Abstract
Background
A peptide mimetic of a ligand for the galactose/N-acetylgalactosamine-specific C-type lectin receptors (GCLR) exhibited monocyte-stimulating activity, but did not extend survival when applied alone against a syngeneic murine malignant glioma. In this study, the combined effect of GCLRP with radiation was investigated.
Methods
C57BL/6 mice underwent stereotactic intracranial implantation of GL261 glioma cells. Animals were grouped based on randomized tumor size by magnetic resonance imaging on day seven. One group that received cranial radiation (4 Gy on days seven and nine) only were compared with animals treated with radiation and GCLRP (4 Gy on days seven and nine combined with subcutaneous injection of 1 nmol/g on alternative days beginning on day seven). Magnetic resonance imaging was used to assess tumor growth and correlated with survival rate. Blood and brain tissues were analyzed with regard to tumor and contralateral hemisphere using fluorescence-activated cell sorting analysis, histology, and enzyme-linked immunosorbent assay.
Results
GCLRP activated peripheral monocytes and was associated with increased blood precursors of dendritic cells. Mean survival increased (P < 0.001) and tumor size was smaller (P < 0.02) in the GCLRP + radiation group compared to the radiation-only group. Accumulation of dendritic cells in both the tumoral hemisphere (P < 0.005) and contralateral tumor-free hemisphere (P < 0.01) was associated with treatment.
Conclusion
Specific populations of monocyte-derived brain cells develop critical relationships with malignant gliomas. The biological effect of GCLRP in combination with radiation may be more successful because of the damage incurred by tumor cells by radiation and the enhanced or preserved presentation of tumor cell antigens by GCLRP-activated immune cells. Monocyte-derived brain cells may be important targets for creating effective immunological modalities such as employing the receptor system described in this study.
Introduction
Immunotherapy with immunostimulants aims to fundamentally alter the basic functions and milieu of the immune system both peripherally and within the brain to attack the tumor.Citation1 Galactose/N-acetylgalactosamine-specific C-type lectin receptors (GCLRPs) have demonstrated their integral role in regulation of cellular differentiation, recognition, and trafficking of monocyte-derived cells.Citation2 Previously, it was demonstrated that a peptide mimetic of a ligand of GCLRP exhibited promising features: (1) increased phagocytosis in vitro of monocytes and microglia isolated from resected human malignant glioma cells;Citation3 (2) increased expression of interleukin-21 in vitro by peripheral blood mononuclear cells, which is known to have antitumoral effect;Citation4 (3) enhanced expression of important functional markers of the microglial and macrophagal cells;Citation3 and (4) increased antigen presentation capacity of blood monocytes and subsequent differentiation of blood monocytes into dendritic cells (DCs) when applied in experimental malignant glioma.Citation3 In Part I of this study, monocytes in animals treated with GCLRP without any other treatment were not able to alter progression of the glioma. These cells were recruited to the tumoral and peritumoral areas more readily and were associated with an increase in tumor size.
It was hypothesized that GCLRP therapy coupled with cranial external radiation would have a synergistic effect.Citation3,Citation5 In addition to the obvious deactivation and destruction of tumor cells, it has been shown that irradiation of glioma cell lines upregulate the expression of numerous immunologically relevant molecules, especially major histocompatibility complex (MHC) class I and β2-microglobulin, which may make the tumor more accessible to immune recognition.Citation6 Moreover, internalization of glioma antigens released after external radiation by antigen presenting cells (APCs), with their subsequent presentation by MHC class II (MHCII) molecules, may elicit effective antigen-specific responses.Citation7 The aim of this study was to explore and assess a beneficial synergistic effect of GCLRP treatment in combination with external radiation in an experimental murine malignant glioma.
Materials and methods
GCLRP synthesis
Synthesis of GCLRP was described previously.Citation4 With a C-terminal amide group, the structure of GCLRP was ([VQATQSNQHTPRGGGS]2K)2K-NH2. The sequence –GGGS– was included as a spacer to extend the mimetic sequence from the core. For some experiments, the C- terminus was extended with β-alanine–cysteine and a dansyl group was incorporated by reaction of the thiol group on C-terminal cysteine with 5-([[[2-iodoacetyl]amino] ethyl]amino)naphthalene-1-sulfonic acid (Life Technologies, Carlsbad, CA). GCLRP was purified on a preparative Jupiter® Proteo C12 column (21.2 mm × 250 mm; Phenomenex, Torrance, CA) using a gradient of 8%–18% acetonitrile in water containing 0.1% trifluoroacetic acid, which was dried under vacuum, dissolved in sterile phosphate-buffered saline (PBS; pH 7.2), and passed through a Sephadex® G-25 or G-15 column (1 × 45 cm) (Sigma-Aldrich, St Louis, MO) in PBS to remove the trifluoroacetic acid. Concentration was determined by absorbance of the dansyl group (millimolar extinction coefficient = 5.7 cm−1 at 336 nm) or by the bicinchoninic acid assay (Thermo Fisher Scientific, Waltham, MA) using known concentrations of the dansylated GCLRP as standard.
