Abstract
Background
This year marks the twentieth anniversary of the approval by the US Food and Drug Administration of interleukin-2 (IL2) for use in cancer therapy, initially for renal cell carcinoma and later for melanoma. IL2 therapy for cancer has stood the test of time, with continued widespread use in Europe, parts of Asia, and the US. Clinical complete responses are variably reported at 5%–20% for advanced malignant melanoma and renal cell carcinoma, with strong durable responses and sustained long-term 5–10-year survival being typical if complete responses are generated.
Methods
The literature was reviewed for the actions and clinical effects of IL2 on subsets of T cells. The influence of IL2 on clinical efficacy was also sought.
Results
The review revealed that IL2 is capable of stimulating different populations of T cells in humans to induce either T effector or T regulatory responses. This apparent “functional paradox” has confounded a clear understanding of the mechanisms behind the clinical effects that are observed during and following administration of IL2 therapy. An average complete response rate of around 7% in small and large clinical trials using IL2 for advanced renal cell carcinoma and malignant melanoma has been shown from a recent review of the literature.
Conclusion
This review considers the published literature concerning the actions and emerging clinical effects of IL2 therapy, spanning its 20-year period in clinical use. It further details some of the recently described “bimodal” effects of IL2 to explain the apparent functional paradox, and how IL2 might be harnessed to emerge rapidly as a much more effective and predictable clinical agent in the near future.
Introduction
Interleukin-2 (IL2) was originally described as an integral T cell growth factor necessary for activation and expansion of T cell populations in vitro and in vivo. It was cloned and synthesized in the early 1980s for laboratory useCitation1 and then subsequently trialed and utilized for human therapy, notably for advanced malignant melanoma and renal cell carcinoma, but has been used for a variety of other cancer types as well, including colorectal, breast, and lung carcinomas, and for mesothelioma. Isolated complete responses have been noted in these cancer types as well, but the larger and more numerous trials have occurred in malignant melanoma and renal cell carcinoma.Citation2 It is the experience in these cancers which we will focus on.
IL2 is a current treatment option for patients with late-stage renal cell carcinoma and those with malignant melanoma. Notably, complete responses, where no evidence of detectable cancer remains in the patient, are reported relatively consistently using IL2 therapies. Complete responses are often durable and associated with long-term survival. IL2 has a remarkable sustained history, being initially approved for clinical use in 1992.Citation2 Back in those days, IL2 was thought to stimulate or initiate the immune response via its stimulation of T effector cells, often being called the “master cytokine”.
Recently, IL2 therapy has been advanced as a potential immune modulator able to “tweak” the immune response selectively to aid transplant tolerance, whilst others reported this in graft versus host disease and chronic hepatitis C infection.Citation3–Citation5 The notion of IL2 possessing a “dual capacity” to promote both activation and tolerance has remained enigmatic and perplexing for some time. Some important issues concerning the earlier reported dual role of IL2 appear to have been somewhat overlooked in much of the understanding, research, and argument surrounding the actions and current clinical use of IL2.
The literature has repeatedly described the effects of IL2 on T effector cells as causing rapid T cell expansion, with subsequent activation or augmentation of an immune response in vitro or in vivo. However, recent attention has also been paid to the capacity of IL2 to stimulate T regulatory cells in a manner analogous to that of T effector cells, pointing to an apparent paradoxical role of IL2 as a cytokine that has the capacity to drive the immune response in both an activated and an inhibitory direction.Citation6–Citation11 The aim of this research was to explore how this apparent paradox can be explained.
Materials and methods
The literature was reviewed for the actions and clinical effects of IL2 on T cell subsets. The influence of IL2 on clinical efficacy was also sought. Search methods included the PubMed, Medline, Google Scholar, and associated data bases, using the terms “interleukin-2”, “IL2”, “complete response”, “clinical trial”, “clinical responses”, “survival”, “T cells”, “T effector cells”, “T regulatory cells”, “cancer”, “melanoma”, “renal cell cancer”, and “renal cell carcinoma”.
