Abstract
Introduction
MiRNAs play a critical role in carcinogenesis, among which miR-10a-5p has been reported in several types of human cancer. Nevertheless, the role of miR-10a-5p remain uncovered in bladder cancer (BCa).
Methods
We recruited 88 BCa patients and 36 healthy controls (HC) to form the training cohort, and other 120 BCa patients to form the validation cohort. The clinical samples were collected for analysis. The expression level of miR-10a-5p was evaluated using RT-qPCR. Receiver operating characteristic (ROC) curves were utilized to calculate diagnostic accuracy. Survival curves were generated to analyze survival outcomes. CCK-8 and transwell assays were conducted to test the cell proliferation, migration, and invasion capacities.
Results
MiR-10a-5p was upregulated in human BCa tissues and closely associated with advanced clinicopathological features, including advanced tumor grade, histological grade, and T stage. High expression of miR-10a-5p was associated with worse survival outcomes in BCa patients. Circulating plasma miR-10a-5p expression had the great performance power to discriminate BCa patients form HC patients before surgery, and to differentiate muscle invasive bladder cancer (MIBC) from non-muscle invasive bladder cancer (NMIBC). In addition, overexpression of miR-10a-5p could promote BCa cell proliferation, migration, and invasion.
Conclusion
This study indicates that miR-10a-5p is a crucial diagnostic and prognostic biomarker for BCa patients, and miR-10a-5p exerted a tumor promoting role during BCa cell progression.
Introduction
Bladder cancer (BCa) is one of the most frequently occurring malignancies worldwide, with about 380,000 new cases and 15,000 deaths each year.Citation1 The incidence of BCa in males is three times that in females.Citation2 BCa incidence and mortality rates vary across countries due to differences in risk factors, diagnostic practices, and availability of treatments.Citation3 BCa has different histological types including urothelial carcinoma, squamous cell carcinoma and adenocarcinoma, and urothelial carcinoma is the most frequent histological type, accounting for more than 95% of all BCa.Citation4 Recently, despite the obvious progress in diagnosis and treatment, the long-term prognosis remains poor for BCa patients, with the 5-year overall survival remaining only 50%-60%.Citation5 The high tumor recurrence rate and a high migratory and invasive ability of BCa have contributed greatly to the unsatisfactory prognosis.Citation6 With the increasing understanding of molecular abnormality, molecular diagnosis and targeted therapy have developed into a crucial part for the diagnosis and treatment of many cancers.Citation7 Moreover, some personalized approaches have grown rapidly for BCa treatment over the past several years, including novel targeted small-molecule and biological treatments, as well as immunotherapies.Citation8 Several immune checkpoint inhibitors that target programmed cell death protein 1 (PD1), its ligand PDL1, and cytotoxic T lymphocyte-associated protein 4 (CTLA4) have already been approved for use in bladder cancer.Citation9 Hence, it is urgent to seek effective and efficient biomarkers for the diagnosis and treatment of BCa patients.Citation10
MicroRNAs (miRNAs) are endogenous small non-coding RNAs at a length of 19 to 25 nucleotide. Recently, researches have elucidated the important roles of miRNAs in carcinogenesis, including cell differentiation, proliferation, apoptosis, and cell metastasis.Citation11–Citation13 Moreover, miRNAs can act as either oncogene or tumor suppressors depending on the genes it targets in various types of cancers.Citation14 Recently, the role of miRNAs has been increasingly determined for the early diagnosis and targeted treatment for tumors of urinary system, including prostate cancer and BCa patients.Citation15,Citation16 MiR-10a-5p, which has been widely reported in recent years, plays critical roles in different kinds of cancers including pancreatic ductal adenocarcinoma,Citation17 acute myeloid leukemia,Citation18 ovarian cancer,Citation19 lung cancer,Citation20 renal cell carcinoma,Citation21 and cervical cancer.Citation22 Nevertheless, the role of miR‐10a-5p still remain uncovered in BCa.