Animals
All experiments were performed in compliance with Institutional Animal Care and Use Committee approval. The murine GL261 glioma cell line (syngeneic glioma cells) was obtained from the National Institute of General Medical Sciences, National Institutes of Health Cell Repository (Bethesda, MD). Cells were isolated, washed with PBS, and resuspended in Dulbecco’s modified Eagle medium at a concentration of 5 × 107 cells/mL. To perform the intracranial inoculation, mice (female C57Bl/six mice, 6–8 weeks old) (Charles River Laboratories, Wilmington, MA) were anesthetized using intraperitoneal injection of ketamine (100 mg/kg) and xylazine (5 mg/kg). Animals were placed into a standard small animal cranial stereotaxic frame. A small hole was made using a 21-gauge needle, 2.3 mm lateral, and 1 mm anterior from the intersection of the sagittal and coronal sutures (bregma). Cells (2 μL) were injected using a microsyringe and microsyringe pump controller (World Precision Instruments, Sarasota, FL). Burr holes were sealed using bone wax and the scalp was sutured closed.
Tumor size in animals was randomized by magnetic resonance imaging (MRI) 7 days after implantation, and the mice were divided into two groups. Thirty-two mice received cranial radiation (4 Gy on days seven and nine) combined with sham subcutaneous injections of saline; a second cohort was treated with cranial radiation (4 Gy on days seven and nine) combined with subcutaneous injections of GCLRP (1 nmol peptide/g) on alternative days beginning on day seven and ending at death.
Seventeen animals from each group were euthanized on day 18 for tissue investigations including flow cytometry, immunohistochemistry, histological staining. Fifty-eight animals from each cohort were used to establish survival rate. Twenty-four mice received radiation therapy only (4 Gy on days seven and nine), twenty-four animals received radiation therapy and peptide injection (4 Gy on days seven and nine and subcutaneously on alternate days with 1 nmol peptide/g GCLRP beginning on day seven and ending at death), and ten animals were used as a control (sham injection of saline on alternative days beginning on day seven and ending at death). The mice were sacrificed when moribund (signs of neurologic dysfunction or distress, dulled response, emaciated figure, skin fold, and cachexia). Kaplan–Meier survival curves with a logrank test were used to compare the survival of the two groups.
MRI
MRI scanning was first done on day seven to assign animals into groups on the basis of matched tumor size to decrease bias and was then repeated on day 19 after implantation. MRI was performed on a 3T GE LightSpeed® Qx/i system (GE Medical Systems, Milwaukee, WI) using a 2-cm birdcage coil. Prior to imaging, the animals were anesthetized with an intraperitoneal injection of xylazine (5 mg/kg) and ketamine (100 mg/kg). The animals received an injection of contrast agent (100 μL) into the tail vein (Magnevist®; Bayer Healthcare Pharmaceuticals, Montville, NJ) and axial images of the brain were acquired within 10 minutes thereafter using the MRI scanner to detect the presence of brain tumors.
Isolation of blood monocytes
Blood was collected in heparin-coated tubes or in syringes containing 1.0 mL of PBS with 8 mM ethylenediaminetetraacetic acid. Peripheral blood mononuclear cells were isolated from heparinized whole blood. After red blood cell lysis with BD Pharm Lyse™ buffer (BD Biosciences, San Jose, CA), cells were washed and filtered through 40-μm nylon strainers.
Isolation of monocyte-derived brain cells (MDCB)
Brain tissue was harvested after perfusion with saline. The tumoral area and contralateral tumor-free hemisphere were dissected separately (). To determine the nature and number of microglial and macrophage cells, brains were harvested from each group and single-cell homogenates were prepared for flow cytometric analysis. Brain tissues were finely minced with a razor blade, and then homogenized in 5 mL of PBS and incubated for 1 hour at 37°C with collagenase (Sigma-Aldrich, St Louis, MO). The homogenate was drawn into a 5-mL syringe fitted with a 21-gauge needle and passed ten times through the needle. The final single-cell suspension was filtered through a 40-μm nylon strainer and centrifuged at 700 × g for 5 minutes. The supernatant containing fully digested tissue was removed. The pellet was resuspended in 5 mL of 30% isotonic Percoll® (Sigma-Aldrich) in Hank’s balanced salt solution and overlaid onto 2 mL of 70% Percoll in Hank’s balanced salt solution in 15-mL conical tubes. The sample was then centrifuged at 700 × g for 20 minutes. The cells at the 30%/70% Percoll interface, which contained the microglia and macrophages, were collected and washed once in media. For cell surface staining, single-cell suspensions were prepared and stained with fluorescently labeled antibodies described below.
