaxis, and CXCR2 plays a role in cell migration. IL-8 binds to CXCR2 with a higher affinity than CXCR1, which is a key receptor for several types of tumor metastasis. IL-8 is a key secretory factor for TNBC tumor growth and metastasis, and CXCR1 and CXCR2 are potential therapeutic targets for metastatic triple-negative breast cancer.Citation114–Citation119 TNBC cell secretion of IL-8, related to the tumor microenvironment in tumor CXCR1 on fibroblast cells and macrophages, CXCR2 receptor interactions, activate CXCR1, CXCR2 receptors, induction of STAT3 phosphorylation, which increases the expression of IL-8 and stromal cells secrete, and the matrix of cancer associated fibroblasts and macrophages secretion of IL-8 with TNBC CXCR1, CXCR2 receptors on the cell interaction, promote TNBC extravasation and engraftment tumor growth and metastasis. Studies have found that TNBC cells co-cultured with fibroblasts or macrophages have higher proliferation and migration ability, while inhibition of IL-8 signaling pathway can reduce the proliferation and migration of TNBC cells.Citation110
In TNBC, IL-2 or IL-15 cytokine stimulation can significantly increase the NK-mediated ADCC in the microenvironment and improve the anti-tumor ability of NK cells.Citation18 IL-15 has emerged as a potential new candidate for immunotherapy for cancer, especially in combination with other drugs. IL-15 is structurally related to IL-2 and has a synergistic effect, promoting IL-2 cell proliferation, promoting T cell differentiation, stimulating B cells to produce immunoglobulin, activating NK cells and enhancing NK cytotoxic function.Citation120,Citation121 Compared with IL-2, IL-15 has no significant effect on Treg cells, does not mediate cell death (AICD), and is less toxic and does not cause capillary leakage syndrome in mice and non-human primates.Citation120 IL-15 can induce NK cell proliferation and survival and enhance its cytotoxic function.Citation122,Citation123
IL-18 is a multipotent cytokine member of the IL-1 family. It is produced by several cells, including macrophages, and plays a pro-inflammatory and anti-inflammatory role. IL-18 is involved in regulating the expression of PD-1 in TNBC microenvironment. IL-18 in tumor microenvironment increases the number of immunosuppressive NK cells and induces PD-1 expression in a subset of NK cells. The level of tumor-derived IL-18 was significantly associated with poor survival in TNBC patients.Citation124,Citation125 In addition, in TNBC, IL-1β interferes with T-cell-mediated immune response, induces tumor angiogenesis, and has carcinogenic effects.Citation108 IL-10 is an important factor for mononuclear macrophages to participate in the immune process of the body, while IL-12 can inhibit tumor growth by inducing a strong cellular immune response.Citation14
TNBC and Cytokine-Induced Killer Cells (CIK)
Cytokine-induced killer cells (CIK), a subset of cytotoxic T lymphocytes with a CD3+ CD56+ immunophenotype, is an Immunoreactive host effector cell and an ideal candidate cell type for adoptive immunotherapy for tumors.Citation126,Citation127 Adoptive immunotherapy collects immune cells from human body, transforms and amplifies them in vitro, and infuses them back into human body for anti-tumor response.Citation128 The CIK is a heterogeneous group of immune-effector cells extracted from PBMCs, stimulated by multiple cytokines such as IFN-β and IL-2, which are amplified to sufficient levels to kill cancer cells without targeting healthy cells or tissues.Citation126,Citation129
CIK proliferates rapidly, which has few side effects and anti-tumor activity. It can directly kill tumor cells and regulate and enhance the immune function of host cells in vivo. CIK cells secrete cytokines such as IFN-γ, IL-2 and TNF-α, activate the anti-tumor activity of macrophages, induce the immune damage caused by chemotherapy drugs, enhance the immune monitoring function of the body, and inhibit the growth of tumor cells.Citation128–Citation132 Tregs in the TNBC microenvironment can inhibit the activation and proliferation of T lymphocytes, prevent the proliferation of NK cells, limit the anti-tumor effect of immune cells, induce inhibitory cytokines, eliminate effector cells, promote the immune escape of tumor cells, and promote the development of tumor. CIK can reduce the Tregs ratio, increase the proportion of CD4+ T cells and CD8+ T cells, and reduce or eliminate the immunosuppressive state of tumor microenvironment.Citation128,Citation133,Citation134
The greatest advantage of CIK cells in the treatment of malignant tumors is safety. Compared with LAK (lymphokine-activated killer cells), CIK enhances tumor cell lysis activity and reduces toxicity.Citation135 In decade of clinical studies, CIK therapy has been shown to provide positive clinical outcomes in patients with multiple types of cancer. At present, CIK has been widely involved in adjuvant and chemotherapy of cancer, and combined treatment of a variety of blood and solid tumors including TNBC, such as renal cell carcinoma, gastric cancer, non-small cell lung cancer, colon cancer and liver cancer has good efficacy and safety.Citation136–Citation140
Conventional chemotherapy combined with CIK immunotherapy is an optimized treatment strategy, which can significantly improve the prognosis of TNBC patients.Citation138 Based on the synergistic effect of chemotherapy, the immunosuppressive factors in TNBC microenvironment were removed, which was beneficial to the anti-tumor function of immune effector cells.Citation141 CIK eliminates potential or residual tumor cells after chemotherapy, including drug-resistant tumor cells.Citation142,Citation143 In addition, CIK has a strong tumor killing effect, reducing the risk of recurrence and metastasis.Citation144,Citation145 CIK therapy combined with chemotherapy can reduce the recurrence and metastasis of TNBC patients after surgery, prolong the overall survival time with minimal side effects, and have a stronger impact in the relatively early stage of the disease. Early TNBC patients benefited the most from CIK therapy, which is consistent with the results of some existing studies on CIK immunotherapy for other early tumors.Citation146,Citation147 On the one hand, the immune function of patients with advanced cancer is inhibited by tumor, which prevents the initial expansion of CIK cells and affects the activity of CIK cells infused. On the other hand, advanced metastatic cancer cells may evolve at the molecular level to evade immune surveillance or immunotherapy.Citation128
The TNBC genome is unstable with strong antigenicity, and the expression of TILs and PD-L1 is high, making it an appropriate target for immunotherapy.Citation126,Citation128 Immune checkpoint inhibitors, such as anti-PD-1 and anti-PD-L1 antibodies, have made breakthroughs in clinical practice, promoting in-depth studies on the efficacy and safety of autologous CIK cell therapy and chemotherapy in TNBC patients. In conclusion, CIK cell immunotherapy may be a new strategy for TNBC-assisted therapy, but not all patients treated with CIK are expected to have improved outcomes. Therefore, how to improve the efficacy of CIK cells in TNBC treatment is worthy of further study.