In this study, we determined that miR-10a-5p was upregulated in human BCa tissues, and its high expression could be an indicator for worse survival. Moreover, circulating plasma miR-10a-5p expression employed outstanding diagnostic value for BCa patients. In addition, enhanced expression of miR-10a-5p promoted proliferation, migration, and invasion of BCa cells.
Materials and Methods
Clinical Samples
A total of 88 BCa patients and 36 healthy controls (HC) were recruited from the Wuxi No.2 Chinese Medicine Hospital between January 2009 and December 2014 to constitute the training cohort. Meanwhile, other 120 BCa patients were enrolled at the same institution between January 2012 and May 2016 to form the validation cohort. Out of 88 bladder cancer patients in training cohort, 32 were diagnosed with non-muscle invasive bladder cancer (NMIBC); while the other 56 were diagnosed with muscle invasive bladder cancer (MIBC). Out of 120 bladder cancer patients in validation cohort, 40 were diagnosed with NMIBC; while the other 80 were diagnosed with MIBC. In the training cohort, plasma samples were extracted on the day of admission (BCa patients and HC patients) and 1 month after surgery (BCa patients); while in the validation cohort, plasma samples were extracted from BCa patients on the day of admission. The tumor tissues and paired adjacent normal tissues were obtained from BCa patients after surgery both in training and validation cohort. The inclusion criteria for BCa patients were:Citation1 all patients were histologically confirmed as BCa;Citation2 no other associated malignancies;Citation3 all patients underwent primary section of bladder cancer (TURBt) or radical cystectomy (RC) without other pre-surgical anti-cancer treatments;Citation4 patients had complete follow-up and clinicopathological information. The peripheral blood samples were collected from all participants in EDTA gel tubes. Each sample was centrifuged at 2000 g for ten minutes to separate plasma and then stored at −80°C until tested. The collected tumor tissues and paired normal tissues were immediately frozen in liquid nitrogen and frozen at −80°C before tested. Some clinical data were collected including age, gender, tumor grade, histological grade, T stage, lymph nodes metastasis, and multiplicity. Written informed consent was obtained from all the patients. This study was approved by the ethics committee of Wuxi No.2 Chinese Medicine Hospital and was conducted in accordance with the Declaration of Helsinki.
Cell Culture
Human BCa cell line 253j and J82 was obtained from Shanghai Chinese Academy of Sciences cell bank (China). 253j and J82 cells were cultured in DMEM medium (Life Technologies, Carlsbad, CA, US) supplemented with 10% fetal bovine serum (FBS, Life Technologies), 100 U/mL penicillin and streptomycin under a humidified incubator at 37 ◦C with 5% CO2.
RNA Extraction and Quantitative Reverse Transcription‑Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted from tissues or cells using the RNA Isolation Kit (Qiagen, USA). Complementary DNA (cDNA) was synthesized using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher, USA) in accordance with the manufacturer’s protocol. RT-qPCR reactions were then conducted on the ABI prism 7500 sequence detection system (Applied Biosystems) using the TaqMan miR assay system (Applied Biosystems, CA, USA). The thermal cycling conditions were: 95°C for 20 sec, followed by 40 cycles of 94°C for 20 sec, 60°C for 40 sec and 72°C for 10 sec. Relative expression of miR-10a-5p were normalized to that of U6 using 2−∆∆Ct method.Citation23 The sequences of oligonucleotides (GenePharma Co., Ltd, Shanghai, China) used in this study are as follows: miR-10a-5p: forward: 5ʹ-CGCTAGAAGCTTTTGGGTTA-3ʹ, reverse: 5ʹ-GCCCTAGACCATGGATTT-3ʹ; U6: forward: 5ʹ-CGCTTCGGCAGCACATATAC-3ʹ, reverse: 5ʹ-TTCACGAATTTGCGTGTCAT-3ʹ.