Fluorescence-activated cell sorting analysis
The following antibodies were employed: cluster of differentiation- 115 (CD115; phycoerythrin-labeled), MHCII (phycoerythrin-cyanine 5-labeled) (eBioscience, San Diego, CA), CD11b (phycoerythrin-cyanine-7-labeled), CD11c (allophycocyanin-labeled), and CD45 (fluorescein isothiocyanate-labeled) (BD Biosciences). Flow cytometric data were collected on a FACSAria™ flow cytometer and analyzed with Diva™ software (BD Biosciences). Identification of the mononuclear cell subpopulations was done on the basis of CD45 (leukocyte common antigen) and CD11b (complement receptor-3).Citation8,Citation9 Microglia were described as a CD45dim/CD11b+ and monocytes/macrophages as a CD45bright/CD11b+ population. Integrin αMβ2 (CD11b) as well as CD45 are not absolutely specific for the monocyte-derived cell lineage.Citation10 Since CD115 is a receptor for macrophage colony-stimulating factor, virtually all mononuclear cells should express this marker. CD115 marker was used to identify MDCB and microglia (CD115+CD45low/CD11b+) and macrophages (CD115+CD45high/CD11b+) in the brain.Citation10
An analysis of tumor-associated MDCB microglial cells (CD115+CD45low/CD11b+) was divided into subsets: resting microglial cells (CD115+CD45lowCD11b+MHCII−CD11c−), activated microglia (CD115+CD45lowCD11b+MHCII+CD11c −), and dendritic-like microglia (CD115+CD45lowCD11b+M HCII+CD11c+). The brain macrophagal population (CD115+ CD45high/CD11b+) was also divided into subsets: inactive macrophages (CD115+CD45highCD11b+MHCII−CD11c−), patrolling macrophages (PMs; CD115+CD45highCD11b+MHCII+CD1 1c−), and DCs (CD115+CD45highCD11b+MHCII+ CD11c+).
Hematoxylin and eosin staining and immunohistochemistry
On day 18 after tumor cell implantation, the animals were perfused transcardially with 4% paraformaldehyde in PBS (pH 7.4). Whole brains were removed, embedded in paraffin, and cryosectioned parasagittally into 10-μm serial sections. Sections were hematoxylin and eosin stained for general morphology. The tissue was then examined for immunocytochemistry with anti-CD68 antibodies (Sigma-Aldrich) to visualize macrophagal/microglial cells.
Cytokine assay
Tumor tissues and cellular extracts were snap frozen. A protease inhibitor cocktail (Sigma-Aldrich) was added to all tissue samples. The concentration of cytokines was measured using the following enzyme-linked immunosorbent assay kits: vascular endothelial growth factor (R&D Systems, Minneapolis, MN), interleukin-1β (Life Technologies), and tumor necrosis factor-α (Life Technologies). The absorbance of each sample at 450 nm and 540 nm was measured with a Bio-Rad 680 plate reader (Bio-Rad Laboratories, Hercules, CA). Absorbance was corrected with reference to a standard curve.
Statistical analysis
The statistical significance was determined using the two-tailed Student’s t-test. P < 0.05 was considered significant.
Results
Combination of GCLRP and radiation increased survival compared to radiation alone
Animals that received GCLRP therapy combined with cranial radiation showed significantly prolonged survival when compared to mice treated with external radiation alone (mean survival: GCLRP + radiation = 38 days versus radiation-only = 30 versus control-sham = 21; P = 0.001; ). MRI was used to study the tumor size in all animals after implantation. The tumor sizes () in animals that received GCLRP therapy combined with cranial external radiation were significantly smaller when compared to the animals from the radiation-only cohort (mean cross-sectional area: GCLRP + radiation = 2.50 ± 0.82 mm2 versus radiation-only = 5.13 ± 0.41 mm2; P = 0.02).
Figure 2 (A) The combination of GCLRP and external radiation increased survival when compared to the radiation-only group (mean survival: galactose/N-acetylgalactosamine-specific C-type lectin receptor + radiation = 38 days; radiation-only = 30; P = 0.001; control-sham animals = 21 days). (B) The survival rate corresponded with the magnetic resonance imaging data that showed a decrease in the tumor cross-sectional area (mean cross-sectional area: galactose/N-acetylgalactosamine-specific C-type lectin receptor + radiation = 2.498 mm2; P = 0.02 radiation-only = 5.126 mm2; P = 0.02 [compared to Control and GCLRP]).
Abbreviations: GCLRP, peptide mimetic of the ligand of the galactose/N-acetylgalactosamine specific C-type lectin receptor; SC, subcutaneous.
