Abstract
Background
We reviewed our experience with 200 patients who underwent video-assisted thoracoscopic day surgery (VATDS) at the Day Surgery Center at West China Hospital to identify the safety and feasibility of VATDS and assess the value of novel management in patients with pulmonary nodules.
Methods
Between June 2019 and December 2020, 200 patients with pulmonary nodules underwent VATDS at the Day Surgery Center at West China Hospital. The medical records of these 200 patients were reviewed for age, sex, preoperative history, operative and pathological findings, amount of daily chest tube drainage, procedure method and duration, length of stay (LOS), visual analog scale (VAS), and postoperative pulmonary complications (PPCs).
Results
There were 45 male and 155 female patients with a median age of 43 years (range 18 to 58 years). A total of 158 (79.00%) patients were diagnosed with lung adenocarcinoma, 35 (17.50%) were diagnosed with chronic inflammation with fibrous hyperplasia, and seven (3.50%) were diagnosed with granulomatous inflammation with necrosis. The mean LOS of the 200 patients was 1.25±0.95 days, and 187 (93.50%) patients were discharged within 24 hours as planned. Thirteen patients were transferred to the thoracic surgery ward for further treatment because of PPCs. The median VAS was 3 points (range 1 to 7 points), and the rate of PPCs was 11.50%.
Conclusion
Two hundred patients underwent VATDS with an acceptable 24-hour discharge rate. However, selection of patients for VATDS is required, and the implementation of VATDS on a larger scale requires further discussion.
Introduction
The advent and widespread acceptance of enhanced recovery after surgery (ERAS) have attracted great interest for a wide range of surgical specialties in the management of patients, aiming to minimize perioperative stress responses and catabolism and morbidity rates, shorten the length of stay (LOS), and enhance postoperative recovery to achieve an early return to normal life.Citation1–Citation3 The ERAS program has been fully implemented in our medical group, and the clinical effects have been shown in previous studies.Citation4,Citation5 Currently, different ERAS management practices are used in different specialties, and further studies are still in progress.Citation6–Citation11 How to make full use of the advantages of ERAS is worth further discussion and research. Therefore, we conducted video-assisted thoracic day surgery (VATDS) for a group of selected patients with pulmonary nodules.
Day surgery is currently a safe and cost-effective medical model that is popular in developed countries, such as America and European countries.Citation12,Citation13 Additionally, day surgery in China has developed gradually.Citation14 The Chinese Ambulatory Surgery Alliance defines day surgery as a planned surgery that is performed, and the patient is discharged within 24 hours, excluding outpatient surgery. The definition of day surgery in West China Hospital is a planned surgery that is performed, and the patient is discharged within 24 hours, excluding outpatient surgery, but the patient can stay overnight, which is similar to extended recovery of day surgery in the United Kingdom.Citation15 Currently, the Day Surgery Center was built in West China Hospital, which has covered many kinds of day surgeries. If patients with pulmonary nodules can undergo VATDS in a day surgery center and be discharged on the first day after surgery, which is within 24 hours after admission, the medical resources may be fully utilized, and the hospital costs will be reduced because of the lower LOS. However, only a few studies have reported the day surgery mode of thoracic surgery.Citation16–Citation18
Day surgery is a pathway of clinical management, not a specific kind of surgery or procedure, which may contribute to the better outcome of the ERAS model. Thus, the implementation of ERAS in thoracic surgery may lead to the success of VATDS for a group of patients with pulmonary nodules. Therefore, the clinical experience of patients undergoing VATDS at the Day Surgery Center at West China Hospital was reviewed to identify the safety and feasibility of VATDS and assess the value of novel management in patients with pulmonary nodules.