Cell Transfection
BCa cells were transfected with miR-10a-5p mimics, miR-10a-5p inhibitors, or their corresponding negative controls (NC mimics or NC inhibitors) (GenePharma Co., Ltd, Shanghai, China). Briefly, BCa cells were seeded in 6-well plates at a density of 1×105 cells per well for 24h. Cell transfection was conducted using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) based on the manufacturer’s instructions. Transfected cells were further cultured for an additional 48 h at 37°C before being used in downstream experiments.
Cell Counting Kit-8 (CCK-8) Assay
Three replicates of transfected BCa cells were seeded into 96-well plates at a density of 1×103 cells per well, following which 10 μL of CCK-8 solution (Dojindo, Kumamoto, Japan) was added to the medium at 1, 2, 3, 4 day before the cells were further incubated at 37 °C for 2 h. Optical density (OD) values were measured at 450 nm using an automatic microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Transwell Assay
The polyethylene membranes (24-well inserts; 8.0 μm; Corning, Inc.) were utilized to detect the migration and invasion abilities of BCa cells. Chambers precoated with 50 µL Matrigel (BD Biosciences) at 37°C for 1 h were used for invasion assays, while uncoated chambers were utilized for migration assays. Cell suspensions containing 1×105 cells in 100 μL FBS-free DMEM were seeded in the upper chamber. Meanwhile, the lower chamber was covered with 500 μL DMEM supplemented with 10% FBS. Cells were cultured at 5% CO2 and 37°C for 48 hours. After 48 hours, cells that have remained in the upper membranes were gently removed using a cotton swab; while cells that have migrated or invaded the bottom of the membrane were fixed with polyoxymethylene at room temperature for 20 min and then stained with 0.5% crystal violet at room temperature for 20 min. Cells were counted in 5 randomly selected fields under a light microscope (Leica Microsystems GmbH, Wetzlar, Germany).
Statistical Analysis
All data are expressed as mean ± standard deviation of at least three experiments. Statistical evaluations were performed using SPSS 20.0 (IBM SPSS Inc., Chicago, IL, USA). Differences between two groups were analyzed using unpaired Student’s t-test, while the expression of miR-10a-5p in tumor tissues and matched normal tissues was compared using paired Student’s t-test. Comparisons of multiple groups were analyzed using the ANOVA followed by Dunnett’s test. Categorical data were compared using chi-square test. Receiver operating characteristic (ROC) curves were utilized to calculate diagnostic accuracy. Overall survival (OS) and recurrence-free (RFS) of patients with BCa were evaluated using Kaplan-Meier curves and compared using Log rank test. Prognostic factors were analyzed by Cox regression proportional hazards analysis. Differences were considered to be significant when P< 0.05.
Results
MiR-10a-5p is Upregulated in BCa Tissues
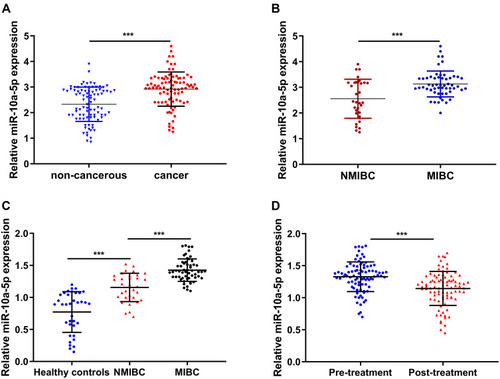
We firstly detected the expression profiles of miR-10a-5p in BCa tissues in the training cohort, and a total of 88 BCa tissues and paired normal tissues were collected for RT-qPCR analysis. Our results showed that miR-10a-5p expression was significantly upregulated in primary BCa tissues compared to that in paired normal tissues (P< 0.001, ). Moreover, RT-qPCR analysis showed a significant upregulation of miR-10a-5p in patient with MIBC compared to those with NMIBC (P< 0.001, ). Subsequently, the relative expression levels of miR-10a-5p were evaluated in plasma samples of the 32 patients with NMIBC, 56 patients with MIBC and 36 HC patients. RT-qPCR analysis