Results and discussion
The literature to date includes numerous studies demonstrating dual roles for IL2 in activating and inhibiting immune responses,Citation6–Citation11 and in producing a range of clinical responses, including a small but important proportion of complete clinical responses.Citation2,Citation6–Citation8
Paradoxical bimodal role of IL2 in immune homeostasis
The literature indicates that IL2 is now recognized as being responsible not only for initiating the immune response, but also for the homeostatic termination of that same immune response by stimulating T regulatory cells. Therefore, it is a “bimodal” cytokine with dual actions and opposing results in the time domain.Citation3–Citation5,Citation9–Citation15 Therefore, we reason that it is this functional “bimodality” which has created a most confounding paradox for medical science and no doubt slowed accurate and successful clinical application of IL2.
“How wonderful that we have met with a paradox. Now we have some hope of making progress” – Niels Bohr (1885–1962) Danish physicist.
The immune response once triggered, is known to be a sequential, time-dependent, homeostatic, physiological process. It is truly “dynamic”, not static, as widely viewed previously. This process requires the coordinated and timely interaction of cytokines, their receptors, and the responding cell populations. These cytokine/receptor interactions have half-lives of minutes to hours. The resulting coordinated, sequential, clonal cellular expansions can take several days to rise and fall. This gives rise to the initiation and then termination of that response. We know this from our experience with vaccinology, and induction of the acute immune response with a rise then fall over several days.Citation11
Both T effector cells and T regulatory cells transiently express the IL2 receptor for only about 8–12 hours, and both require IL2 for their activation/expansion and maintenance ( and ). Recently, McNally et al described the existence of an IL2 “feedback loop” between T effector cells and T regulatory cells, which can homeostatically promote or limit the intensity, respectively, of the immune response.Citation12 Further, both of these opposing arms of the immune response are exquisitely sensitive to IL2, including both endogenous and exogenous (therapeutic) IL2 sources. In addition, Knoechel et al previously described the sequential development of T effector cells and then T regulatory cells under the influence of IL2 in an autoimmune mouse model.Citation10 These observations were in the acute state, but little has been done to monitor closely and sequentially map the immune response in the chronic state using serial daily measurements.
Figure 1 Progressive interleukin-2 (IL2R) receptor expression over time on T effector cells then regulatory T cells, and respective time-dependent activation of effector cells and regulatory cells.
Notes: This sequential rise and fall of receptor density and expansion of alternating opposing T cell populations creates the homeostatic feedback loop of initiation then termination of the immune response. These cytokine and cellular kinetics are well described in the literature. The relative “refractory” period is predicted from the literature and biological principles observed in other systems, where restimulation is impeded.
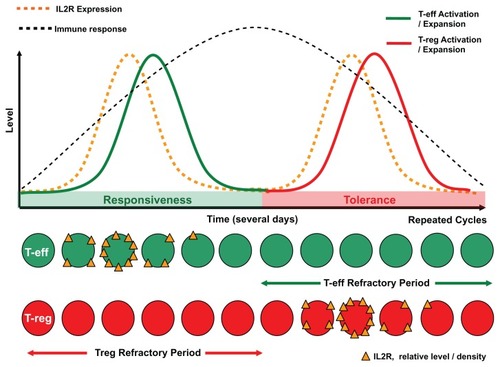
Figure 2 Alternating interleukin-2 “feedback” induced homeostatic oscillation of T effector cells followed by T regulatory cells.
Notes: This “functional unit” of the (acute) immune response consists of responsiveness, then tolerance, to antigen stimulation. Chronic antigen persistence and stimulation produces the observed alternating inflammatory/immune oscillation or cycle (upper figure). The repeating cycle is hypothesized to create recurring narrow therapeutic windows (of approximately 12 hours wide) where the immune response can be driven in the direction of responsiveness or tolerance. The typical sigmoidal alternating T effector cell and T regulatory cell rapid sigmoidal expansion curves showing the predicted respective positions of maximal susceptibility for therapeutic intervention using agents such as interleukin-2 (lower figure). On the basis of mathematical probability this potentially explains why timing of administration of IL2 therapy may ultimately govern and restrict complete response efficacy to approximately 7% of patients.