TNBC with Immune Checkpoint
TNBC has the worst prognosis among the different subtypes of breast cancer. Currently, radiotherapy and chemotherapy are the main treatments for TNBC, but patients have residual disease after chemotherapy, showing a worse overall survival rate than the luminal type of breast cancer.Citation148,Citation149 These results indicate that chemotherapy alone is not enough to treat TNBC, and more advanced treatment methods are needed to improve the prognosis of this specific patient subgroups. For many years, immunotherapy has not been considered suitable for TNBC, until recently as some studies have found various effective immunotherapy drugs.Citation150 Currently, immunotherapy is being developed as a new treatment regimen for TNBC. Cancer immunotherapy includes immune checkpoint inhibitors, cytokine/adoptive cell therapy, and cancer vaccines. Immune checkpoints are a group of different regulatory points in the adaptive immune system and play an important role in self-tolerance and anti-tumor immunity. Most clinical trials of immune checkpoint blockade have focused on cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and PD-1/PD-L1 ( and ).Citation148,Citation151,Citation152
Table 2 Clinical Trial of Immunotherapy for TNBC
Figure 3 The main immune cells involved in immune checkpoint regulation and their interactions in tumor microenvironment.
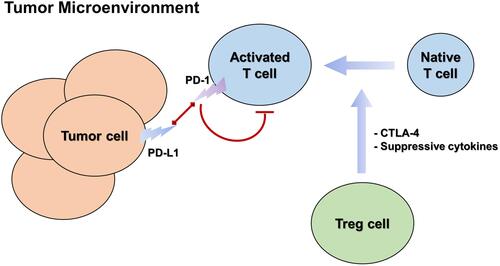
Among all breast cancer subtypes, PD-L1 accounts for 20–50%, and its expression rate in TNBC is relatively high.Citation148,Citation150,Citation153–Citation155 PD-L1 positivity in immune cells correlates with a favorable prognosisCitation156–Citation158 Stovgaard et al 2021 found that PD-L1 expression was associated with improved survival, perhaps due to the more active immune response of lymphocytes with high PD-L1 expression in the TNBC microenvironment.Citation159 The pathological complete response rate of TNBC patients with high PD-L1 expression was as high as 44.4%, while that of TNBC patients without PD-L1 positive immune cells was 2.6%.Citation148 Therefore, TNBC expression of PD-L1 can be used as a potential biomarker for clinical applicationsCitation154 and targeting PD-1 or PD-L1 may be an option for treating TNBC.Citation160
As negative regulators of immune activation, CTLA-4 and PD-1 inhibit the antitumor effects of the tumor microenvironment.Citation161–Citation164 A number of clinical trials are currently evaluating checkpoint inhibitor combinations (blocking CTLA-4 and PD-1/PD-L1 pathways) to improve response rates. For example, a phase 1b/2 clinical study of KN046 combined with nab-paclitaxel in patients with TNBC was recently announced by Alphamab Oncology at the American Association for Cancer Research (AACR 2021) (Clinical Trial Number: KN046-203; NCT03872791). KN046 is a diabody of PD-L1/CTLA-4, independently developed by Alphamab Oncology. The results showed that the combination of KN046 with nab-paclitaxel was well tolerated and effective, showing potentially clinically significant benefits in progression-free survival (PFS) and overall survival (OS), especially in patients with PD-L1 positive TNBC. The results showed that the combination of CTLA-4 and PD-1/PD-L1 immune checkpoint inhibitors is a promising immunotherapy method for TNBC. The combination of two different classes of antibody drugs overcame the immunosuppressive effect of tumor tissues and transformed the immunosuppressive condition of the tumor microenvironment into a matrix environment with anticancer function, showing a significant effect in the treatment of TNBC.Citation148,Citation150,Citation153,Citation154,Citation165,Citation166
Discussion
TNBC is the most aggressive and metastatic subtype of breast cancer with high intrinsic heterogeneity. The lack of estrogen receptor (ER), progesterone receptor (PR), and HER-2 expression means that there are few targeted drugs available to patients with TNBC. Thus, chemoradiotherapy is the first choice of systemic treatment for patients with TNBC. Anthracyclines and taxoid anticancer drugs are still the preferred treatment for patients with early cancer. For patients with terminal cancer, new chemotherapeutic agents, such as paclitaxel injection (albumin bound) and eribulin mesylate, have made some progress. However, only 10–15% of patients with TNBC respond to standard chemotherapy, with remission lasting only 2–3 months, which makes the treatment of TNBC a difficult problem. Therefore, it is necessary to develop new treatment strategies, such as TNBC immunotherapy and cell therapy, and combine traditional radiotherapy and chemotherapy to comprehensively improve the efficacy of TNBC patients. In a recent study, Prof. Schmid et al demonstrated that immunotherapy can significantly reduce the risk of TNBC recurrence. Among patients with early TNBC, the percentage with a pathological complete response was significantly higher among those who received pembrolizumab plus neoadjuvant chemotherapy than among those who received placebo plus neoadjuvant chemotherapy. Other studies have shown that atezolizumab plus nab-paclitaxel prolonged progression-free survival among patients with metastatic TNBC in both the intention-to-treat population and the PD-L1-positive subgroup.Citation151,Citation152 These results indicate that immunotherapy is gradually being applied in the treatment of TNBC and has achieved certain results.