![Figure 2 (A) The combination of GCLRP and external radiation increased survival when compared to the radiation-only group (mean survival: galactose/N-acetylgalactosamine-specific C-type lectin receptor + radiation = 38 days; radiation-only = 30; P = 0.001; control-sham animals = 21 days). (B) The survival rate corresponded with the magnetic resonance imaging data that showed a decrease in the tumor cross-sectional area (mean cross-sectional area: galactose/N-acetylgalactosamine-specific C-type lectin receptor + radiation = 2.498 mm2; P = 0.02 radiation-only = 5.126 mm2; P = 0.02 [compared to Control and GCLRP]).Abbreviations: GCLRP, peptide mimetic of the ligand of the galactose/N-acetylgalactosamine specific C-type lectin receptor; SC, subcutaneous.](/cms/asset/a2d36020-fd91-464f-a4da-a9eeb7573e0f/dcmr_a_33355_f0002_c.jpg)
External radiation with GCLRP administration is associated with the generation of DC precursors in the blood
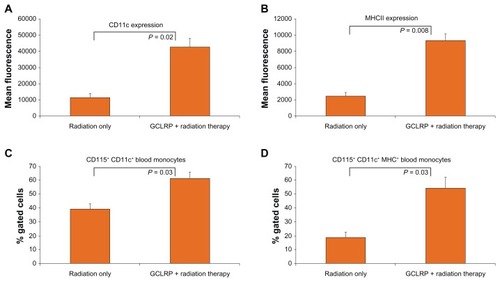
Optimal antigen presentation requires maturation of DCs; this event is critical to mount an antitumoral immune response. It was previously demonstrated that GCLRP treatment of animals with glioma was associated with the generation of DC precursors in the blood (Part I).Citation11 In order to determine the effects of GCLRP on blood monocytes in glioma-bearing mice that received external radiation, the phenotype of peripheral blood monocytes in the radiation-only and GCLRP + radiation groups was analyzed. GCLRP administration did not increase the number of circulating blood monocytes, but phenotypic analysis of these cells through flow cytometry revealed an increased expression of CD11c and MHCII on circulating mononuclear cells (). A detailed examination of these cells established an increase in the number of CD11c+ cells among blood monocytes (59.8% ± 5.1% versus 40.1% ± 4.3% in the radiation-only group; P = 0.03) which are considered to be precursors of DCs (). Moreover, the GCLRP + radiation group showed an increased number of double positive CD11c+MHCII+ cells, which are DCs with a certain degree of maturation (54.4% ± 7.6% versus 18.9% ± 3.6% in the radiation-only group; P = 0.03; ). Since glioma cells suppress the maturation of DCs, GCLRP-associated DC maturation is an important step in eliciting an effective antiglioma immune response.Citation12 In previous experiments, animals that received GCLRP without radiation also showed GCLRP-associated generation of precursor DCs in the blood. These data showed that GCLRP with or without radiation was strongly associated with the generation of DCs from peripheral blood monocyte precursors.
Figure 3 With regard to the analysis of blood monocytes, external radiation with GCLRP administration was associated with the generation of dendritic cell precursors in the blood. Fluorescence-activated cell sorting analysis of these cells revealed the increased expression of (A) cluster of differentiation-11c and (B) major histocompatibility complex class II on circulating mononuclear cells. (C) Galactose/N-acetylgalactosamine-specific C-type lectin receptor increased the amount of precursor dendritic cells (cluster of differentiation-115+ cluster of differentiation-11c+) from blood monocytes. (D) Galactose/N-acetylgalactosamine-specific C-type lectin receptor increased the amount of precursor dendritic cells with a certain degree of maturation (cluster of differentiation-115+ cluster of differentiation-11c+ major histocompatibility complex class+) from blood monocytes.
Abbreviations: CD, cluster of differentiation; GCLRP, peptide mimetic of the ligand of the galactose/N-acetylgalactosamine specific C-type lectin receptor; MHC, major histocompatibility complex class II.
Accumulation of DCs in GCLRP + radiation treated animals
The combination of cranial external radiation with administration of GCLRP was found to prolong the survival rate of the animals. A phenotypic examination of the cells of the tumoral hemisphere did not reveal any changes in number of MDCB (). It is important to note that when GCLRP was applied without radiation, the number of infiltrative cells within the tumor dramatically increased (see Part I).Citation11
Figure 4 Analysis of monocyte-derived brain cells within the tumoral hemisphere. Immunohistochemistry with anti-cluster of differentiation 68 confirmed fluorescence-activated cell sorting data: (A) the combination of cranial external radiation with administration of GCLRP was not associated with an increased number of monocyte-derived brain cells within glioma tissue as compared to (B) radiation alone. Hematoxylin and eosin staining did not reveal any changes in vascularization between (C) GCLRP + radiation versus (D) radiation-only animals.