Patients and Methods
Ethical Review
This study was registered in the Chinese Clinical Trial Registry before submission (Chi-CTR 2000034999). This study was approved by Ethics Committee on Biomedical Research, West China Hospital of Sichuan University (Number: 2020-341) and the Chinese Ethics Committee of Registering Clinical Trials and adhered to the tenets of the Declaration of Helsinki. Additionally, written informed consent was obtained from the patients. The work has been reported in line with the STROCSS criteria.Citation19
Inclusion and Exclusion Criteria
Patients were included if they met the following inclusion criteria: 1) were between 18 and 60 years old; 2) undergoing VATS anatomical pulmonary resection; 3) no severe comorbidities, such as chronic obstructive pulmonary disease (COPD) or hypertension; 4) an American Standards Association (ASA) score of ≤2 points; and 5) a pulmonary nodule diameter that was less than 3 cm.
Patients were excluded if they met the following exclusion criteria: 1) informed consent was not signed or 2) radiotherapy, chemotherapy or pulmonary operation were received before the surgery.
Patient Selection and Education
Data from patients with pulmonary nodules who underwent thoracoscopic anatomical pulmonary resection in the same medical group at the Day Surgery Center of our hospital from June 2019 to December 2020 were continuously collected. Two weeks before surgery, patients were examined by head, chest and upper abdomen-enhanced CT, pulmonary function tests, blood tests, blood biochemical tests, and electrocardiogram as preoperative evaluations.Citation20 On the day before surgery, patients and their families were informed in detail of surgical expectations and the risk of complications. Informed consent was signed in the surgeon’s office.
Surgical Approach
VATS was mainly performed via the three-portal thoracoscopic technique and double-lumen endotracheal intubation, combined with intravenous anesthesia and single lung ventilation.Citation21 The thoracoscopy entrance was selected to be 1.5 cm in the 7th intercostal space anterior to the midaxillary line; the main operation port was in the 3rd or 4th intercostal space; and the auxiliary operation port was located at the 9th intercostal space behind the axillary line. When performing systemic lymph node dissection, the left nodes were dissected in groups 5, 6, 7, 8, 9 and 10, and the right nodes were dissected in groups 2, 4, 7, 8, 9 and 10.
ERAS Program Management
Perioperative Fluid Management
Fluid management encompassed the pre-, intra- and post-operative periods.Citation22,Citation23 Carbohydrate loading and the avoidance of starvation ensured that patients were not dehydrated prior to the induction of anesthesia preoperatively.Citation24 Balanced crystalloids were used for liquid input during and after the operation, and the volume was controlled at approximately 1–2 mL/kg/hour. The positive liquid balance was maintained at <1500 mL (or 20 mL/kg/24 hours) during the perioperative period.Citation25
Chest Drainage Management
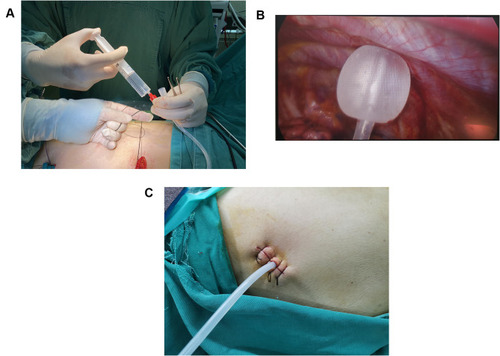
Single chest tube drainage was performed after surgery.Citation4 An 18F silicone Foley catheter was inserted through a port wound in the third or 4th intercostal middle axillary line and then descended toward the dorsal region (). When the patient regained consciousness 4 hours after returning to the ward, chest tube removal was performed if the lung remained fully expanded from the chest X-ray and no air leak was observed in the water seal chamber. The chest tube could be removed safely even if the daily serous effusion was of a high volume (up to 450 mL/24 h).Citation26
No Catheterization Management
Patients were instructed to empty their bladders before surgery, and no urinary catheters were inserted during the operation.Citation27 When patients had difficulty urinating after the surgery, they were promptly informed of several methods, including taking a semi-recumbent or sitting position with warm compression, rinsing the vulva, and listening to the sound of running water. Patients urinated intermittently using a urinary tube if necessary.
