?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Papillary thyroid microcarcinoma (PTMC) has indolent features and low mortality. Recently, active surveillance (AS) instead of early surgery (ES) has been introduced as one treatment option but economical preference has not been established. The study objective was to systemically review the literature relating to cost-effectiveness of AS compared to ES for PTMC. Keywords were selected through PICO (Population, Intervention, Comparison, and Outcomes) tools. The search was conducted using PubMed, Cochrane, EMBASE, and Elsevier databases. Papers that had irrelevant titles were written in foreign languages, or had no original results were excluded. Out of the 62 papers extracted, five relevant to the subject matter of this study were identified. Three papers made their own decision models and proceeded with cost-effectiveness analysis (CEA), but the remaining two simply compared costs rather than cost-effectiveness. In terms of cost-effectiveness, three papers preferred AS, one preferred ES, and one preferred neither. The major differences in the CEA might arise from variations in each country’s medical insurance system, the utility score systems, and decision models used. In subgroup analysis, two papers preferred AS to ES for patients at a younger age at diagnosis in terms of cost-effectiveness as well as tumor biological characteristics. Although AS has been generally more cost-effective than ES in previous publications, younger age at diagnosis could be one factor contributing to preference for ES. The CEA of prospective cohorts based on the decision model and utility score for thyroid cancer should be undertaken to confirm the cost-effectiveness of AS.
Introduction
Although thyroid tumorigenesis is a complex process, most patients with differentiated thyroid cancer (DTC) exhibit slow tumor growth and have a good prognosis.Citation1 In Korea, while the incidence of thyroid cancer has increased rapidly since 2000, the 10-year survival rate of 94% is relatively high according to the 2018 Korean National Cancer registration data.Citation2 This trend is similar to that of worldwide data.Citation3 Papillary thyroid microcarcinoma (PTMC) with a diameter of less than 1 cm progresses slowly, is less aggressive, and has been detected more frequently in recent years.Citation4,Citation5 Due to indolent features, low mortality of small thyroid cancer, and fear of surgical complications, active surveillance (AS) could be provided as a surrogate of early surgery (ES). In one meta-analysis about AS for PTMC, the maximum percentage of delayed operation in AS population was 32%, but the reason for delayed operation was others rather than size increase or lymph node metastasis.Citation6 And according to one retrospective study, the surgical complication rate was 1% for lobectomy and 17% for total thyroidectomy.Citation7 However, it is vague that how these factors influence the cost-effectiveness of AS for PTMC. And it is not clear that AS is cost-effective compared to ES considering the good prognosis and long-term follow-up nature of DTC. This nature also makes it essential to consider cost-effectiveness from a long-term perspective for patients, physicians, payers, and the government. However, as it is difficult to find well-designed conclusive studies on the cost-effectiveness analysis (CEA) of AS versus ES for PTMC in Korea and at a global level, a systemic review on the topic is necessary.
Methods
Search Tools
Keywords were selected through PICO (Population, Intervention, Comparison, and Outcomes) tools. The search was conducted using PubMed, Cochrane, EMBASE, and Elsevier databases. The selected keywords included “thyroid cancer patient” (population), “surgical management” (intervention), “active surveillance” (comparison), and “cost-effectiveness” (outcomes). Detailed search words are shown in .
Table 1 PICO (Patient, Intervention, Comparison, Outcome) Method Keywords Used to Search the Literature on Cost-Effectiveness Analysis of Active Surveillance versus Early Surgery in Papillary Thyroid Cancer
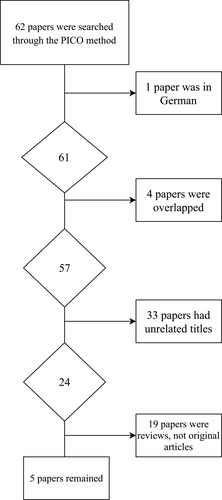
Using the aforementioned keywords, 62 papers were found. One of them was excluded as it was written in German. Four papers were found to be overlapping. Among the 57 remaining papers, 33 papers were excluded as they had unrelated or inapplicable titles. For example, the titles covered other cancers such as esophageal or lung cancer, or the papers did not include AS. Among the final 24 papers, 19 were excluded upon checking the abstracts, as they were reviews ().