IL2 therapy in cancer
Since 1992, repeated trials using IL2 have delivered complete response rates which have remained remarkably consistent at approximately 7% in two very different (embryologically derived) advanced cancer types, namely renal cell carcinoma (mesodermal) and malignant melanoma (neural crest).Citation2,Citation6–Citation8,Citation16 Although complete responses occur randomly and unpredictably in a trial population, the approximate average 7% complete response barrier has been fixed across many years in both large and small clinical trials. This has been difficult to comprehend clinically until now.
Of major clinical importance is that many of these complete responses have been durable, out beyond 10 years. With our current and emerging understanding of the suppressed tumor-specific immune response in the cancer patient, IL2 when efficacious, clearly appears to be successfully manipulating a pre-existing or endogenous antitumor immune response in these few lucky patients. Indeed, with IL2 therapy alone, no tumor antigen is being supplied. How can we make complete responses a reality for most or all patients?
In the case of IL2 therapy for renal cell carcinoma and malignant melanoma, the schedule of high-dose infusions over 15 minutes intermittently every 8 hours for up to 4–5 days (with a break of a number of days before repeating)Citation2,Citation6–Citation8,Citation16 has probably evolved clinically to overcome the natural physiological IL2/IL2R half-life restrictions, and the narrow time-dependent influence of IL2 on the effector arm of the tumor immune response, while also attempting to reduce toxicity. This evolution occurred perhaps without due appreciation in the past of the biological bimodal activatory/ proinflammatory, then immune regulatory/inhibitory function of IL2, in the time domain. Consequently, it is not surprising that IL2 is only successful therapeutically in a minority of patients, ie, some 7% or so. Moreover, some investigators have observed effective clinical responses using intermittent short bolus IL2 dosing, rather than longer IL2 infusions.Citation2,Citation17
Historically, cytokine and cellular subpopulation measures of immune reactivity have been used in an ad hoc fashion and intermittently to observe points in time during therapy. This has perhaps led to some misleading inferences about how the immune system is behaving at the time of therapy and afterwards in the cancer patient. Interpretation of the interaction between the immune dynamics during IL2 therapy under the influence of chronic tumor antigen exposure has been greatly lacking. This is because regular, frequent, serial daily measurements of inflammation or immune reactivity have largely not been done in the past, either before, during, or after therapy. This has meant that detailed information concerning the timing of therapy and the effects of therapeutic interventions has not been able to be elucidated or appreciated fully in terms of clinical outcome.
Therapeutic observations from the preclinical IL2 mouse model
Since the mid 1980s, when the first published preclinical accounts of the immune modulatory effects of IL2 were reported, a number of researchers have noticed a timing effect with respect to IL2 administration and efficacy of therapy.Citation18–Citation21 This led Shrikant and Mescher in 2002 to comment, “The timing and extent of exposure to IL-2 can clearly have dramatic effects on whether or not it is efficacious in activating, or reactivating, tumor-specific CD8+ T cell responses, making it difficult to know how to use it clinically in an optimal manner”.Citation20 In addition, Knoechel et al commented, “the timing of administration may be the key to its successful clinical application”.Citation10 However, no method to achieve this for maximum therapeutic benefit in the human situation appears to have been offered to date, despite the afore mentioned observations indicating timing was important.
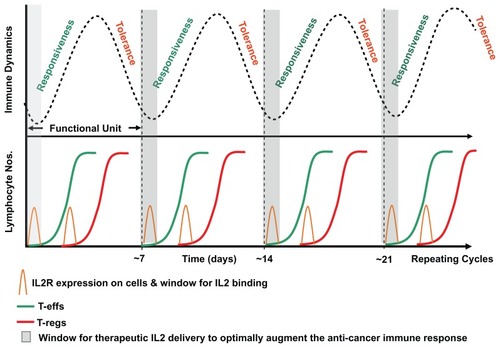
Furthermore, more recently, Feinerman et al have demonstrated with their T effector/T regulatory cell modeling system at the single cell level that the timing and molecular surface density of IL2/IL2 receptor interaction are significant contributors to discrimination between self and nonself.Citation22 How these factors play out at the whole tissue/systemic level is less well known, and again serial daily measurements are likely to hold many of the crucial answers. These preclinical data strongly suggest that the cell types predominantly expressing the IL2 receptor at the time of treatment with IL2, ie, T regulatory cells and T effector cells, will determine the direction in which the immune response is driven ( and ).