Regional Anesthesia and Pain Relief
Patients were asked about their drug allergy history in detail. Parecoxib sodium (40 mg, Pharmacia & Upjohn Company LLC, batch number: H20171071) was injected 1 hour before surgery for preemptive anesthesia.Citation28 At the end of the surgery, thoracoscopic intercostal nerve blocks (TINBs) were administered by infiltration of a local anesthetic mixture (15 mL for each intercostal space) from the third to the 9th intercostal nerves under the parietal pleura, 2 cm lateral to the sympathetic chain ().Citation29 When returning to the ward, 40 mg parecoxib sodium was injected 8 hours after surgery.Citation30 If necessary, nonsteroidal anti-inflammatory drugs (NSAIDs), oral sustained-release ibuprofen capsules or ammonia phenolic oxycodone tablets were provided to the patient.
MCT Treatment
Patients were treated with a short-term medium-chain triglyceride (MCT) diet after surgery.Citation31,Citation32 Four hours after the operation, when the patient was conscious, 100 mL of warm water was taken. If there was no nausea or vomiting after 6 to 8 hours, the patient drank 250 mL of appetizing liquid, which was used to accelerate the recovery of gastrointestinal function. The patient took 50 g of nutritional powder and 250 mL of warm water 10 to 12 hours after surgery. On the first day after surgery, the nutrition department provided dietary guidance.
Discharge Criterion
The discharge standards for the patient were set in five categories: 1) vital signs, which included blood pressure and pulse; 2) activity and mental status; 3) nausea and vomiting; 4) surgical bleeding; and 5) pain. The possibility of discharge was evaluated using the post anesthesia discharge scoring system (PADSS) score until patients had a score of 9 or higher, allowing their discharge.Citation33 Details are provided in Supplement 1.
Post Discharge Management
To better ensure patient condition after discharge, the nurse communicated with patients and their family by telephone every day during the first week and on the 14th and 28th days after discharge. If there was an emergency requiring medical intervention, the follow-up nurse notified the thoracic surgeon as soon as possible to deal with it in a timely manner. When patients experienced complications, such as bleeding or breaking pain, during the follow-up after day surgery, the patient or his family members were guided to perform simple treatments or transferred to the community hospital contracted by our hospital for treatment. If necessary, an ambulance from the emergency department was arranged to take the patient to the hospital, and the surgeons were informed to stay in the emergency department to treat the patient. Then, the patient was admitted to the thoracic surgery ward for further treatment.
Results
Characteristics of the Patients
There were 45 male and 155 female patients, with a median age of 43 years old (range 18 to 58 years). Four patients had a history of hypertension, four patients had a history of surgery, which included two appendectomies, one cholecystectomy and one benign breast nodule resection, and two patients had diabetes. No one had a history of current smoking.
The basic information on the patients with VATDS is shown in . In 87 (43.50%) cases, the tumor was in the left lung, while 113 (56.50%) cases were in the right lung. Lobectomy was performed in 73 patients, segmentectomy was performed in 118 patients, and wedge excision was performed in nine patients. The mean length of operation was 72.47±21.59 minutes, and the average blood loss was 42.43±1.43 mL. Out of 200 patients, 158 (79.00%) patients were diagnosed with lung adenocarcinoma, 35 (17.50%) were diagnosed with benign nodules, including chronic inflammation with fibrous hyperplasia, and seven (3.50%) were diagnosed with granulomatous inflammation with necrosis. The mean LOS of the 200 patients was 1.25±0.95 days, and 187 (93.50%) patients were discharged within 24 hours as planned. A total of 13 patients were transferred to the thoracic surgery ward for further treatment because of postoperative complications (PPCs). The median visual analog scale (VAS) was 3 points (range 1 to 7 points).
Table 1 Preoperative, Operative, and Postoperative Characteristics of Patients Included in the Study
Surgical Approaches
Patients who underwent VATDS were all admitted because of pulmonary nodules. Since different types of surgery may influence the prognosis of patients, a comparison between different surgical approaches was conducted. Lobectomy was performed in 73 patients, segmentectomy was performed in 118 patients and wedge excision in nine. Considering the PPCs, no significant difference was found among the three groups of patients, as shown in . In addition, although the operative approaches were different, there was no statistically significant difference in the operative time, mean length of stay, blood loss or hospital cost, as shown in .