Word Definition
Cost-effectiveness analysis (CEA) is typically defined as a comparative assessment of two or more interventions in terms of costs and consequences.Citation8 Medical procedures require heavy focus on the “consequences.” For example, if a new antidiabetic agent is developed, its effectiveness is measured in terms of reducing blood sugar levels. For a CEA, quality-adjusted life-years (QALYs) are recommended to measure the appropriateness of the outcome after one medical intervention. This is called the utility score. For QALY, consequences can be measured by defining alive states as 1 (one year in perfect health) and death as 0. QALY could be calculated in many ways. For one example, QALY data can be obtained from the quality of life (QoL) questionnaires answered by each patient.Citation9 Furthermore, the incremental cost-effectiveness ratio (ICER) can be calculated using the following formula: . ICER is defined as how much money is needed to obtain one QALY improvement by one medical intervention over one control care. If the medical intervention’s ICER is less than a specific threshold (eg $100,000/QALY), this intervention is thought to be cost-effective.
The threshold of ICER is called willingness to pay (WTP). In 2001, the World Health Organization’s Commission on Macroeconomics in Health suggested cost-effectiveness thresholds based on multiples of a country’s per capita gross domestic product (GDP). Despite the controversy over a threshold based on GDP, a CEA study conducted in Korea set the threshold at approximately $27,000–35,000/QALY. This varied remarkably from studies based on the USA, which usually raise the threshold to $50,000–100,000/QALY.Citation10–Citation14
When conducting a CEA, the analysis perspective being considered must be clarified. Five perspectives are generally cited: institution, third party, patient, government, and society.Citation15 There are several types of costs used in performing the CEA. Direct medical costs include physician services, diagnostic tests, and hospitalization expenses. Direct nonmedical costs are expenditures due to illness (eg travel, accommodation), and do not include medical services. Indirect costs are defined as expenses arising from cessation or reduction of work productivity because of illness (eg, work loss and worker replacement).Citation16
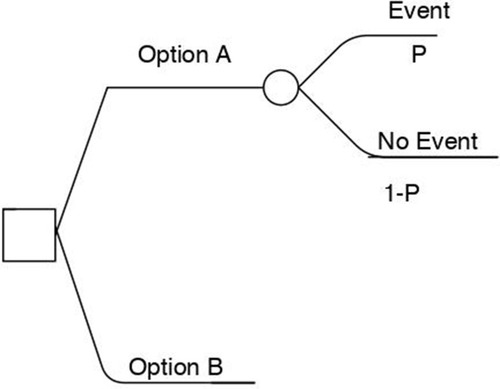
The two “decision models” most commonly applied in CEA are a decision tree and Markov model. In general, a decision tree consists of nodes and branches (). Each node can represent decision options (such as whether to opt for a particular treatment) and it is connected to branches. The chance of following one branch versus another is decided by the probabilities of events estimated with data.Citation17,Citation18
Figure 2 Decision tree example. Each node can represent decision options (such as whether to opt for a particular treatment) and it is connected to branches. The chance of going down one branch versus another is decided by the probabilities of events estimated with data. “P” represents the probabilities.
Notes: Adapted from: 2011 Medical Economic Evaluation Guideline. Health Insurance Review & Assessment Service (HIRA); 2016. Available from: http://www.hira.or.kr/cms/participation/05/07/__icsFiles/afieldfile/2013/04/01/3.pdf. Korean. 2016 Copyright HIRA.Citation31
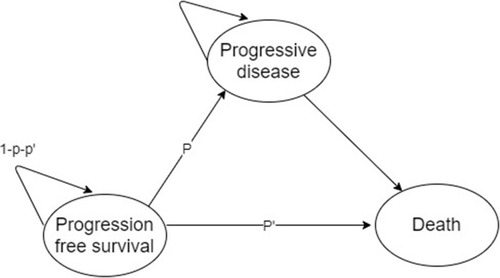
A Markov model is a stochastic model used to deal with randomly changing systems (). It assumes that future states depend only on the current state, not on the events that occurred before it. This assumption is called the Markov property.Citation17,Citation18
Figure 3 Markov model example. Each circle represents a specific medical condition. “P” represents the probability that a medical event can happen.