results showed that miR-10a-5p expression was significantly higher in the MIBC group compared to the NMIBC or HC group (P< 0.001, ). The plasma levels of miR-10a-5p were also measured by RT‐qPCR at 1 month after surgery, and the results showed that plasma levels of miR-10a-5p were significantly downregulated at 1 month after treatment when compared to the pre-surgery levels of miR-10a-5p (P< 0.001, ). We analyzed the relationship between miR-10a-5p expression in BCa tissues and the clinicopathological features of these BCa patients. We firstly separated the 88 BCa patients into miR-10a-5p low expression group (n=44) and miR-10a-5p high expression group (n=44) based on the median values of miR-10a-5p expression in BCa tissues. MiR-10a-5p expression in tumor tissues was significantly correlated with tumor grade, histological grade, and T stage (). Moreover, we further confirmed the above findings with miR-10a-5p expression in validation cohort and GEPIA online database. As shown in Figure S1A–C, significant upregulation of miR-10a-5p was also observed in primary BCa tissues and patients with MIBC. Plasma miR-10a-5p expression level was significantly higher in the MIBC group compared to the NMIBC or HC group (P< 0.001, Figure S1D). The clinicopathological analysis showed that miR-10a-5p expression was significantly correlated with tumor grade, histological grade, and T stage in validation cohort (Table S1).
Table 1 The Relationship Between miR-10a-5p Expression and Clinical Features in Patients with Bladder Cancer
Figure 1 Upregulation miR-10a-5p in BCa tissues. (A) RT-qPCR analysis of miR-10a-5p expression in 88 pairs of BCa tissues and adjacent normal tissues. (B) RT-qPCR analysis of miR-10a-5p expression in patients with MIBC or NMIBC. (C) RT-qPCR analysis of miR-10a-5p expression in plasma samples of 60 MIBC, NMIBC, and healthy controls (HC) patients. (D) Downregulation of miR-10a-5p was observed after surgical resection. ***P< 0.001.
The Diagnostic Value of miR-10a-5p in BCa Patients
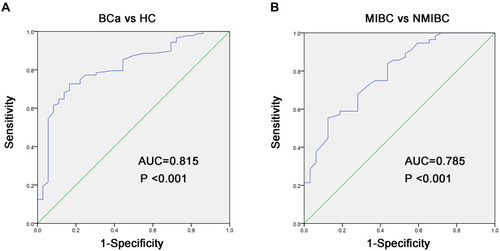
To evaluate the diagnostic value of miR-10a-5p in BCa patients, we analyzed the performance of plasma miR-10a-5p in distinguishing BCa patients from HC patients using ROC analysis in the training cohort. As shown in and , the optimal diagnostic cut-off value for miR-10a-5p was 1.09, and the AUC value for miR-10a-5p was 0.815 (95% confidence interval [CI], 0.734–0.896), with a sensitivity and specificity of 79.5% and 65.6%, respectively, in distinguishing the BCa patients from HC patients. Next, the ROC curve was used to explore the potential of utilizing miR-18a as a biomarker for differentiating MIBC from NMIBC. As shown in and , the optimal diagnostic cut-off value for miR-10a-5p was 1.28, and the AUC value for miR-10a-5p was 0.785 (95% CI, 0.688–0.883), with a sensitivity and specificity of 75.0% and 64.2%, respectively, in distinguishing the MIBC patients from NMIBC patients. Subsequently, the diagnostic power of miR-10a-5p was confirmed in the validation cohort. As demonstrated in Figure S2, miR-10a-5p still had the great value to distinguish BCa patients form HC patients with the AUC of 0.954 (0.688–0.883), and further to differentiate MIBC patients from NMIBC patients with the AUC of 0.796 (0.688–0.883).
Table 2 ROC Curves Analysis for the Diagnostic Power of Plasma miR-10a-5p in BCa
Figure 2 Diagnostic value assessment of miR-10a-5p in bladder cancer. (A) ROC curves indicate the ability of plasma miR-10a-5p to distinguish BCa patients from HC patients. (B) ROC curves indicate the ability of plasma miR-10a-5p to distinguish MIBC patients from NMIBC patients.