Immunotherapy and cell therapy are closely related to the functional characteristics of tumor microenvironmental components. TNBC immunotherapy and cell therapy targets are mostly derived from the unique immune microenvironment of TNBC, and previous studies have shown that the prognosis of TNBC is closely related to biomarkers in the microenvironment.Citation7,Citation9,Citation10,Citation16,Citation17,Citation19 For example, CD8+ T lymphocytes and CD4+ T lymphocytes are positive predictors of the long-term prognosis of TNBC. TNBC with higher TILs showed better short-term and long-term prognoses. High levels of TILs (CD8+, CD4+, and FOXP3+)-specific phenotypes positively predicted long-term progression. However, TAMs are an independent factor for poor prognosis of TNBC. They promote the occurrence and development of tumors, directly and indirectly regulating the expression of PD-1/PD-L1 in the tumor environment, and are significantly correlated with tumor size, lymph node metastasis, and degree of differentiation. CAFs, TANs, and CAAs, as important predictors of poor prognosis in TNBC, also play important regulatory functions in tumor occurrence, development, and metastasis. In addition, the TNBC microenvironment has an important impact on the malignant behavior and growth of tumor cells and surrounding cells, and its unique microenvironment can reprogram surrounding cells, offset the progress of cancer cells, develop unique signaling pathways, and form a positive feedback loop, thus affecting the targeted therapy for TNBC tumor cells.Citation66 The TNBC microenvironment is also associated with the characteristics of TNBC, immune system suppression, avoidance of immune detection, and drug resistance. In the microenvironment, various immune cells, immune factors, and tumor cells interact with each other. On the one hand, specific immune responses and innate immune responses are used to monitor TNBC and inhibit the occurrence, development, and metastasis of tumor cells. In contrast, immunosuppressive cells, immunosuppressive factors, phenotypic remodeling of tumor cells, and secreted immunosuppressive factors can cause immune escape of tumor cells.Citation7,Citation9,Citation13,Citation14,Citation16,Citation17
At present, research on the TNBC immune microenvironment is mainly limited to TILs, TAMS, and CAFs, while there are few basic studies on other cells in the microenvironment, such as TANs, NK cells, and CAAs, and the biological characteristics such as the distribution, function, and interaction of immune cells and cytokines in the TNBC microenvironment, have not been fully clarified. There is still a lack of more powerful bioinformatics analysis tools, leading to the personalized precision treatment of TNBC patients still facing many challenges. Therefore, more in vitro and in vivo experiments are needed to clarify the relationship between the components in the TNBC microenvironment, in order to further study the function and characteristics of the components in the TNBC microenvironment. It is believed that with the further advancement of functional genomics such as TNBC proteomics and immunohistology, as well as the development of new bioinformatics tools and immunotherapy technologies, the TNBC microenvironment will be further explored and studied to promote the precise and individualized treatment of TNBC.
Conclusion
In conclusion, an in-depth study of the components and functions of the TNBC microenvironment will aid in further understanding of the unique characteristics of TNBC, reveal the heterogeneity of TNBC, provide accurate prognosis prediction, improve the accuracy of TNBC clinical immunity and cell therapy, and develop new treatment strategies for TNBC.
Disclosure
The authors report no conflicts of interest for this work.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394. doi:10.3322/caac.21492
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi:10.3322/caac.21583
- Heer E, Harper A, Escandor N, et al. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020;8(8):1027–1037. doi:10.1016/S2214-109X(20)30215-1
- Liang H, Li H, Xie Z, et al. Quantitative multiplex immunofluorescence analysis identifies infiltratingPD1(+)CD8(+)and CD8(+)T cells as predictive of response to neoadjuvant chemotherapy in breast cancer. Thorac Cancer. 2020;11(10):2941–2954. doi:10.1111/1759-7714.13639
- Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26(15):2568–2581. doi:10.1200/JCO.2007.13.1748
- Irshad S, Ellis P, Tutt A. Molecular heterogeneity of triple-negative breast cancer and its clinical implications. Curr Opin Oncol. 2011;23(6):566–577. doi:10.1097/CCO.0b013e32834bf8ae
- Isakoff SJ. Triple-negative breast cancer: role of specific chemotherapy agents. Cancer J. 2010;16(1):53–61. doi:10.1097/PPO.0b013e3181d24ff7
- Perdiguero EG, Geissmann F. Identifying the infiltrators. Science. 2014;344(6186):801–802. doi:10.1126/science.1255117
- Yu X, Zhang Z, Wang Z, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin Transl Oncol. 2016;18(5):497–506. doi:10.1007/s12094-015-1391-y
- Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–113. doi:10.1200/JCO.2009.23.7370
- Sharma P. Immune checkpoint therapy as a weapon against cancer. Cancer J. 2016;22(2):67. doi:10.1097/PPO.0000000000000184
- Kim J, Bae J. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016;2016:6058147. doi:10.1155/2016/6058147
- Costa A, Kieffer Y, Scholer-Dahirel A, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33(3):463. doi:10.1016/j.ccell.2018.01.011
- Santoni M, Romagnoli E, Saladino T, et al. Triple negative breast cancer: key role of tumor-associated macrophages in regulating the activity of anti-PD-1/PD-L1 agents. Biochim Biophys Acta Rev Cancer. 2018;1869(1):78–84. doi:10.1016/j.bbcan.2017.10.007