Notes: Adapted from: 2011 Medical Economic Evaluation Guideline. Health Insurance Review & Assessment Service (HIRA); 2016. Available from: www.hira.or.kr. Korean. 2016 Copyright HIRA.Citation31
Validation
The CEA should be validated. In other words, the quality of a cost-effectiveness model should be measured.Citation19 To validate results of the CEA and the quality of the CEA model used in each study, we adopted a grading system suggested by Chiou et al.Citation20 They used a questionnaire consisting of 16 items to measure the model’s construction, proper cost data adoption, and appropriate health outcome measures (Supplementary Table 1). Each question was answered dichotomously by yes or no to simplify the process.
Results
From the initial 62 papers, only five fit the criteria required by the current study (). Three papers made their own decision models and proceeded with CEA, but the remaining two papers simply compared costs rather than cost-effectiveness. All five papers only considered direct medical costs. Two papers clarified the perspective of analysis, but the others did not.
Table 2 Selected Five Key Papers About Cost-Effectiveness Analysis of Active Surveillance versus Early Surgery in Papillary Thyroid Cancer
A study conducted by Lang et al in Hong Kong concluded that adopting AS was not only cost saving in the initial 16 years but also remained cost-effective thereafter.Citation21 The authors constructed a decision tree and conducted a CEA on the base case hypothesis. They used cost data from public access files of the Centers for Medicare and Medicaid Services and examined the cost from an institution’s perspective. Indirect costs were not included. Utilities and probabilities used for the decision tree were based on literature. They used QALY as a utility score to measure cost-effectiveness. In contrast to other studies, only three cases were scored: alive without permanent complication (1.00), alive with permanent complication (0.54), and death (0) (). The results showed that more money was spent on AS than ES ($7204 vs $6521) but also that more QALY was gained (15.23 vs 14.97) (). They set the WTP as $50,000/QALY. ICER per patient for AS and ES was 473.1$/QALY and 435.7$/QALY, respectively, and both were lower than the WTP. As both the cost and QALY of AS were higher than those of ES, the authors calculated the incremental (AS minus ES) values of the base case to see if AS is really superior to ES in terms of cost-effectiveness. In this case, the ICER was 2623$/QALY, which meant that AS was more cost-effective. In sub-group analysis, those under the age of 40 showed a larger ICER than those over 40 (11,501$/QALY vs 1263$/QALY), indicating that AS required more money to achieve the same QALY difference in those under 40 and they preferred ES over AS. Nevertheless, the ICERs of AS in both age groups were below the WTP threshold of $50,000. This means adopting AS might not only be economic but also cost-effective regardless of patient age. The CEA structure was well organized and they used proper cost data. Despite the use of a simplified utility score system, the analysis and results were reliable ().
Table 3 Utility Score of the Three Key Reference Papers About Cost-Effectiveness Analysis of Active Surveillance versus Early Surgery in Papillary Thyroid Cancer
Table 4 Method of Modeling, Cost Sources, Utility Source, and Validation Level of Selected Papers
Venkatesh et al concluded that the cost-effectiveness of ES is highly dependent on patients’ utility score differences.Citation22 They constructed the Markov model as a decision model, and the CEA was based on the case hypothesis. The authors determined the cost from the perspective of the third-party payer. Medical costs were obtained from published Medicare reimbursement rates as well as the Healthcare Costs and Utilization Project. They also used QALYs for the utility score calculated according to changes in QoL. However, they used the utility score for prostate cancer because there was no published literature on the utility of thyroid cancer at that time and prostate cancer is the only other solid cancer in which AS is an acceptable management option instead of ES (). The WTP was set at $100,000/QALY. If a patient had small thyroid cancer that had not been removed by surgery (indicating AS), this would negatively affect QoL by “living” long-term with the diagnosis of a cancer and worsen the patient’s utility score. When a health utility difference between AS and a disease-free state was smaller, AS was found less expensive than ES and provided more QALY. On the other hand, when the health utility difference was larger between AS and a completely disease-free state, ES was cost-effective. Thus, they concluded that the cost-effectiveness of AS or ES was highly dependent on the patient’s utility score, without preference for either. In terms of validity, the authors constructed a well-designed CEA model, and used proper cost data. Although they applied the utility score for prostate cancer, the justification for usage of it was given because utility score for thyroid cancer was not available at that time ().