Table 2 Comparison of PPCs Among Different Surgical Approaches
Table 3 Treatment-Related Costs and Resource Consumption Among Different Surgical Approaches
Pathological Diagnosis
In these 200 patients who underwent VATDS, a total of 158 (79.00%) patients were diagnosed with lung adenocarcinoma, and benign pathological diagnosis was diagnosed in 42 (21.00%) patients, which included chronic inflammation with fibrous hyperplasia and granulomatous inflammation with necrosis. The primary TNM stage of the 158 patients was stage I, and only one patient was stage II because of mediastinal lymph node metastasis. Among the 42 patients with a benign diagnosis, chronic inflammation with fibrous hyperplasia was observed in 35 (17.50%) patients, and granulomatous inflammation with necrosis was observed in 7 (3.50%) patients. When comparing the PPCs between the two groups, although persistent air leakage, pneumothorax, bleeding and pulmonary infection occurred more easily in the malignant group, no significant difference was found. Hoarseness and chylothorax are specially related complications that result from lymph node dissection, which may explain the difference. Details are shown in .
Table 4 Comparison of PPCs Between Different Pathological Diagnoses
Failure Cases
Of the 200 VATDS patients, a total of 187 (93.50%) patients were discharged within 24 hours as planned, but 13 patients were transferred to the thoracic surgery ward for further treatment because of PPCs. Among the 13 patients who were not discharged within 24 hours, nine patients had air leakage, and four patients had postoperative bleeding. In these nine air leakage patients, six patients underwent right-side surgery, and the fissure was undeveloped in three of them, two patients had extensive pleural adhesion, and one patient had right upper lobe posterior segment (S2) resection and right lower lobe dorsal segment (S6) resection, which may have contributed to persistent air leakage (PAL). For these four bleeding patients, one patient’s pulmonary artery was broken during the operating process and repaired with hemostatic clamps after thoracotomy. The patient was transferred to the thoracic surgery ward for further observation. The other three patients’ chest drainage volume per hour was more than 200 mL and persisted for three hours after surgery. Among the three postoperative bleeding patients, one patient underwent thoracoscopic hemostasis surgery because of surgical incision bleeding and was transferred to the thoracic ward, and the other two patients were transferred after using hemostatic methods, such as carbazochrome sodium sulfonate. Four patients’ vital signs recovered, and they were discharged after several days of observation. The information on the 13 patients is listed in .
Table 5 Information of Failure Cases
Discussion
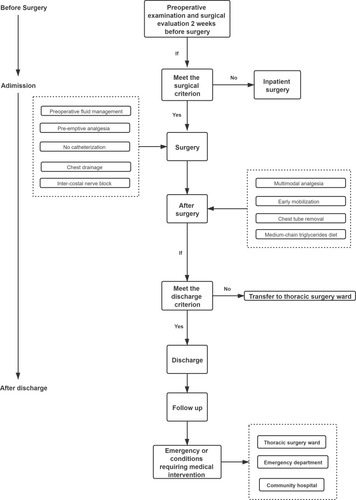
The VATDS study flow is shown in , which briefly explains the VATDS mode. To our knowledge, this is the first report of VATDS in a day surgery center with such large number of patients.
Most of the 200 patients with VATDS were female, young, without serious comorbidities or a history of smoking, and they were likely to have a shorter postoperative recovery period than older patients. In addition, surgeries were mainly segmentectomy with lymph node sampling or dissection, which is related to the popularity of current lung cancer screening methods. More lung tumors can be detected early and removed surgically in a timely manner. However, it also means that the implementation of VATDS is limited because of the high selection of patients with pulmonary nodules. Most patients in our study were non-smoker young women, which is clearly not representative of the daily work of other centers for lung cancer. Older men with a history of smoking have always been the main group of lung cancer patients. Thus, careful selection of patients for VATDS is highly required, and the implementation of VATDS on a larger scale needs further discussion.