- Wu Q, Li B, Li Z, et al. Cancer-associated adipocytes: key players in breast cancer progression. J Hematol Oncol. 2019;12(1):1–15. doi:10.1186/s13045-019-0778-6
- Lotfinejad P, Asghari Jafarabadi M, Abdoli Shadbad M, et al. Prognostic role and clinical significance of tumor-infiltrating lymphocyte (TIL) and programmed death ligand 1 (PD-L1) expression in triple-negative breast cancer (TNBC): a systematic review and meta-analysis study. Diagnostics (Basel, Switzerland). 2020;10(9). doi:10.3390/diagnostics10090704
- Sahin Ozkan H, Ugurlu MU, Yumuk PF, et al. Prognostic role of immune markers in triple negative breast carcinoma. Pathol Oncol Res. 2020;26(4):2733–2745. doi:10.1007/s12253-020-00874-4
- Julia EP, Mordoh J, Mariel Levy E. Cetuximab and IL-15 promote NK and dendritic cell activation in vitro in triple negative breast cancer. Cells. 2020;9(7):1573. doi:10.3390/cells9071573
- Deepak KGK, Vempati R, Nagaraju GP, et al. Tumor microenvironment: challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol Res. 2020;153:104683. doi:10.1016/j.phrs.2020.104683
- Bianchini G, Balko JM, Mayer IA, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674–690. doi:10.1038/nrclinonc.2016.66
- Park JH, Ahn JH, Kim SB. How shall we treat early triple-negative breast cancer (TNBC): from the current standard to upcoming immuno-molecular strategies. ESMO Open. 2018;3(Suppl 1):e000357. doi:10.1136/esmoopen-2018-000357
- Mahmoud SMA, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–1955. doi:10.1200/JCO.2010.30.5037
- Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two Phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–2966. doi:10.1200/JCO.2013.55.0491
- Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi:10.1093/annonc/mdu112
- Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016;2(10):1354–1360. doi:10.1001/jamaoncol.2016.1061
- Pruneri G, Vingiani A, Bagnardi V, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. 2016;27(2):249–256. doi:10.1093/annonc/mdv571
- Leon-Ferre RA, Polley M-Y, Liu H, et al. Impact of histopathology, tumor-infiltrating lymphocytes, and adjuvant chemotherapy on prognosis of triple-negative breast cancer. Breast Cancer Res Treat. 2018;167(1):89–99. doi:10.1007/s10549-017-4499-7
- Lee J, Kim DM, Lee A. Prognostic role and clinical association of tumor-infiltrating lymphocyte, programmed death ligand-1 expression with neutrophil-lymphocyte ratio in locally advanced triple-negative breast cancer. Cancer Res Treat. 2019;51(2):649–663. doi:10.4143/crt.2018.270
- Ku YJ, Kim HH, Cha JH, et al. Correlation between MRI and the level of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer. Am J Roentgenol. 2016;207(5):1146–1151. doi:10.2214/AJR.16.16248
- Mao Y, Qu Q, Chen X, et al. The prognostic value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. PLoS One. 2016;11(4):e0152500. doi:10.1371/journal.pone.0152500
- Ravelli A, Roviello G, Cretella D, et al. Tumor-infiltrating lymphocytes and breast cancer: beyond the prognostic and predictive utility. Tumour Biol. 2017;39(4):1010428317695023. doi:10.1177/1010428317695023
- Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi:10.1016/S1470-2045(17)30904-X
- Dong D, Zhang F, Zhong L-Z, et al. Development and validation of a novel MR imaging predictor of response to induction chemotherapy in locoregionally advanced nasopharyngeal cancer: a randomized controlled trial substudy (NCT01245959). BMC Med. 2019;17(1):190. doi:10.1186/s12916-019-1422-6
- Badiu DC, Zgura A, Gales L, et al. Modulation of immune system – strategy in the treatment of breast cancer. In Vivo (Brooklyn). 2021;35(5):2889–2894. doi:10.21873/invivo.12578
- Bauer KR, Brown M, Cress RD, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer. 2007;109(9):1721–1728. doi:10.1002/cncr.22618
- Moore OJ, Foote FJ. The relatively favorable prognosis of medullary carcinoma of the breast. Cancer. 1949;2(4):635–642. doi:10.1002/1097-0142(194907)2:4<635::AID-CNCR2820020411>3.0.CO;2-Q
- Emens LA. Breast cancer immunobiology driving immunotherapy: vaccines and immune checkpoint blockade. Expert Rev Anticancer Ther. 2012;12(12):1597–1611. doi:10.1586/era.12.147
- Galon J, Angell H, Bedognetti D, et al. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39(1):11–26. doi:10.1016/j.immuni.2013.07.008
- West NR, Kost SE, Martin SD, et al. Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer. 2013;108(1):155–162. doi:10.1038/bjc.2012.524
- Zheng Y. A rogue Foxp3 mutant undermines treg cell function. Immunity. 2017;47(2):211–214. doi:10.1016/j.immuni.2017.07.024
- Liu F, Lang R, Zhao J, et al. CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat. 2011;130(2):645–655. doi:10.1007/s10549-011-1647-3
- Liu S, Lachapelle J, Leung S, et al. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14(2):R48. doi:10.1186/bcr3148
- Song IH, Heo S-H, Bang WS, et al. Predictive value of tertiary lymphoid structures assessed by high endothelial venule counts in the neoadjuvant setting of triple-negative breast cancer. Cancer Res Treat. 2017;49(2):399–407. doi:10.4143/crt.2016.215
- Sanchez K, Page D, McArthur HL. Immunotherapy in breast cancer: an overview of modern checkpoint blockade strategies and vaccines. Curr Probl Cancer. 2016;40(2–4):151–162. doi:10.1016/j.currproblcancer.2016.09.009
- Stovgaard ES, Nielsen D, Hogdall E, et al. Triple negative breast cancer - prognostic role of immune-related factors: a systematic review. Acta Oncol. 2018;57(1):74–82. doi:10.1080/0284186X.2017.1400180
- Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–5380. doi:10.1200/JCO.2006.05.9584