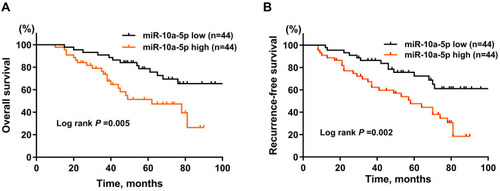
The Prognostic Value of miR-10a-5p in BCa Patients
Next, by generating Kaplan-Meier curves of OS, our results showed that BCa patients with a high level of miR-10a-5p was associated with worse OS (; P= 0.005) and RFS (; P= 0.002) compared with patients with a low level of miR-10a-5p in the training cohort. By using univariate and multivariate COX regression analysis ( and ), we found that tumor grade (HR: 2.02, 95% CI: 1.32–2.85, P= 0.028), T stage (HR: 1.81, 95% CI: 1.51–2.13, P= 0.001), and high miR-10a-5p expression (HR: 1.74, 95% CI: 1.31–2.01, P= 0.002) were independent indicators of poor OS in BCa patients; and tumor grade (HR: 2.52, 95% CI: 1.16–3.31, P= 0.035), T stage (HR: 2.11, 95% CI: 1.21–3.05, P= 0.001), and high miR-10a-5p expression (HR: 1.95, 95% CI: 1.20–2.64, P= 0.001) were independent indicators of poor RFS in BCa patients. Additionally, upregulation of miR-10a-5p correlated with suboptimal OS (Figure S3A; P= 0.008) and RFS (Figure S3B; P= 0.016) in the validation cohort. The COX regression analysis (Tables S2 and S3) further verified the prognostic role miR-10a-5p for BCa in the validation cohort. Taken together, our data indicated that miR-10a-5p is an independent favorable prognostic factor in BCa patients.
Table 3 Univariate and Multivariate Analyses of Prognostic Factors Associated with Overall Survival
Table 4 Univariate and Multivariate Analyses of Prognostic Factors Associated with Recurrence-Free Survival
Relationship Between miR-10a-5p and the Proliferation, Migration, and Invasion Ability of BCa Cells
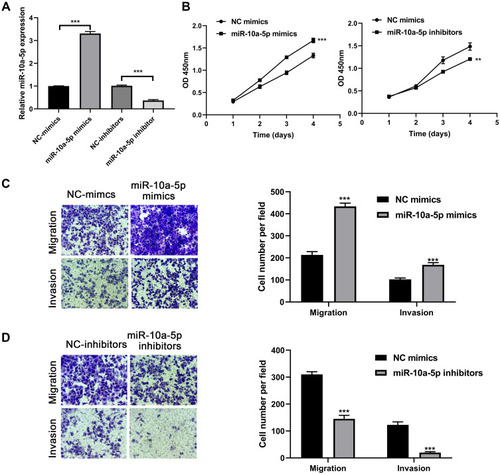
Next, we further investigated the proliferation, migration, and invasion abilities of miR-10a-5p in BCa tumor progression using CCK-8 and Transwell assays. Firstly, we overexpressed and knocked down the expression level of miR-10a-5p in 253j cells using miR-10a-5p mimics and inhibitors (P< 0.001, ). The CCK-8 assay results showed that the proliferation of the 253j cell lines transfected with the miR-10a-5p mimics was significantly increased compared with that of the cells transfected with the negative control, while the proliferation of the 253j cell lines transfected with the miR-10a-5p inhibitors was significantly inhibited (). Transwell assays demonstrated that miR-10a-5p mimics led to a significant increase in the migratory and invasive capability of 253j cells (P< 0.001, ), whereas miR-10a-5p inhibitors markedly suppressed the migration and invasion of 253j cells compared to that in respective controls (P< 0.001, ). In addition, as revealed in , the promoting role of miR-10a-5p overexpression as regards cell proliferation, migration, and invasion was confirmed in another BCa cell lines J82.