When the surgical approaches and pathological diagnoses were reviewed, we found that segmentectomy and adenocarcinoma accounted for a large part. Since one of the inclusion criteria of VATDS was that the diameter of the pulmonary nodules was less than 3 cm, in which patients with advanced lung cancer may have been ruled out, most patients had a diagnosis of micro-invasive adenocarcinoma (MIA) or adenocarcinoma in situ (AIS) after surgery. To minimize the damage to lung tissue during surgery on the premise of sufficient surgical margin, segmentectomy is preferred, thus ensuring that VATDS is relatively easier to perform and patients in a day surgery center may recover faster than advanced lung cancer patients to meet the discharge criteria of a day surgery center. According to our study, LOS, PPCs and hospital cost were not different between the lobectomy group and segmentectomy group, and although the operative approaches were different, there was no statistically significant difference in operative time. After the surgery records review, we found that one patient in the wedge excision group underwent pleural adhesion cautery because of extensive adhesion in the thoracic cavity, which led to a longer surgery duration (115 minutes) and postoperative complications (persistent air leakage). Another patient in the wedge section group underwent a 120-minute surgery because of pulmonary artery bleeding, which took much time for hemostasis. Two patients were transferred to the thoracic surgery ward and discharged after a few days when their condition was stable. Due to the small sample size of wedge resection patients, the data from these two patients may have a significant impact on the operative time and medical costs, which may explain the outcome. In addition, a more comprehensive selection of patients and more careful steps in surgery need to be done for the sake of patients.
Among the 200 day surgery patients in this study, although the discharge rate within 24 hours (93.50%) was acceptable, the factors that made the 6.50% patients in this study unable to be discharged require further clarification Among the nine patients who were not discharged within 24 hours, nine patients had air leakage, and four patients had postoperative bleeding. Persistent air leakage (PAL) is one of the major complications after lung surgery. According to previous studies,Citation34,Citation35 the incidence of PAL is approximately 10%, which is more common in male patients and patients with preoperative pulmonary dysfunction (such as emphysema and COPD), lobotomy, pleural adhesion, low body mass index (BMI), right-side surgery, and underdeveloped fissures.Citation36–Citation41 Of the nine patients with air leakage, six underwent surgery on the right side, of which three had underdeveloped fissures. Two patients had pleural adhesion that could lead to PAL. The ERAS team in ItalyCitation42 recommended that prevention of air leakage is an important component of ERAS and that high-risk patients should take certain measures, such as pleural tent, surgical sealant, and staple-line reinforcement. In the day surgery center in particular, the discharge of day surgery patients was limited by air leakage. Therefore, a method to prevent air leakage should be developed when necessary. In addition, Bao and his team reported the safety of thoracic tube discharge in selected patientsCitation43 after pulmonary surgery. Further studies are needed to validate this approach, and it would be meaningful in the management of VATDS.
Due to China’s population and medical resources, it is important to make full and rational use of medical resources. This novel approach for early-stage NSCLC patients with fewer conditions may lessen medical pressure in China. However, VATDS is still in exploration; thus, it may not be made in all selected patients even though they meet the inclusion criteria. Among the 200 patients that underwent day surgery, a total of 23 patients had postoperative complications: nine patients had PAL, four patients developed pneumothorax, four patients developed hemorrhage after surgery, two patients had hoarseness after surgery, two patients had pleural effusion, one had chylothorax, and one had pulmonary infection. The rate of PPCs in VATDS patients (11.5%) was acceptable compared with standard VATS inpatients.Citation44 Thus, it is feasible to encourage this management for selected patients with pulmonary nodules.
Perhaps a “short” length of stay is not an accurate indicator to evaluate the quality of surgery. However, the safety and feasibility may be confirmed by the clinical outcomes of these 200 VATDS patients. For patients without serious complications, VATDS may achieve the effect of in-hospital surgery without increasing the perioperative risk.