Notes: These observations were in contrast to the authors’ previous experiment in which the administration of GCLRP alone was strongly associated with an increased number of monocyte-derived brain cells and angiogenesis in glioma tissue (Part I).
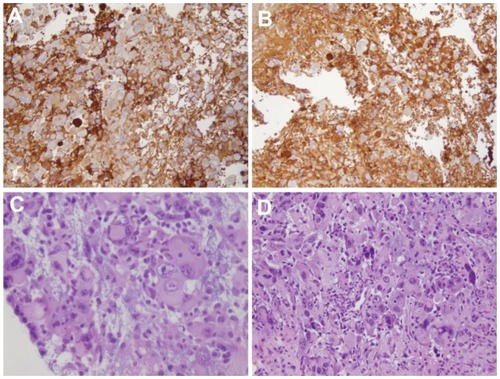
There were no significant differences among hematoxylin and eosin staining of the radiation and GCLRP + radiation samples (). No morphologically distinguished vascular structures were identified in animals that received GCLRP treatment in combination with external radiation. This observation was in contrast to the authors’ previous experiment in which administration of GCLRP alone was associated with angiogenesis within glioma tissue (see Part I).Citation11
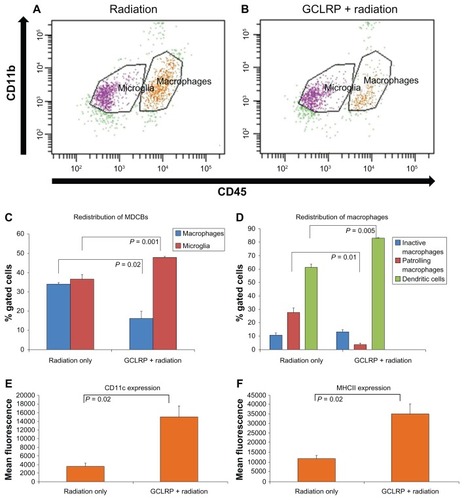
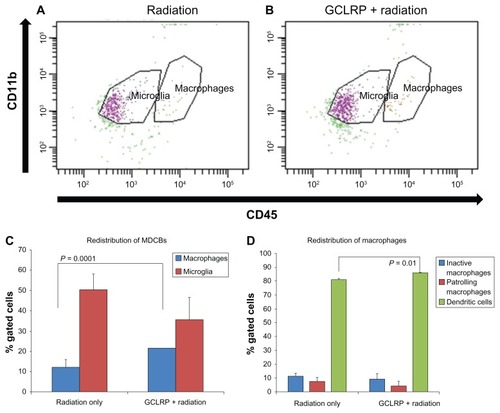
A comparative analysis of the tumoral hemispheres between groups revealed that GCLRP + radiation was associated with a redistribution of the major populations of MDCB, with an increased number of microglial cells (47.9% ± 0.6% versus 36.5% ± 3.1% in the radiation-only group; P = 0.001) and decreased number of macrophagal cells (16.3% ± 4.1% versus 34.1% ± 1.3% in the radiation-only group; P = 0.02; ). There were no significant changes among microglial subsets, but examination of the macrophagal population showed an increase in DCs (83.1% ± 1.1% versus 61.6% ± 2.4% in the radiation-only group; P = 0.005) and a decrease in PMs (3.6% ± 1.2% versus 27.7% ± 3.5% in the radiation-only group; P = 0.01; ). This might reflect the predominate differentiation of GCLRP-activated monocytes into DCs rather than into macrophages. Cytokine assays did not reveal any significant changes in the expression level of tumor necrosis factor-α, vascular endothelial growth factor, and interleukin-1β, which suggests that the GCLRP-mediated activation of MDCB was not associated with proinflammatory and proangiogenic cytokines. Moreover, these findings were associated with an increased survival rate of GCLRP + radiation animals. The macrophagal population in GCLRP + radiation animals exhibited increased expression of CD11c and MHCII as compared to the radiation-only group, which may indicate an increase in the antigen presenting capacity of these cells ().
Figure 5 Analysis of monocyte-derived brain cells within the tumoral hemisphere. Dot plots represent the population of cluster of differentiation-115+ cells in the (A) radiation-only and (B) GCLRP + radiation treated animals. (C) The combination of GCLRP with radiation was associated with the redistribution of microglial and macrophagal populations in the tumor hemisphere: the number of macrophages significantly decreased and microglial cells increased. (D) GCLRP + radiation, which increased survival rate, was also associated with significant accumulation of dendritic cells as compared to the radiation-only group. The accumulation of dendritic cells corresponded with a significant decrease in the percentage of patrolling macrophages in the GCLRP + radiation group. It was also demonstrated that the whole macrophagal population of the monocyte-derived brain cells of animals that received GCLRP + radiation exhibited increased expression of (E) cluster of differentiation-11c and (F) major histocompatibility complex class II compared to the radiation-only group.