Oda et al from Japan concluded that the total cost of ES was 4.1 times higher than that of AS.Citation23 Rather than a decision model, the authors created a model of the flow of thyroid cancer management. They used Kuma Hospital’s patient data, which included an observational study of 2153 patients with PTMC. From the data, they calculated “the simple cost” of AS and ES and compared both. Whether conversion surgeries (for example, patients having a change of mind) were considered or not, the simple cost of ES was about 4.1 to 6.5 times the cost of AS ($7225–$9873 vs $1525–$2052) depending on the type of surgery and postoperative medication. The costs were calculated according to the Japanese Health Care Insurance System. The authors did not calculate the utility score; “cost-effectiveness” was not estimated. They used the prospective cohort population but they did not make the CEA model and did not use the utility score ().
White et al from the USA compared cost-effectiveness of the 2009 and 2015 American Thyroid Association (ATA) guidelines. The 2009 ATA guidelines suggested total thyroidectomy for malignant nodules of any size and lobectomy for very specific nodules: AS was not an option. On the other hand, under the ATA 2015 guidelines, small (< 1cm) solitary nodules with no lymph node metastasis could be managed with AS. The cost-effectiveness outcomes showed that the ATA 2015 strategy was better than that of 2009 for ES. Specifically, ATA 2015 delivered greater QALY (13.09 vs 12.43) at lower cost ($14,752 vs $20,126),Citation24 meaning that including the AS strategy brought better QoL at a lower price. The authors created a Markov simulation model and used cost data from the published literature, the Centers for Medicare and Medicaid Services Fee Schedule, the fee schedule at a major academic hospital, and the National Inpatient Sample database of the Centers for Medicare and Medicaid Services. They did not state the cost perspective. Complicated utility scores were used based on at least more than 20 studies of the QOL and diagnosis/treatment processes in patients with PTC.Citation25 The authors presented a well-organized CEA model and clarified the cost data sources but not the cost perspective. They used the utility score derived from studies about thyroid cancer, not other kinds of malignancy ().
Lin et al from Australia concluded that ES might have a long-term economic advantage for younger patients who are likely to require more than 16.2 years of follow-up in an AS scheme.Citation26 They used prospective surgical cohort data from between 1985 and 2017 and compared the total cost of surgical treatment to hypothetical AS. However, they did not use the decision tree or Markov model, and did not calculate the utility score. Therefore, the validity of their model is lower than the other studies ().
In short, from a cost-effectiveness point of view, three of the aforementioned studies preferred AS, one preferred ES, and one did not prefer either.
Discussion
In light of high thyroid cancer incidence and little information on cost-effectiveness of treatment, the current study aimed to search and review studies relating to cost-effectiveness of AS compared to ES for small PTC. In a mini-review of AS of PTMC in Korea, the authors insisted that the conclusions of CEA from one country with country-specific medical costs should not be applied to other countries but believed that ES might be more cost-effective in Korea due to lower surgery costs. However, in the absence of cost-effectiveness data, it might be too early to reach that conclusion because the costs of physician visits and sonography are also lower in Korea.Citation27
Because individual countries have varying medical cost systems, it was inevitable that the CEA results would be heterogeneous. Oda et al from Japan reported that the total cost of ES was 4.1 times higher than that of AS. However, the cost of thyroid sonography was only $32, which is much cheaper than costs mentioned in other studies ($100–$124). The physician visit fee was also cheaper than other costs ($20 vs $96.96–117).Citation21–24,Citation26 On the other hand, the cost of surgery was $2801–$3165, which is similar to that of other studies (). As the AS costs mainly consist of thyroid sonography and physician visit fee, AS is deservedly cost-effective in Japan. But it is not clear how much each medical cost contributes to the results. Although Korea and Japan share a similar medical cost system, it is not possible to conclude that the CEA results would also be similar.