Limitations
There are some limitations that we cannot ignore. First, this was a retrospective study rather than a randomized controlled trial and may inevitably have been confounded by other factors. A control group of traditional VATS will be made in our further study to compare the safety, feasibility and medical economics between day surgery and in-hospital surgery. Second, the study selected patients receiving VATS for pulmonary nodules, which may have limited us to generalization of relevant conclusions. Furthermore, we only discussed the patients in the day surgery center after strict selection. As shown in the discussion section, most patients were nonsmoker young women, which may be inapplicable to all other lung cancer centers, and careful selection of patients for VATDS is highly required; thus, the spread of VATDS is limited. Finally, patients should have a longer follow-up period with a comprehensive assessment, including a quality-of-life assessment, which needs to be considered in future research. A multicenter, prospective study is needed to further confirm the safety and feasibility of VATDS.
Conclusion
Two hundred patients underwent VATDS with an acceptable 24-hour discharge rate. Thirteen delayed-discharge patients recovered well and were discharged after treatment for several days. Thus, careful selection of patients for VATDS is required, and the implementation of VATDS on a larger scale needs further study.
Data Sharing Statement
Clinical trial data that underlie the results reported in this article (text, tables, figures, and appendices) and other study documents like Study Protocol, Informed Consent Form, Clinical Study Report are available to regulators, researchers, and trial participants upon request. And these data will be made available beginning 3 months and ending 5 years following article publication. Requestors can send a data access email to Dong Yingxian’s email, which is [email protected].
Consent for Publishing
All the authors consent to publish the paper.
Disclosure
Yingxian Dong and Jialong Li have shared first authorship. The authors have no potential conflicting interests to declare.
References
- SmithTW, WangX, SingerMA, GodellasCV, VainceFT. Enhanced recovery after surgery: a clinical review of implementation across multiple surgical sub-specialties. Am J Surg. 2020;219(3):530–534. doi:10.1016/j.amjsurg.2019.11.00931761300
- SemenkovichTR, HudsonJL, SubramanianM, KozowerBD. Enhanced Recovery After Surgery (ERAS) in thoracic surgery. Semin Thorac Cardiovasc Surg. 2018;30(3):342–349. doi:10.1053/j.semtcvs.2018.06.00129940227
- BatchelorTJP, LjungqvistO. A surgical perspective of ERAS guidelines in thoracic surgery. Curr Opin Anaesthesiol. 2019;32(1):17–22. doi:10.1097/aco.000000000000068530589662
- LaiY, WangX, ZhouH, KunzhouPL, CheG. Is it safe and practical to use a Foley catheter as a chest tube for lung cancer patients after lobectomy? A prospective cohort study with 441 cases. Int J Surg. 2018;56:215–220. doi:10.1016/j.ijsu.2018.06.02829936194
- LaiY, WangX, ZhouK, SuJ, CheG. The feasibility and safety of no placement of urinary catheter following lung cancer surgery: a retrospective cohort study with 2495 cases. J Invest Surg. 2019;34:1–8. doi:10.1080/08941939.2019.166337730898041
- GustafssonUO, ScottMJ, HubnerM, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) society recommendations: 2018. World J Surg. 2019;43(3):659–695. doi:10.1007/s00268-018-4844-y30426190
- SimpsonJC, BaoX, AgarwalaA. Pain management in Enhanced Recovery after Surgery (ERAS) protocols. Clin Colon Rectal Surg. 2019;32(2):121–128. doi:10.1055/s-0038-167647730833861
- BatchelorTJP, RasburnNJ, Abdelnour-BerchtoldE, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 2019;55(1):91–115. doi:10.1093/ejcts/ezy30130304509
- WainwrightTW, GillM, McDonaldDA, et al. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Acta Orthop. 2020;91(1):3–19. doi:10.1080/17453674.2019.168379031663402
- MałczakP, PisarskaM, PiotrM, WysockiM, BudzyńskiA, PędziwiatrM. Enhanced recovery after bariatric surgery: systematic review and meta-analysis. Obes Surg. 2017;27(1):226–235. doi:10.1007/s11695-016-2438-z27817086
- LjungqvistO. ERAS–enhanced recovery after surgery: moving evidence-based perioperative care to practice. JPEN J Parenter Enteral Nutr. 2014;38(5):559–566. doi:10.1177/014860711452345124567343
- British Association of Day Surgery. BADS Directory of Procedures. 5th ed. London, UK: BADS; 2016.