Figure 4 MiR-10a-5p promotes BCa cell proliferation, migration, and invasion. (A) RT-qPCR analysis of miR-10a-5p expression levels in 253j cells after transfection with miR-10a-5p mimics/inhibitors and respective negative controls. (B) CCK8 assays were performed to test the effect of miR-10a-5p mimics or inhibitors on cell proliferation of 253j cells. (C) Transwell assays were performed to test the effect of miR-10a-5p mimics on cell migration and invasion of 253j cells (magnification: 200×). (D) Transwell assays were performed to test the effect of miR-10a-5p inhibitors on cell migration and invasion of 253j cells (magnification: 200×). **P< 0.01, ***P< 0.001.
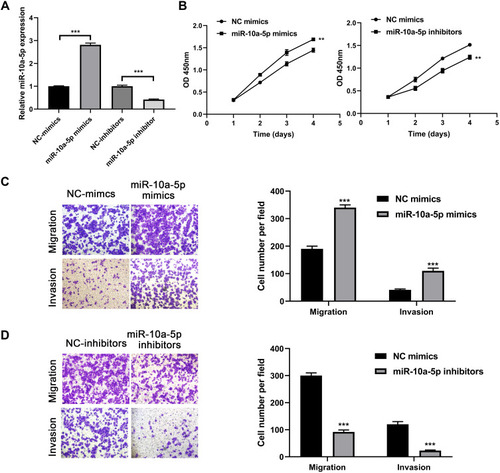
Figure 5 MiR-10a-5p promotes BCa cell proliferation, migration, and invasion. (A) RT-qPCR analysis of miR-10a-5p expression levels in J82 cells after transfection with miR-10a-5p mimics/inhibitors and respective negative controls. (B) CCK8 assays were performed to test the effect of miR-10a-5p mimics or inhibitors on cell proliferation of J82 cells. (C) Transwell assays were performed to test the effect of miR-10a-5p mimics on cell migration and invasion of J82 cells (magnification: 200×). (D) Transwell assays were performed to test the effect of miR-10a-5p inhibitors on cell migration and invasion of J82 cells (magnification: 200×). **P< 0.01, ***P< 0.001.
Discussion
Currently, a great number of studies have shown that miRNAs are abnormally expressed and may play crucial roles in diagnostic, prognostic, or biological functions in BCa progression. For instance, Wataru et al reported a miRNA panel, including miR-6087, miR-6724-5p, miR-3960, miR-1343-5p, miR-1185-1-3p, miR-6831-5p and miR-4695-5p, which could discriminate bladder cancer from healthy volunteers.Citation24 Moreover, Lin et al established a predictive model to predict the overall survival of bladder cancer based on 7 miRNAs including miR-185-5p, miR-663a, miR-30c-5p, miR-3648, miR-1270, miR-200c-3p, and miR-29c-5p.Citation25
Various studies have implicated miR-10a-5p in tumor progression. For example, Takayuki et al revealed the antitumor role of miR-10a-5p in renal cell carcinoma that low expression of miR-10a-5p was correlated to worse overall survival and overexpression of miR-10a-5p could inhibit cancer cell migration and invasion.Citation21 Moreover, Zhai et al reported that downregulation of miR‑10‑5p inhibited the cell viability and promoted cell cycle arrest of cervical cancer cells.Citation22 In addition, Xiong et al determined that miR-10a-5p was an independent adverse prognostic factor in patients with pancreatic ductal adenocarcinoma (PDAC) and promoted progression of PDAC cells in vitro and vivo.Citation17 In this study, through analyzing the clinical samples, we found that miR-10a-3p was upregulated in BCa tissues and further increased in patient with MIBC compared to those with NMIBC both in tumor samples and plasma samples. Our study also revealed the close relationship between miR-10a-5p expression and advanced tumor grade, histological grade, and T stage. High expression of miR-10a-5p closely correlated with advanced clinicopathological characteristics and poor survival outcomes.