Table 5 Costs Mentioned in the Five Key Reference Papers on Cost-Effectiveness Analysis of Active Surveillance versus Early Surgery in Papillary Thyroid Cancer
Most importantly, the differences in the CEA of each study come from the utility score. Lang et al assumed that the utility of a healthy state in the model was 1 regardless of the degree of a patient’s QoL, with only three semi-quantitative categories.Citation21 On the other hand, Venkatesh et al divided the utility levels into more details, but they used the utility score from prostate cancer, not thyroid cancerCitation20,Citation22 As the authors mentioned, because the population of thyroid cancer has a different age, sex, and QoL compared to that of prostate cancer, a separate utility score for thyroid cancer is needed for CEA. White et al used the utility score calculated from the study results based on the QoL of PTC patients.Citation24 However, White et al compared the 2009 and 2015 ATA guidelines, not the actual AS and ES. Therefore, future studies should attempt to develop a standard utility scoring system and apply it to studies that compare AS and ES.
Of the five papers, two created a decision tree and one a Markov model. However, they all created their own unique decision models.Citation21,Citation22,Citation24 This may cause confusion about whether the differences derive from the cost, the utility score, or the actual performance differences between ES and AS. The Markov model was used to compare the two guidelines, not specifically AS vs ES. On the other hand, the decision tree was created to reflect the clinical decision. Although there were minor differences, the three papers that developed the CEA model showed high validity.
In addition, variations in study results might derive from the differences between the decision tree and the Markov model. The decision tree seems logical but is not suitable for application to recurring events because of its linear fashion. However, it is better for generalizing and applying learned data. On the other hand, the Markov model can handle many clinical problems related to time, such as life expectancy or disease over time, but it is limited due to its assumption, the Markov property. In other words, the Markov model is memoryless.Citation17 With a limited number of studies using CEA, the most suitable model for thyroid cancer remains uncertain. Therefore, a well-organized standard decision model for thyroid cancer is needed for CEA.
Although some studies have dealt with the cost-effectiveness of AS, it is difficult to reach a conclusion because of the different ways the studies conducted the CEA. Considering this, future studies on the following subjects are needed.
First, a consistent decision model should be developed. Although heterogeneity inevitably derives from varying costs in different countries, a uniformly useful model, such as a Markov model or decision tree, is needed. Second, a utility score specific to thyroid cancer and a QALY system based on prospective cohort studies are needed. Lastly, appropriate follow-up and decision protocols for AS of thyroid cancer should be made. Although many studies on AS for PTMC have been conducted in recent years, tools for detecting disease progression have not been confirmed.Citation27,Citation28
Particularly, when the appropriate patients for AS are selected, age should be considered. In a multi-center cohort study in Korea, the risk of tumor volume increase in patients <45 years was twice higher than that of older patients during AS for low-risk PTMC.Citation29 Besides, Lang et al showed that those under 40 years would prefer ES over AS based on sub-group analysis.Citation21 This suggests that a different AS strategy according to age would be required in terms of not only tumor biological characteristics but also cost-effectiveness.
Our study has some limitations. Although we used the systemic method for searching the literature, there was a lack in the number of papers selected. In addition, among the five papers, two papers did not even construct the CEA model. Hence, it is difficult to make a clear conclusion about CEA for AS of PTC. In addition, the aforementioned studies almost used retrospective data. This limitation could be improved through accumulation of research about thyroid cancer CEA, especially in a prospective cohort population in the future. Recently, a Korean multicenter prospective study of AS or surgery (KoMPASS) has been initiated.Citation30 The collection of adequate data and development of a CEA model will provide a better understanding of the cost-effectiveness of AS and ES in Korea.