- CarloC, LuigiB, DraceBU, MartinCM. Day surgery: making it happen. Policy brief: European Observatory on health systems; 2007. Available from: www.euro.who.int/document/e90295.pdf. Accessed 627, 2014.
- JiangL, HoustonR, LiC, et al. Day surgery program at west china hospital: exploring the initial experience. Cureus. 2020;12(7):e8961. doi:10.7759/cureus.896132766004
- BaileyCR, AhujaM, BartholomewK, et al. Guidelines for day-case surgery 2019: guidelines from the Association of Anaesthetists and the British Association of Day Surgery. Anaesthesia. 2019;74(6):778–792. doi:10.1111/anae.1463930963557
- BlewettCJ, BennettWF, MillerJD, UrschelJD. Open lung biopsy as an outpatient procedure. Ann Thorac Surg. 2001;71(4):1113–1115. doi:10.1016/s0003-4975(00)02657-611308145
- TovarEA. One-day admission for major lung resections in septuagenarians and octogenarians: a comparative study with a younger cohort. Eur J Cardiothorac Surg. 2001;20(3):449–454. doi:10.1016/s1010-7940(01)00835-111509262
- TovarEA, RoetheRA, WeissigMD, LloydRE, PatelGR. One-day admission for lung lobectomy: an incidental result of a clinical pathway. Ann Thorac Surg. 1998;65(3):803–806. doi:10.1016/S0003-4975(97)01381-79527217
- AghaR, Abdall-RazakA, CrossleyE, DowlutN, IosifidisC, MathewG; for the STROCSS Group. The STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int J Surg. 2019;72:156–165. doi:10.1016/j.ijsu.2019.11.00231704426
- LakshminarasimhacharA, SmetanaGW. Preoperative evaluation: estimation of pulmonary risk. Anesthesiol Clin. 2016;34(1):71–88. doi:10.1016/j.anclin.2015.10.00726927740
- LiuL, CheG, PuQ, et al. A new concept of endoscopic lung cancer resection: single direction thoracoscopic lobectomy. Surg Oncol. 2010;19(2):e71–e77. doi:10.1016/j.suronc.2009.04.00519500971
- IijimaT, BrandstrupB, RodheP, AndrijauskasA, SvensenCH. The maintenance and monitoring of perioperative blood volume. Perioper Med (Lond). 2013;2:9. doi:10.1186/2047-0525-2-924472160
- NavarroLH, BloomstoneJA, AulerJO, et al. Perioperative fluid therapy: a statement from the International Fluid Optimization Group. Perioper Med. 2015;4:3. doi:10.1186/s13741-015-0014-z
- WeimannA, BragaM, CarliF, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36:623–650. doi:10.1016/j.clnu.2017.02.01328385477
- EvansRG, NaiduB. Does a conservative fluid management strategy in the perioperative management of lung resection patients reduce the risk of acute lung injury. Interact CardioVasc Thorac Surg. 2012;15:498–504. doi:10.1093/icvts/ivs17522617510
- BjerregaardLS, JensenK, PetersenRH, HansenHJ. Early chest tube removal after video-assisted thoracic surgery lobectomy with serous fluid production up to 500 mL/day. Eur J Cardiothorac Surg. 2014;45:241–246. doi:10.1093/ejcts/ezt37623872457
- MatotI, DeryE, BulgovY, CohenB, PazJ, NesherN. Fluid management during video-assisted thoracoscopic surgery for lung resection: a randomized, controlled trial of effects on urinary output and postoperative renal function. J Thorac Cardiovasc Surg. 2013;146:461–466. doi:10.1016/j.jtcvs.2013.02.01523558303