Up to date, cystectomy is still the most reliable and accessible method for the detection of bladder cancer, which is expensive, invasive, time-consuming, and cannot be widely used especially for developing countries.Citation26 With the development of microarray technology, a great number of biomarkers, including miRNAs, have been developed for the early diagnosis of multiple cancers.Citation27 Specific serum miRNAs are very stable in blood plasma and serum, which is more convenient and noninvasive to predict the initiation and progression of BCa.Citation28 As reported in acute myeloid leukemia (AML), miR-10a-5p had important diagnostic value in differentiating AML from normal subjects.Citation18 In addition, Bao et al showed that miR-10a-5p was upregulated in non-small cell lung cancer (NSCLC) and also had great value for the clinical diagnosis of patients with NSCLC.Citation20 However, the diagnostic and biological role of miR-10a-5p have not been elucidated in BCa. In this study, our results indicated that circulating plasma miR-10a-5p expression had the great performance power to discriminate BCa patients form HC patients before surgery, and further to differentiate MIBC from NMIBC. In addition, our biological function assays determined the promoting role of miR-10a-5p in cell proliferation, migration, and invasion of BCa cells. However, this study had its limitation that the underlying mechanism of miR-10a-5p biological function is unclear.
In conclusion, our results demonstrate that miR-10a-5p was upregulated in BCa patients, and miR-10a-5p could act as a promising diagnostic and prognostic biomarker for BCa patients. Moreover, miR-10a-5p exerted a tumor promoting role during the BCa cell progression.
Data Sharing Statement
All data generated in this study will be made available on reasonable request.
Disclosure
The authors report no conflicts of interest in this work.
References
- Malats N, Real FX. Epidemiology of bladder cancer. Hematol Oncol Clin North Am. 2015;29(2):177–189. doi:10.1016/j.hoc.2014.10.00125836927
- Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder Cancer Incidence and Mortality: a Global Overview and Recent Trends. Eur Urol. 2017;71(1):96–108. doi:10.1016/j.eururo.2016.06.01027370177
- Babjuk M, Burger M, Compérat EM, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update. Eur Urol. 2019;76(5):639–657. doi:10.1016/j.eururo.2019.08.01631443960
- Moschini M, D’Andrea D, Korn S, et al. Characteristics and clinical significance of histological variants of bladder cancer. Nat Rev Urol. 2017;14(11):651–668. doi:10.1038/nrurol.2017.12528895563
- DeGeorge KC, Holt HR, Hodges SC. Bladder Cancer: diagnosis and Treatment. Am Fam Physician. 2017;96(8):507–514.29094888
- Fankhauser CD, Mostafid H. Prevention of bladder cancer incidence and recurrence: nutrition and lifestyle. Curr Opin Urol. 2018;28(1):88–92. doi:10.1097/MOU.000000000000045229211694
- McConkey DJ, Choi W. Molecular Subtypes of Bladder Cancer. Curr Oncol Rep. 2018;20(10):77. doi:10.1007/s11912-018-0727-530128829
- Felsenstein KM, Theodorescu D. Precision medicine for urothelial bladder cancer: update on tumour genomics and immunotherapy. Nat Rev Urol. 2018;15(2):92–111. doi:10.1038/nrurol.2017.17929133939
- Sweis RF, Galsky MD. Emerging role of immunotherapy in urothelial carcinoma-Immunobiology/biomarkers. Urol Oncol. 2016;34(12):556–565. doi:10.1016/j.urolonc.2016.10.00627836246