Author Contributions
Han-sang Baek mainly designed the study and wrote the review. Chul-Min Kim and Dong-Jun Lim supervised the study and are corresponding authors. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Author Declaration
A portion of this study was presented in abstract form at the AOCE-SICEM 2020 (Asia Oceanian Congress of Endocrinology and Seoul International Congress of Endocrinology and Metabolism 2020) in Seoul, Korea.
Disclosure
The authors declare that they have no conflicts of interest for this work.
Acknowledgments
The literature search was supported and conducted by Na Jin Kim of the Medical Library at The Catholic University of Korea, Seoul, Republic of Korea. We would like to thank Editage (www.editage.co.kr) for English language editing.
References
- YiHS, ChangJY, KimKS, ShongM. Oncogenes, mitochondrial metabolism, and quality control in differentiated thyroid cancer. Korean J Intern Med. 2017;32(5):780–789. doi:10.3904/kjim.2016.42028823142
- HongS, WonY-J, ParkYR, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 2020;52(2):335–350. doi:10.4143/crt.2020.20632178489
- DaviesL, WelchHG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–322. doi:10.1001/jamaoto.2014.124557566
- ItoY, MiyauchiA, KiharaM, HigashiyamaT, KobayashiK, MiyaA. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24(1):27–34. doi:10.1089/thy.2013.036724001104
- AhnHS, KimHJ, WelchHG. Korea’s thyroid-cancer “epidemic”–screening and overdiagnosis. N Engl J Med. 2014;371(19):1765–1767. doi:10.1056/NEJMp140984125372084
- ChoSJ, SuhCH, BaekJH, et al. Active surveillance for small papillary thyroid cancer: a systematic review and meta-analysis. Thyroid. 2019;29(10):1399–1408. (1557–9077 (Electronic)). doi:10.1089/thy.2019.015931368412
- KwonH, JeonMJ, KimWG, et al. A comparison of lobectomy and total thyroidectomy in patients with papillary thyroid microcarcinoma: a retrospective individual risk factor-matched cohort study. Eur J Endocrinol. 2017;176(4):371–378. (1479–683X (Electronic)). doi:10.1530/EJE-16-084528089996
- GuptaN, VermaR, DhimanRK, RajsekharK, PrinjaS. Cost-effectiveness analysis and decision modelling: a tutorial for clinicians. J Clin Exp Hepatol. 2020;10(2):177–184. doi:10.1016/j.jceh.2019.11.00132189934
- WhiteheadSJ, AliS. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96(1):5–21. (1471–8391 (Electronic)). doi:10.1093/bmb/ldq03321037243
- BertramMY, LauerJA, De JoncheereK, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94(12):925–930. doi:10.2471/BLT.15.16441827994285
- ChoiMJ, KangSO, OhJJ, ParkSB, KimMJ, CheongHJ. Cost-effectiveness analysis of 13-valent pneumococcal conjugate vaccine versus 23-valent pneumococcal polysaccharide vaccine in an adult population in South Korea. Hum Vaccin Immunother. 2018;14(8):1914–1922. doi:10.1080/21645515.2018.145660229953307
- LeeD, ShinHY, ParkSM. Cost-effectiveness of antiviral prophylaxis during pregnancy for the prevention of perinatal hepatitis B infection in South Korea. Cost Eff Resour Alloc. 2018;16:6. doi:10.1186/s12962-018-0088-929467596
- YunJW, ChoiMJ, ShinGS, et al. Cost-effectiveness of influenza vaccine strategies for the elderly in South Korea. PLoS One. 2019;14(1):e0209643. doi:10.1371/journal.pone.020964330682030
- NeumannPJ, CohenJT, WeinsteinMC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi:10.1056/NEJMp140515825162885
- GarrisonLPJr, PaulyMV, WillkeRJ, NeumannPJ. An overview of value, perspective, and decision context-A health economics approach: an ISPOR special task force report [2]. Value Health. 2018;21(2):124–130. doi:10.1016/j.jval.2017.12.00629477389
- BoccuzziSJ. Indirect health care costs. In: WeintraubWS, editor. Cardiovascular Health Care Economics. Contemporary Cardiology. Totowa, NJ: Humana Press; 2003. doi:10.1007/978-1-59259-398-9_5
- KuntzK, SainfortF, ButlerM, et al. Decision and Simulation Modeling in Systematic Reviews. Vol. 11. Rockville, MD: Agency for Healthcare Research and Quality; 2013.