Abbreviations: CD, cluster of differentiation; GCLRP, peptide mimetic of the ligand of the galactose/N-acetylgalactosamine-specific C-type lectin receptor; MDCB, monocyte-derived brain cells; MHC, major histocompatibility complex.
GCLRP + radiation increased the portion of DCs in the contralateral tumor-free hemisphere
In order to determine the effect of GCLRP in combination with radiation on MDCB within the contralateral tumor-free hemisphere, an analysis of the cell populations in this area was performed. This analysis revealed an increased number of macrophages (21.6% ± 0.4% versus 12.3% ± 3.1% in the radiation-only group; P = 0.0001) with increasing DCs in GCLRP + radiation animals (86.1% ± 0.7% versus 81.2% ± 1.4% in the radiation-only group; P = 0.01; ).
Figure 6 Analysis of monocyte-derived brain cells within the contralateral tumor-free hemisphere.
Notes: Dot plots represent the population of cluster of differentiation-115+ cells in the (A) radiation-only and (B) GCLRP + radiation treated animals. (C) In the tumor-free hemisphere, the number of macrophages significantly increased in the GCLRP + radiation group compared to the radiation-only group. (D) GCLRP + radiation treatment was associated with an increased amount of dendritic cells in the contralateral tumor-free hemisphere compared to the radiation-only group.
Abbreviations: CD, cluster of differentiation; GCLRP, peptide mimetic of the ligand of the galactose/N-acetylgalactosamine-specific C-type lectin receptor; MDCB, monocyte-derived brain cells.
Discussion
There has been a growing interest in the interactions between ionizing radiation and the immune system. In this study, the therapeutic combination of GCLRP treatment with cranial radiation was tested. The previous experiments in Part I established that GCLRP was associated with the generation DC precursors in the blood. It was found that GCLRP-activated monocytes may not be able to overcome the immunosuppressive effect of the malignant glioma once recruited into the tumor and peritumoral area, becoming PMs in these areas. It was also found that growth of the malignant glioma and survival rate were associated with the predominance of a specific population of MDCB (ie, PM or DC) and amount of the infiltrative monocyte-derived cells within the glioma.
The results of the present study show that GCLRP with cranial radiation increased the survival rate of animals with implanted glioma cells. Blood monocytes and discrete brain regions were analyzed with respect to the MDCB populations. This study showed that GCLRP administration was associated with an increased expression of CD11c and MHCII on circulating mononuclear cells, increased number of CD11c+ cells (precursors of DCs), and increased number of double positive CD11c+MHCII+ cells (DCs with a certain degree of maturation).
The increased survival rate in GCLRP + radiation animals was associated with a decreased macrophagal population within the tumoral hemisphere tissue and an accumulation of DCs. Also, PMs within the tumoral hemisphere decreased and a proportion of DCs within the macrophagal population of the contralateral tumor-free hemisphere increased. The tumoral hemisphere of GCLRP + radiation compared to radiation-only animals contained half the percentage of macrophages, while microglial cell percentage increased to 15% of radiation-only animals, with an increased percentage of DCs and decreased PMs compared to the radiation-only group. The contralateral tumor-free hemisphere of GCLRP + radiation animals contained a two-fold percentage increase of DCs compared to the radiation-only group.
Survival advantage was established when peptide treatment began immediately after applying cranial radiation. GCLRP may generate DCs in the blood to migrate to the glioma, while radiation creates an environment with decreased tumoral immunosuppression more conducive to the operation of activated MDCB.Citation13–Citation18 Several studies have shown that irradiation of human and murine tumor cell lines upregulates the expression of numerous immunologically relevant molecules – including Fas, intercellular adhesion molecule-1, and the human carcinoma-associated antigens, carcinoembryonic antigen and mucin-1, cell surface associated – which may make them better targets for immune recognition.Citation6,Citation19–Citation22 Importantly, external radiation also upregulates MHC class I expression on GL261 glioma cells that can be recognized by APCs.Citation16,Citation18 Radiation-damaged glioma cells lead to the release of a considerable amount of tumor antigens and cell debris that may enhance the uptake and presentation of these antigens by tumor-associated APCs, including GCLRP-activated DCs.Citation23,Citation24 Activation of the immune system may also potentially recognize the unique radiation-induced antigenic peptides or enhanced tumor-related self-antigens resulting in better tumor responses, and then stimulate apoptosis or directly attack the altered tumor cells.Citation15