- Lee S, Cho EY, Park YH, et al. Prognostic impact of FOXP3 expression in triple-negative breast cancer. Acta Oncol. 2013;52(1):73–81. doi:10.3109/0284186X.2012.731520
- Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4(1):59. doi:10.1186/s40425-016-0165-6
- Miyashita M, Sasano H, Tamaki K, et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res. 2015;17(1):124. doi:10.1186/s13058-015-0632-x
- Zhang Q, Ying J, Li J, et al. Aberrant promoter methylation of DLEC1, a critical 3p22 tumor suppressor for renal cell carcinoma, is associated with more advanced tumor stage. J Urol. 2010;184(2):731–737. doi:10.1016/j.juro.2010.03.108
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi:10.1016/j.ccr.2012.02.022
- Lu J, Ma L. The role of tumor-associated macrophages in the development, metastasis and treatment of breast cancer. Pathol Res Pract. 2020;216(9):153085. doi:10.1016/j.prp.2020.153085
- Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol. 2012;167(2):195–205. doi:10.1111/j.1365-2249.2011.04515.x
- Yuan ZY, Luo R-Z, Peng R-J, et al. High infiltration of tumor-associated macrophages in triple-negative breast cancer is associated with a higher risk of distant metastasis. Onco Targets Ther. 2014;7:1475–1480. doi:10.2147/OTT.S61838
- Sami E, Paul BT, Koziol JA, et al. The immunosuppressive microenvironment in BRCA1-IRIS-overexpressing TNBC tumors is induced by bidirectional interaction with tumor-associated macrophages. Cancer Res. 2020;80(5):1102–1117. doi:10.1158/0008-5472.CAN-19-2374
- Laoui D, Movahedi K, Van Overmeire E, et al. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol. 2011;55(7–9):861–867. doi:10.1387/ijdb.113371dl
- Annaratone L, Cascardi E, Vissio E, et al. The multifaceted nature of tumor microenvironment in breast carcinomas. Pathobiology. 2020;87(2):125–142. doi:10.1159/000507055
- Coffelt SB, Hughes R, Lewis CE. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta. 2009;1796(1):11–18. doi:10.1016/j.bbcan.2009.02.004
- Ding M, Fu X, Tan H, et al. The effect of vascular endothelial growth factor C expression in tumor-associated macrophages on lymphangiogenesis and lymphatic metastasis in breast cancer. Mol Med Rep. 2012;6(5):1023–1029. doi:10.3892/mmr.2012.1043
- Mahmoud SM, Lee AHS, Paish EC, et al. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012;65(2):159–163. doi:10.1136/jclinpath-2011-200355
- Mhawech-Fauceglia P, Wang D, Ali L, et al. Intraepithelial T cells and tumor-associated macrophages in ovarian cancer patients. Cancer Immun. 2013;13:1.
- Shen KY, Song Y-C, Chen I-H, et al. Depletion of tumor-associated macrophages enhances the anti-tumor immunity induced by a Toll-like receptor agonist-conjugated peptide. Hum Vaccin Immunother. 2014;10(11):3241–3250. doi:10.4161/hv.29275
- Kamoshida G, Ogawa T, Oyanagi J, et al. Modulation of matrix metalloproteinase-9 secretion from tumor-associated macrophage-like cells by proteolytically processed laminin-332 (laminin-5). Clin Exp Metastasis. 2014;31(3):285–291. doi:10.1007/s10585-013-9627-0
- Hollmén M, Roudnicky F, Karaman S, et al. Characterization of macrophage–cancer cell crosstalk in estrogen receptor positive and triple-negative breast cancer. Sci Rep. 2015;5:9188. doi:10.1038/srep09188
- Shiga K, Hara M, Nagasaki T, et al. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel). 2015;7(4):2443–2458. doi:10.3390/cancers7040902
- Yu T, Di G. Role of tumor microenvironment in triple-negative breast cancer and its prognostic significance. Chin J Cancer Res. 2017;29(3):237–252. doi:10.21147/j.issn.1000-9604.2017.03.10
- Dotto GP, Weinberg RA, Ariza A. Malignant transformation of mouse primary keratinocytes by Harvey sarcoma virus and its modulation by surrounding normal cells. Proc Natl Acad Sci U S A. 1988;85(17):6389–6393. doi:10.1073/pnas.85.17.6389
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi:10.1038/nrc1877
- Mao Y, Keller ET, Garfield DH, et al. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013;32(1–2):303–315. doi:10.1007/s10555-012-9415-3
- Luo H, Tu G, Liu Z, et al. Cancer-associated fibroblasts: a multifaceted driver of breast cancer progression. Cancer Lett. 2015;361(2):155–163. doi:10.1016/j.canlet.2015.02.018
- Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev. 2016;30(9):1002–1019. doi:10.1101/gad.279737.116
- Gentric G, Mieulet V, Mechta-Grigoriou F. Heterogeneity in cancer metabolism: new concepts in an old field. Antioxid Redox Signal. 2017;26(9):462–485. doi:10.1089/ars.2016.6750
- Magesh P, Thankachan S, Venkatesh T, et al. Breast cancer fibroblasts and cross-talk. Clin Chim Acta. 2021;521:158–169. doi:10.1016/j.cca.2021.07.011
- Wang M, Zhang J, Huang Y, et al. Cancer-associated fibroblasts autophagy enhances progression of triple-negative breast cancer cells. Med Sci Monit. 2017;23:3904–3912. doi:10.12659/MSM.902870
- Takai K, Le A, Weaver VM, et al. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget. 2016;7(50):82889–82901. doi:10.18632/oncotarget.12658
- Camp JT, Elloumi F, Roman-Perez E, et al. Interactions with fibroblasts are distinct in Basal-like and luminal breast cancers. Mol Cancer Res. 2011;9(1):3–13. doi:10.1158/1541-7786.MCR-10-0372
- Allaoui R, Bergenfelz C, Mohlin S, et al. Cancer-associated fibroblast-secreted CXCL16 attracts monocytes to promote stroma activation in triple-negative breast cancers. Nat Commun. 2016;7(1):13050. doi:10.1038/ncomms13050
- Mantovani A, Cassatella MA, Costantini C, et al. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–531. doi:10.1038/nri3024
- Huang H, Zhang H, Onuma AE, Tsung A. Neutrophil elastase and neutrophil extracellular traps in the tumor microenvironment. Adv Exp Med Biol. 2020;1263:13–23.