- Martinez Rodriguez RH, Buisan Rueda O, Ibarz L. Bladder cancer: present and future. Med Clin (Barc). 2017;149(10):449–455. doi:10.1016/j.medcli.2017.06.00928736063
- Tutar Y. miRNA and cancer; computational and experimental approaches. Curr Pharm Biotechnol. 2014;15(5):429. doi:10.2174/13892010150514082816133525189575
- Qadir MI, Faheem A. miRNA: a Diagnostic and Therapeutic Tool for Pancreatic Cancer. Crit Rev Eukaryot Gene Expr. 2017;27(3):197–204. doi:10.1615/CritRevEukaryotGeneExpr.201701949429199604
- Sun Z, Shi K, Yang S, et al. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17(1):147. doi:10.1186/s12943-018-0897-730309355
- Barbato S, Solaini G, Fabbri M. MicroRNAs in Oncogenesis and Tumor Suppression. Int Rev Cell Mol Biol. 2017;333:229–268.28729026
- Wieczorek E, Reszka E. mRNA, microRNA and lncRNA as novel bladder tumor markers. Clin Chim Acta. 2018;477:141–153. doi:10.1016/j.cca.2017.12.00929224950
- Cochetti G, Vermandois JA, Maulà V, et al. Role of miRNAs in prostate cancer: do we really know everything? Urol Oncol. 2020;38(7):623–635. doi:10.1016/j.urolonc.2020.03.00732284256
- Xiong G, Huang H, Feng M, et al. MiR-10a-5p targets TFAP2C to promote gemcitabine resistance in pancreatic ductal adenocarcinoma. J Exp Clin Cancer Res. 2018;37(1):76. doi:10.1186/s13046-018-0739-x29615098
- Zhi Y, Xie X, Wang R, et al. Serum level of miR-10-5p as a prognostic biomarker for acute myeloid leukemia. Int J Hematol. 2015;102(3):296–303. doi:10.1007/s12185-015-1829-626134365
- Guo L, Li Y, Zhao C, et al. RECQL4, Negatively Regulated by miR-10a-5p, Facilitates Cell Proliferation and Invasion via MAFB in Ovarian Cancer. Front Oncol. 2020;10:524128. doi:10.3389/fonc.2020.52412833014878
- Bao M, Pan S, Yang W, Chen S, Shan Y, Shi H. Serum miR-10a-5p and miR-196a-5p as non-invasive biomarkers in non-small cell lung cancer. Int J Clin Exp Pathol. 2018;11(2):773–780.31938164
- Arai T, Okato A, Kojima S, et al. Regulation of spindle and kinetochore-associated protein 1 by antitumor miR-10a-5p in renal cell carcinoma. Cancer Sci. 2017;108(10):2088–2101. doi:10.1111/cas.1333128746769
- Zhai L, Li Y, Lan X, Ai L. MicroRNA-10a-5p suppresses cancer proliferation and division in human cervical cancer by targeting BDNF. Exp Ther Med. 2017;14(6):6147–6151.29285171
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.126211846609
- Usuba W, Urabe F, Yamamoto Y, et al. Circulating miRNA panels for specific and early detection in bladder cancer. Cancer Sci. 2019;110(1):408–419. doi:10.1111/cas.1385630382619
- Lin GB, Zhang CM, Chen XY, et al. Identification of circulating miRNAs as novel prognostic biomarkers for bladder cancer. Mathematical Biosci Eng. 2019;17(1):834–844. doi:10.3934/mbe.2020044
- Takata R, Katagiri T, Kanehira M, et al. Predicting response to methotrexate, vinblastine, doxorubicin, and cisplatin neoadjuvant chemotherapy for bladder cancers through genome-wide gene expression profiling. Clin Cancer Res. 2005;11(7):2625–2636. doi:10.1158/1078-0432.CCR-04-198815814643
- Shi HB, Yu JX, Yu JX, et al. Diagnostic significance of microRNAs as novel biomarkers for bladder cancer: a meta-analysis of ten articles. World J Surg Oncol. 2017;15(1):147. doi:10.1186/s12957-017-1201-928774300
- Tölle A, Blobel CC, Jung K. Circulating miRNAs in blood and urine as diagnostic and prognostic biomarkers for bladder cancer: an update in 2017. Biomark Med. 2018;12(6):667–676. doi:10.2217/bmm-2017-039229896971