DCs are the most powerful APCs in the body.Citation25 In Part I, it was found that accumulation of DCs within glioma and peritumoral area was associated with longer survival as compared with GCLRP-treated animals where PMs predominated. In this study, it was also established that the predominance of DCs in the GCLRP + radiation group was also associated with favorable prognosis. The direct interaction of APCs with tumor cells could result in an effective tumor-specific immunogen, either by conferring sufficient APC function to tumor cells for T-cell activation or by facilitating the delivery of tumor antigens to DCs for processing and presentation.Citation25 However, it is well established that glioma cells suppress the maturation of DCs.Citation12,Citation26 In addition, glioma-mediated defects in APC populations in glioblastoma multiforme patients may contribute to severe immunosuppression conditions including lymphopenia and T-cell hyporesponsiveness.Citation12 The present results support other studies where effective stimulation of APCs can prolong survival of experimental animals when glioma antigens are available for phagocytosis. Wallenfriedman et al showed the efficacy of granulocyte–macrophage colony-stimulating factor that stimulates DCs in combination with inoculations of inactivated tumor antigens against malignant gliomas.Citation27 Such a technique can initiate a long-lasting systemic response that protects the animal from a distant neoplasm, halts tumor growth, stimulates regression, and provides protection against further tumor recurrences. Jean et al found that stimulation of DCs with granulocyte–macrophage colony-stimulating factor in combination with subcutaneous administration of irradiated 9L cells increased survival rate in rats with intracranial 9L tumors when compared to untreated animals.Citation28
Newcomb et al demonstrated that the peripheral vaccination of mice with modified autologous tumor cells secreting granulocyte–macrophage colony-stimulating factor combined with ionizing radiation to the whole brain could cure well-established brain tumors in roughly half of their experimental animals.Citation6 In a subsequent study, ionizing radiation in combination with anti-CD137 immunotherapy was used to elicit an effective immune response against glioma within a murine model.Citation29
The strategy of using a peptide mimetic that employs the GCLRs to activate immune cells in combination with radiation- induced local accumulation of DCs, which are believed to induce tumor-specific cellular immunity, suppressed the growth of preexisting tumors. Such a therapeutic strategy may be considered as an internal dendritic vaccination. This strategy may have potential to selectively target residual tumor cells that have invaded the normal brain tissue.
Although this study presents data from a syngeneic murine malignant glioma model, a similar environment likely exists within the human glioma milieu. Proposed therapeutic modalities will need to consider these crucial glioma-associated and influenced immune cell populations. A treatment strategy using GCLRPs in combination with radiotherapy seems to have potential and may present viable options for new perspectives to existing cancer treatments.Citation30
Conclusion
Although this study was conducted in a well-known syngeneic murine glioma model, the results suggest that targeting the GCLRs may have potential relevance to clinical scenarios. This receptor system has important relationships with MDCBs, and the present study found important associations between the presence of certain populations of MDCB and malignant glioma. The main effect seems to be related to the accumulation of DCs, which – unlike the accumulation of PMs – is strongly associated with better prognosis in the mice.
In the central nervous system, the turnover of MDCB is thought to be due to the entrance of peripheral monocytes and not active proliferation of resident microglial cells.Citation31 The present experiment showed that GCLRP is associated with peripheral monocyte activation and the generation of precursor DCs. This concept was established in experiments where GCLRP was the only method of the treatment and where GCLRP was combined with radiation. The fate of the GCLRP-activated monocyte precursors of DCs is related to the trafficking area (ie, the brain-tumor tissue region studied). Migration of these cells into the contralateral tumor-free hemisphere replenishes the population of the DCs. This occurred with GCLRP use alone or in combination with radiation. When GCLRP was used alone, and likely related to the overwhelming immunosuppressive effect of the glioma, GCLRP-activated monocyte precursors of DCs recruited to the glioma and peritumoral areas were likely transformed into PMs that infiltrated the tumor and consequently increased its size.
External radiation decreased the immunosuppressive effect of the glioma and provided the migratory capability for GCLRP-activated monocyte precursors of DCs to move into the tumoral hemisphere, preserving their differentiation into mature DCs within the tumoral hemisphere, which is associated with decreased tumor size and improved survival. The antitumoral activity observed in this murine glioma model provides rationale for investigating GCLRP modalities that specifically activate MDCB, but also provides important insight into the reorganization and crucial functional relationships and interactions of MDCB in gliomas.
Acknowledgments
This study was supported by funds to Dr Preul, Dr Hoober, and Dr Eggink in a biinstitutional grant from the Arizona Biomedical Research Commission. Additional support was furnished by the Barrow Neurological Foundation and the Newsome Endowed Chair in Neurosurgery Research.
Disclosure
LLE and JKH declare that they are inventors of the technology contained in this report. Intellectual property has been licensed to Susavion Biosciences, Inc, in which these authors hold shares. Other authors have no conflicts of interest to report.