- Nagaraj S, Schrum AG, Cho H-I, et al. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184(6):3106–3116. doi:10.4049/jimmunol.0902661
- Jablonska J, Leschner S, Westphal K, et al. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120(4):1151–1164. doi:10.1172/JCI37223
- Droeser RA, Hirt C, Eppenberger-Castori S, et al. High myeloperoxidase positive cell infiltration in colorectal cancer is an independent favorable prognostic factor. PLoS One. 2013;8(5):e64814. doi:10.1371/journal.pone.0064814
- Rymaszewski AL, Tate E, Yimbesalu JP, et al. The role of neutrophil myeloperoxidase in models of lung tumor development. Cancers (Basel). 2014;6(2):1111–1127. doi:10.3390/cancers6021111
- Queen MM, Ryan RE, Ryan GH, et al. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65(19):8896–8904. doi:10.1158/0008-5472.CAN-05-1734
- Mamessier E, Sylvain A, Bertucci F, et al. Human breast tumor cells induce self-tolerance mechanisms to avoid NKG2D-mediated and DNAM-mediated NK cell recognition. Cancer Res. 2011;71(21):6621–6632. doi:10.1158/0008-5472.CAN-11-0792
- Lance DM, Johanna S, Joshy G. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci U S A. 2005;102(38):13550–13555. doi:10.1073/pnas.0506230102
- Desmedt C, Piette F, Loi S, et al. Strong time dependence of the 76-gene prognostic signature for node-negative breast cancer patients in the TRANSBIG multicenter independent validation series. Clin Cancer Res. 2007;13(11):3207–3214. doi:10.1158/1078-0432.CCR-06-2765
- Fujisaki K, Fujimoto H, Sangai T, et al. Cancer-mediated adipose reversion promotes cancer cell migration via IL-6 and MCP-1. Breast Cancer Res Treat. 2015;150(2):255–263. doi:10.1007/s10549-015-3318-2
- Dirat B, Bochet L, Dabek M, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71(7):2455–2465. doi:10.1158/0008-5472.CAN-10-3323
- D’Esposito V, Liguoro D, Ambrosio MR, et al. Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget. 2016;7(17):24495–24509. doi:10.18632/oncotarget.8336
- De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17(8):457–474. doi:10.1038/nrc.2017.51
- Bochet L, Meulle A, Imbert S, et al. Cancer-associated adipocytes promotes breast tumor radioresistance. Biochem Biophys Res Commun. 2011;411(1):102–106. doi:10.1016/j.bbrc.2011.06.101
- Balaban S, Shearer RF, Lee LS, et al. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017;5(1):1. doi:10.1186/s40170-016-0163-7
- Casbas-Hernandez P, Sun X, Roman-Perez E, et al. Tumor intrinsic subtype is reflected in cancer-adjacent tissue. Cancer Epidemiol Biomarkers Prev. 2015;24(2):406–414. doi:10.1158/1055-9965.EPI-14-0934
- Dore-Savard L, Lee E, Kakkad S, et al. The angiogenic secretome in VEGF overexpressing breast cancer xenografts. Sci Rep. 2016;6(1):39460. doi:10.1038/srep39460
- Welti J, Loges S, Dimmeler S, et al. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. 2013;123(8):3190–3200. doi:10.1172/JCI70212
- Shweiki D, Itin A, Soffer D, et al. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–845. doi:10.1038/359843a0
- Saharinen P, Eklund L, Pulkki K, et al. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol Med. 2011;17(7):347–362. doi:10.1016/j.molmed.2011.01.015
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi:10.1038/35025220
- Hu X, Zhang J, Xu B, et al. Multicenter Phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135(8):1961–1969. doi:10.1002/ijc.28829
- Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res. 2015;3(1):1–11. doi:10.1158/2326-6066.CIR-14-0209
- Sa-Nguanraksa D, Chuangsuwanich T, Pongpruttipan T, et al. High vascular endothelial growth factor gene expression predicts poor outcome in patients with non-luminal A breast cancer. Mol Clin Oncol. 2015;3(5):1103–1108. doi:10.3892/mco.2015.574
- Linderholm BK, Hellborg H, Johansson U, et al. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol. 2009;20(10):1639–1646. doi:10.1093/annonc/mdp062
- Lee TH, Avraham HK, Jiang S, et al. Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem. 2003;278(7):5277–5284. doi:10.1074/jbc.M210063200
- Bender RJ, Mac GF, Schönbach C. Expression of VEGF and semaphorin genes define subgroups of triple negative breast cancer. PLoS One. 2013;8(5):e61788. doi:10.1371/journal.pone.0061788
- Zhang X, Zeng Y, Qu Q, et al. PD-L1 induced by IFN-γ from tumor-associated macrophages via the JAK/STAT3 and PI3K/AKT signaling pathways promoted progression of lung cancer. Int J Clin Oncol. 2017;22(6):1026–1033. doi:10.1007/s10147-017-1161-7
- Ayers M, Lunceford J, Nebozhyn M, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi:10.1172/JCI91190
- Wang J, Chen H, Chen X, et al. Expression of tumor-related macrophages and cytokines after surgery of triple-negative breast cancer patients and its implications. Med Sci Monit. 2016;22:115–120. doi:10.12659/MSM.895386
- Hartman ZC, Poage GM, den Hollander P, et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. 2013;73(11):3470–3480. doi:10.1158/0008-5472.CAN-12-4524-T
- Jin K, Pandey NB, Popel AS. Crosstalk between stromal components and tumor cells of TNBC via secreted factors enhances tumor growth and metastasis. Oncotarget. 2017;8(36):60210–60222. doi:10.18632/oncotarget.19417
- Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16(Suppl 1):1–11. doi:10.1634/theoncologist.2011-S1-01
- Lee E, Fertig EJ, Jin K, et al. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat Commun. 2014;5(1):4715. doi:10.1038/ncomms5715
- Long X, Ye Y, Zhang L, et al. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways (Review). Int J Oncol. 2016;48(1):5–12. doi:10.3892/ijo.2015.3234
- Yang L, Huang J, Ren X, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13(1):23–35. doi:10.1016/j.ccr.2007.12.004
- Wuyts A, Van Osselaer N, Haelens A, et al. Characterization of synthetic human granulocyte chemotactic protein 2: usage of chemokine receptors CXCR1 and CXCR2 and in vivo inflammatory properties. Biochemistry. 1997;36(9):2716–2723. doi:10.1021/bi961999z
- Wilson S, Wilkinson G, Milligan G. The CXCR1 and CXCR2 receptors form constitutive homo- and heterodimers selectively and with equal apparent affinities. J Biol Chem. 2005;280(31):28663–28674. doi:10.1074/jbc.M413475200
- Saintigny P, Massarelli E, Lin S, et al. CXCR2 expression in tumor cells is a poor prognostic factor and promotes invasion and metastasis in lung adenocarcinoma. Cancer Res. 2013;73(2):571–582. doi:10.1158/0008-5472.CAN-12-0263
- Chuntharapai A, Kim KJ. Regulation of the expression of IL-8 receptor A/B by IL-8: possible functions of each receptor. J Immunol. 1995;155(5):2587–2594.