- BongCL, SamuelM, NgJM, Ip-YamC. Effects of preemptive epidural analgesia on post-thoracotomy pain. J Cardiothorac Vasc Anesth. 2005;19:786–793. doi:10.1053/j.jvca.2005.08.01216326309
- JoshiGP, BonnetF, ShahR, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg. 2008;107:1026–1040. doi:10.1213/01.ane.0000333274.63501.ff18713924
- DahlV, RaederJC. Non-opioid postoperative analgesia. Acta Anaesthesiol Scand. 2000;44:1191–1203. doi:10.1034/j.1399-6576.2000.441003.x11065198
- Rego CostaAC, RosadoEL, Soares-MotaM. Influence of the dietary intake of medium chain triglycerides on body composition, energy expenditure and satiety: a systematic review. Nutr Hosp. 2012;27(1):103–108. doi:10.1590/s0212-1611201200010001122566308
- LiR, MaJ, YuK, WangL. Dietary or enteral medium-chain triglyceride usage in a Chinese general hospital. Asia Pac J Clin Nutr. 2015;24(3):387–393. doi:10.6133/apjcn.2015.24.3.1826420178
- ChungF. Discharge criteria–a new trend. Can J Anaesth. 1995;42(11):1056–1058. doi:10.1007/bf030110838590498
- VarelaG, JimenezMF, NovoaN, et al. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg. 2005;27:329–333. doi:10.1016/j.ejcts.2004.11.00515691691
- RiveraC, BernardA, FalcozPE, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg. 2011;92:1062–1068. doi:10.1016/j.athoracsur.2011.04.03321871301
- StolzAJ, SchutznerJ, LischkeR, et al. Predictors of prolonged air leak following pulmonary lobectomy. Eur J Cardiothorac Surg. 2005;27:334–336. doi:10.1016/j.ejcts.2004.11.00415691692
- BrunelliA, CassiviSD, HalgrenL. Risk factors for prolonged air leak after pulmonary resection. Thorac Surg Clin. 2010;20:359–364. doi:10.1007/s12055-019-00827-w20619226
- DeCampMM, BlackstoneEH, NaunheimKS, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg. 2006;82:197–206. doi:10.1016/j.athoracsur.2006.02.05016798215
- CerfolioRJ, BassCS, PaskAH, et al. Predictors and treatment of persistent air leaks. Ann Thorac Surg. 2002;73:1727–1730. doi:10.1016/s0003-4975(02)03531-212078760
- FilossoPL, RuffiniE, SolidoroP, et al. Digital air leak monitoring after lobectomy for primary lung cancer in patients with moderate COPD: can a fast-tracking algorithm reduce postoperative costs and complications?J Cardiovasc Surg (Torino). 2010;51:429–433.
- Gómez-CaroA, CalvoMJ, LanzasJT, et al. The approach of fused fissures with fissureless technique decreases the incidence of persistent air leak after lobectomy. Eur J Cardiothorac Surg. 2007;31:203–208. doi:10.1016/j.ejcts.2006.11.03017175163
- GonfiottiA, ViggianoD, VoltoliniL, et al. Enhanced recovery after surgery and video-assisted thoracic surgery lobectomy: the Italian VATS Group surgical protocol. J Thorac Dis. 2018;10:S564–S570. doi:10.21037/jtd.2018.01.15729629203
- BaoF, DimitrovskaNT, HuS, ChuX, LiW. Safety of early discharge with a chest tube after pulmonary segmentectomy. Eur J Cardiothorac Surg. 2020;58(3):613–618. doi:10.1093/ejcts/ezaa09732236542
- BoffaDJ, DhamijaA, KosinskiAS, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg. 2014;148(2):637–643. doi:10.1016/j.jtcvs.2013.12.045. PMID: 24529729.24529729