References
- ButowskiNImmunostimulants for malignant gliomasNeurosurg Clin N Am2010211536519944966
- HigashiNFujiokaKDenda-NagaiKThe macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cellsJ Biol Chem200227723206862069311919201
- AbdelwahabMGKuschayevSVKuschayevaYSSynthetic peptide mimetic of macrophage-activating factor in a murine glioma model [abstract]Neuro Oncol2009115613
- EgginkLLHooberJKA biologically active peptide mimetic of N-acetylgalactosamine/galactoseBMC Res Notes200922319284521
- NewcombEWLymberisSCLukyanovYRadiation sensitivity of GL261 murine glioma model and enhanced radiation response by flavopiridolCell Cycle200651939916319534
- NewcombEWTamasdanCEntzmingerYFlavopiridol inhibits the growth of GL261 gliomas in vivo: implications for malignant glioma therapyCell Cycle20043223023414712094
- YamanakaRCell- and peptide-based immunotherapeutic approaches for gliomaTrends Mol Med200814522823518403264
- BadieBSchartnerJMFlow cytometric characterization of tumor-associated macrophages in experimental gliomasNeurosurgery200046495796110764271
- ParneyIFWaldronJSParsaATFlow cytometry and in vitro analysis of human glioma-associated macrophages. Laboratory investigationJ Neurosurg2009110357258219199469
- RandolphGJJakubzickCQuCAntigen presentation by monocytes and monocyte-derived cellsCurr Opin Immunol2008201526018160272
- KuschayevSVEgginkLLKuschayevaYSMonocyte galactose/N-acetylgalactosamine-specific C-type lectin receptor stimulant immunotherapy of an experimental glioma. Part I: stimulatory effects on blood monocytes and monocyte-derived cells of the brainCancer Manag Res Forthcoming2012
- OgdenATHorganDWaziriADefective receptor expression and dendritic cell differentiation of monocytes in glioblastomasNeurosurgery200659490290917038955
- HallahanDEStaba-HoganMJVirudachalamSKolchinskyAX-ray-induced P-selectin localization to the lumen of tumor blood vesselsCancer Res19985822521652209823335
- HallahanDEVirudachalamSKuchibhotlaJNuclear factor κB dominant negative genetic constructs inhibit X-ray induction of cell adhesion molecules in the vascular endotheliumCancer Res19985823548454889850083
- McBrideWHCombining radiation therapy with immunotherapy for treatment of brain tumorsLiauLMBeckerDPCloughesyTFBignerDDBrain Tumor ImmunotherapyTotowa, NJHumana Press2001345362
- NewcombEWLukyanovYKawashimaNRadiotherapy enhances antitumor effect of anti-CD137 therapy in a mouse glioma modelRadiat Res2010173442643220334514
- QinDXZhengRTangJLiJXHuYHInfluence of radiation on the blood–brain barrier and optimum time of chemotherapyInt J Radiat Oncol Biol Phys1990196150715102262373
- SavillJFadokVHensonPHaslettCPhagocyte recognition of cells undergoing apoptosisImmunol Today19931431311368385467
- De VleeschouwerSFieuwsSRutkowskiSPostoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiformeClin Cancer Res200814103098310418483377
- MaesWVan GoolSWExperimental immunotherapy for malignant glioma: lessons from two decades of research in the GL261 modelCancer Immunol Immunother201160215316021120655
- WattersJJSchartnerJMBadieBMicroglia function in brain tumorsJ Neurosci Res200581344745515959903
- WheelerCJBlackKLLiuGVaccination elicits correlated immune and clinical responses in glioblastoma multiforme patientsCancer Res200868145955596418632651
- CavalloFDi PierroFGiovarelliMProtective and curative potential of vaccination with interleukin-2-gene-transfected cells from a spontaneous mouse mammary adenocarcinomaCancer Res19935321506750708221636
- RaychaudhuriSMorrowWJCan soluble antigens induce CD8+ cytotoxic T-cell responses? A paradox revisitedImmunol Today19931473443488363723
- CelluzziCMFaloLDJrPhysical interaction between dendritic cells and tumor cells results in an immunogen that induces protective and therapeutic tumor rejectionJ Immunol19981607308130859531260
- KikuchiTAbeTOhnoTEffects of glioma cells on maturation of dendritic cellsJ Neurooncol200258212513012164683
- WallenfriedmanMAConradJADelaBarreLEffects of continuous localized infusion of granulocyte-macrophage colony-stimulating factor and inoculations of irradiated glioma cells on tumor regressionJ Neurosurg19999061064107110350253
- JeanWCSpellmanSRWallenfriedmanMAEffects of combined granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2, and interleukin-12 based immunotherapy against intracranial glioma in the ratJ Neurooncol2004661–2394915015768
- NewcombEWDemariaSLukyanovYThe combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomasClin Cancer Res200612154730473716899624
- ReitsEAHodgeJWHerbertsCARadiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapyJ Exp Med200620351259127116636135
- PonomarevEDShriverLPMareszKDittelBNMicroglial cell activation and proliferation precedes the onset of CNS autoimmunityJ Neurosci Res200581337438915959904