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–550. doi:10.1038/nrc1388
- Waldmann TA. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol Res. 2015;3(3):219–227. doi:10.1158/2326-6066.CIR-15-0009
- Cornish GH, Sinclair LV, Cantrell DA. Differential regulation of T-cell growth by IL-2 and IL-15. Blood. 2006;108(2):600–608. doi:10.1182/blood-2005-12-4827
- Ferlazzo G, Pack M, Thomas D, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004;101(47):16606–16611. doi:10.1073/pnas.0407522101
- Anguille S, Van Acker HH, Van den Bergh J, et al. Interleukin-15 dendritic cells harness NK cell cytotoxic effector function in a contact- and IL-15-dependent manner. PLoS One. 2015;10(5):e0123340. doi:10.1371/journal.pone.0123340
- Wawrocki S, Druszczynska M, Kowalewicz-Kulbat M, et al. Interleukin 18 (IL-18) as a target for immune intervention. Acta Biochim Pol. 2016;63(1):59–63. doi:10.18388/abp.2015_1153
- Fabbi M, Carbotti G, Ferrini S. Context-dependent role of IL-18 in cancer biology and counter-regulation by IL-18BP. J Leukoc Biol. 2015;97(4):665–675. doi:10.1189/jlb.5RU0714-360RR
- Pan MR, Wu -C-C, Kan J-Y, et al. Impact of FAK expression on the cytotoxic effects of CIK therapy in triple-negative breast cancer. Cancers (Basel). 2019;12(1):94. doi:10.3390/cancers12010094
- Gao X, Mi Y, Guo N, et al. Cytokine-induced killer cells as pharmacological tools for cancer immunotherapy. Front Immunol. 2017;8:774. doi:10.3389/fimmu.2017.00774
- Zhang Y, Shuaibing W, Beibei Y, et al. Adjuvant treatment for triple-negative breast cancer: a retrospective study of immunotherapy with autologous cytokine-induced killer cells in 294 patients. Cancer Biol Med. 2019;16(2):350–360. doi:10.20892/j.issn.2095-3941.2018.0378
- Schmidt-Wolf IG, Lefterova P, Mehta BA, et al. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol. 1993;21(13):1673–1679.
- Verneris MR, Kornacker M, Mailänder V, et al. Resistance of ex vivo expanded CD3 + CD56 + T cells to Fas-mediated apoptosis. Cancer Immunol Immunother. 2000;49(6):335–345. doi:10.1007/s002620000111
- Zhou ZQ, Zhao -J-J, Pan Q-Z, et al. PD-L1 expression is a predictive biomarker for CIK cell-based immunotherapy in postoperative patients with breast cancer. J Immunother Cancer. 2019;7(1):228. doi:10.1186/s40425-019-0696-8
- Li M, Wang Y, Wei F, et al. Efficiency of cytokine-induced killer cells in combination with chemotherapy for triple-negative breast cancer. J Breast Cancer. 2018;21(2):150–157. doi:10.4048/jbc.2018.21.2.150
- Zhao X, Ji C-Y, Liu G-Q, et al. Immunomodulatory effect of DC/CIK combined with chemotherapy in multiple myeloma and the clinical efficacy. Int J Clin Exp Pathol. 2015;8(10):13146–13155.