- BoeckJD, VerpoortenK, LuytenK, ConinxK. A comparison between decision trees and markov models to support proactive interfaces. Paper presented at: 18th International Workshop on Database and Expert Systems Applications (DEXA 2007); September 3–7’ 2007; Regensburg.
- McCabeC, DixonS. Testing the validity of cost-effectiveness models. Pharmacoeconomics. 2000;17(5):501–513. doi:10.2165/00019053-200017050-0000710977390
- ChiouCF, HayJW, WallaceJF, et al. Development and validation of a grading system for the quality of cost-effectiveness studies. Med Care. 2003;41(1):32–44. doi:10.1097/00005650-200301000-0000712544542
- LangBHH, WongCKH. A cost-effectiveness comparison between early surgery and non-surgical approach for incidental papillary thyroid microcarcinoma. Eur J Endocrinol. 2015;173(3):367–375. doi:10.1530/EJE-15-045426104754
- VenkateshS, PasternakJD, BeninatoT, et al. Cost-effectiveness of active surveillance versus hemithyroidectomy for micropapillary thyroid cancer. Surgery. 2017;161(1):116–126. doi:10.1016/j.surg.2016.06.07627839930
- OdaH, MiyauchiA, ItoY, et al. Comparison of the costs of active surveillance and immediate surgery in the management of low-risk papillary microcarcinoma of the thyroid. Endocr J. 2017;64(1):59–64. doi:10.1507/endocrj.EJ16-038127667647
- WhiteC, WeinsteinMC, FingeretAL, et al. Is less more? A microsimulation model comparing cost-effectiveness of the revised american thyroid association’s 2015 to 2009 guidelines for the management of patients with thyroid nodules and differentiated thyroid cancer. Ann Surg. 2020;271(4):765–773. doi:10.1097/SLA.000000000000307430339630
- LubitzCC, De GregorioL, FingeretAL, et al. Measurement and variation in estimation of quality of life effects of patients undergoing treatment for papillary thyroid carcinoma. Thyroid. 2017;27(2):197–206. doi:10.1089/thy.2016.026027824301
- LinJF, JonkerPKC, CunichM, et al. Surgery alone for papillary thyroid microcarcinoma is less costly and more effective than long term active surveillance. Surgery. 2020;167(1):110–116. doi:10.1016/j.surg.2019.05.07831543327
- KimTY, ShongYK. Active surveillance of papillary thyroid microcarcinoma: a mini-review from Korea. Endocrinol Metab.2017;32(4):399–406. doi:10.3803/EnM.2017.32.4.399
- LohiaS, HansonM, TuttleRM, MorrisLGT. Active surveillance for patients with very low-risk thyroid cancer. Laryngoscope Investig Otolaryngol. 2020;5(1):175–182. doi:10.1002/lio2.356
- OhHS, HaJ, KimHI, et al. Active surveillance of low-risk papillary thyroid microcarcinoma: a Multi-Center Cohort Study in Korea. Thyroid. 2018;28(12):1587–1594. doi:10.1089/thy.2018.026330226447
- JeonMJ, KangYE, MoonJH, et al. Protocol for a Korean Multicenter Prospective Cohort Study of Active Surveillance or Surgery (KoMPASS) in papillary thyroid microcarcinoma. Endocrinol Metab. 2021;36(2):359. (2093–5978 (Electronic)). doi:10.3803/EnM.2020.890
- Health Insurance Review & Assessment Service (HIRA). 2011 Medical Economic Evaluation Guideline; 2016. Available from: http://www.hira.or.kr/cms/participation/05/07/__icsFiles/afieldfile/2013/04/01/3.pdf. Korean.