- Andersen MH, Sørensen RB, Brimnes MK, et al. Identification of heme oxygenase-1-specific regulatory CD8+ T cells in cancer patients. J Clin Invest. 2009;119(8):2245–2256. doi:10.1172/JCI38739
- Hontscha C, Borck Y, Zhou H, et al. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol. 2011;137(2):305–310. doi:10.1007/s00432-010-0887-7
- Yang L, Ren B, Li H, et al. Enhanced antitumor effects of DC-activated CIKs to chemotherapy treatment in a single cohort of advanced non-small-cell lung cancer patients. Cancer Immunol Immunother. 2013;62(1):65–73. doi:10.1007/s00262-012-1311-8
- Shi L, Zhou Q, Wu J, et al. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother. 2012;61(12):2251–2259. doi:10.1007/s00262-012-1289-2
- Pan K, Guan -X-X, Li Y-Q, et al. Clinical activity of adjuvant cytokine-induced killer cell immunotherapy in patients with post-mastectomy triple-negative breast cancer. Clin Cancer Res. 2014;20(11):3003–3011. doi:10.1158/1078-0432.CCR-14-0082
- Liu L, Zhang W, Qi X, et al. Randomized study of autologous cytokine-induced killer cell immunotherapy in metastatic renal carcinoma. Clin Cancer Res. 2012;18(6):1751–1759. doi:10.1158/1078-0432.CCR-11-2442
- Li W, Wang Y, Kellner DB, et al. Efficacy of RetroNectin-activated cytokine-induced killer cell therapy in the treatment of advanced hepatocelluar carcinoma. Oncol Lett. 2016;12(1):707–714. doi:10.3892/ol.2016.4629
- Herber DL, Nagaraj S, Djeu JY, et al. Mechanism and therapeutic reversal of immune suppression in cancer. Cancer Res. 2007;67(11):5067–5069. doi:10.1158/0008-5472.CAN-07-0897
- Zhang YS, Yuan FJ, Jia GF, et al. CIK cells from patients with HCC possess strong cytotoxicity to multidrug-resistant cell line Bel-7402/R. World J Gastroenterol. 2005;11(22):3339–3345. doi:10.3748/wjg.v11.i22.3339
- Schmidt-Wolf IG, Lefterova P, Johnston V, et al. Sensitivity of multidrug-resistant tumor cell lines to immunologic effector cells. Cell Immunol. 1996;169(1):85–90. doi:10.1006/cimm.1996.0094
- Sangiolo D, Mesiano G, Gammaitoni L, et al. Cytokine-induced killer cells eradicate bone and soft-tissue sarcomas. Cancer Res. 2014;74(1):119–129. doi:10.1158/0008-5472.CAN-13-1559
- Gammaitoni L, Giraudo L, Leuci V, et al. Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features. Clin Cancer Res. 2013;19(16):4347–4358. doi:10.1158/1078-0432.CCR-13-0061
- Li H, Huang L, Liu L, et al. Selective effect of cytokine-induced killer cells on survival of patients with early-stage melanoma. Cancer Immunol Immunother. 2017;66(3):299–308. doi:10.1007/s00262-016-1939-x
- Li DP, Li W, Feng J, et al. Adjuvant chemotherapy with sequential cytokine-induced killer (CIK) cells in stage IB non-small cell lung cancer. Oncol Res. 2015;22(2):67–74. doi:10.3727/096504014X14024160459168
- Vikas P, Borcherding N, Zhang W. The clinical promise of immunotherapy in triple-negative breast cancer. Cancer Manag Res. 2018;10:6823–6833. doi:10.2147/CMAR.S185176
- Mehraj U, Dar AH, Wani NA, et al. Tumor microenvironment promotes breast cancer chemoresistance. Cancer Chemother Pharmacol. 2021;87(2):147–158. doi:10.1007/s00280-020-04222-w
- Li Z, Qiu Y, Lu W, et al. Immunotherapeutic interventions of triple negative breast cancer. J Transl Med. 2018;16(1):147. doi:10.1186/s12967-018-1514-7
- Cetin B, Gumusay O. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:e108.
- Altundag K. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2019;380(10):986–987.
- Razazan A, Behravan J. Single peptides and combination modalities for triple negative breast cancer. J Cell Physiol. 2020;235(5):4089–4108. doi:10.1002/jcp.29300
- Oner G, Altintas S, Canturk Z, et al. Triple-negative breast cancer-role of immunology: a systemic review. Breast J. 2020;26(5):995–999. doi:10.1111/tbj.13696
- Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361–370. doi:10.1158/2326-6066.CIR-13-0127
- Sun S, Fei X, Mao Y, et al. PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol Immunother. 2014;63(4):395–406. doi:10.1007/s00262-014-1519-x
- Li X, Wetherilt CS, Krishnamurti U, et al. Stromal PD-L1 expression is associated with better disease-free survival in triple-negative breast cancer. Am J Clin Pathol. 2016;146(4):496–502. doi:10.1093/ajcp/aqw134
- Kim HM, Lee YK, Koo JS, et al. Expression of PD-L1 in triple-negative breast cancer based on different immunohistochemical antibodies. Virchows Arch. 2016;4691:S55.
- Stovgaard ES, Kümler I, List-Jensen K, et al. Prognostic and clinicopathologic associations of LAG-3 expression in triple-negative breast cancer. Appl Immunohistochem Mol Morphol. 2022;30(1):62–71.
- Lotfinejad P, Kazemi T, Mokhtarzadeh A, et al. PD-1/PD-L1 axis importance and tumor microenvironment immune cells. Life Sci. 2020;259:118297. doi:10.1016/j.lfs.2020.118297
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239
- Nascimento C, Urbano AC, Gameiro A, et al. Serum PD-1/PD-L1 levels, tumor expression and PD-L1 somatic mutations in HER2-positive and triple negative normal-like feline mammary carcinoma subtypes. Cancers. 2020;12(6):13866. doi:10.3390/cancers12061386
- Kassardjian A, Shintaku PI, Moatamed NA, Ahmad A. Expression of immune checkpoint regulators, cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death-ligand 1 (PD-L1), in female breast carcinomas. PLoS One. 2018;13(4):e01959584. doi:10.1371/journal.pone.0195958
- Acs B, Madaras L, Tőkés A-M, et al. PD-1, PD-L1 and CTLA-4 in pregnancy-related - and in early-onset breast cancer: a comparative study. Breast. 2017;35:69–77. doi:10.1016/j.breast.2017.06.013
- Saleh R, Toor SM, Khalaf S, Elkord E. Breast cancer cells and PD-1/PD-L1 blockade upregulate the expression of PD-1, CTLA-4, TIM-3 and LAG-3 immune checkpoints in CD4(+) T cells. Vaccines. 2019;7:1494. doi:10.3390/vaccines7040149
- Peng Z, Su P, Yang Y, et al. Identification of CTLA-4 associated with tumor microenvironment and competing interactions in triple negative breast cancer by co-expression network analysis. J Cancer. 2020;11(21):6365–6375. doi:10.7150/jca.46301