We posit that artificiality of the mouse IL2 experiments increased the probability of a successful therapeutic outcome. We suspect a convenient timeframe (in days) between primary tumor inoculation and initiation of therapy favored effector responses in successful outcomes (immune-mediated tumor regression), and regulatory/immune suppression (tolerogenic) at later time points in animals that experienced disease progression.
These conflicting and confounding observations can now be explained in light of the contemporary appreciation of the discrete, time-dependent, and opposing dual or “bimodal” role that IL2 plays in immune homeostasis and apparent chronic antigen stimulation.Citation13 The implications of knowledge gained over 20 years has meant that the earlier preclinical and clinical experience of limited spectacular, but random, successes (complete responses) seen in late-stage cancer patients can now be explained rationally. This could potentially open up the way for redesigning the IL2 protocols to maximize efficacy in most patients.
Other mechanisms could explain the historic nature and spectrum of random responses seen in late-stage cancer patients treated with IL2. Genetic variations in tumors and in individual patient immune constitution/capability have been investigated in attempts to either predict responses or select patients for therapy. Although several factors, such as low serum levels of fibronectin and vascular endothelial growth factor, have been associated with improved responses to IL2, it has remained difficult to predict responders.Citation23,Citation24
Perhaps the simplest explanation for random efficacy, namely the timing of therapy, is the easiest to explore with the greatest immediate translational potential. This has been largely overlooked in human clinical application, despite it clearly playing a role in the earlier mouse experiments. The recent advances in our understanding of immune homeostasis and the bimodal attributes of IL2 suggests researchers should be looking more closely and accurately at the “time domain”. Single time-point or limited sampling analysis is clearly inadequate for accurately mapping a dynamic physiological process (the immune response) that is known to operate homeostatically and sequentially in minutes, hours, days, and weeks. As an example, imagine how our understanding would have been retarded in mapping the hormonal kinetics or transcriptional profile of the menstrual cycle with only one or two blood samples taken from 100 fertile women.
IL2 and IL2 receptors and cancer cells
Other factors that have confounded our understanding of the roles of IL2 in cancer have been the knowledge that in some situations either IL2 and/or IL2 receptors can be released or expressed by cancer cells themselves.Citation25–Citation29 How this affects the growth of cancers in vivo and how it interplays with exogenous IL2 therapies is currently unclear. However, the broad aim of exogenous IL2 therapy is to coordinate effector T cell immune responses to enhance the pre-existing antitumor immune response direction towards that of killing cancer cells. Therefore, evaluation of IL2 and IL2R expression on tumor cells from biopsies prior to therapy might potentially be of clinical relevance.
Wider role of IL2 in chronic disease
Our work suggests that under a chronic persistent tumor antigen load, as exists for a late-stage surgically nonresectable cancer patient, the IL2 feedback loop described by McNally et alCitation12 continuously oscillates between a state of activation and suppression.Citation30–Citation34 In essence, what happens in the acute inflammatory state simply repeats itself continuously unless antigen is removed from the system. Therefore, it is not unreasonable to speculate that due to the time-dependent, bimodal functions of IL2 and the IL2 receptor, the time and dose could influence the clinical outcome in different disease states, ie, cancer, autoimmunity, chronic infections, and transplantation, as also described previously in the mouse experiments.
Improving clinical efficacy of IL2 therapy
We suggest that because IL2 is capable of driving the immune system in either of two opposing directions, namely either responsiveness/activation or tolerance/inhibition, the timing of IL2 administration with respect to the status of immune system dynamics at the time of administration will critically direct the immune response and determine outcome. The influence of any underlying natural bimodal homeostatic dynamic will therefore likely be the critical principal determinant of clinical efficacy. We suspect that the way forward, at least for cancer therapy, will be to analyze accurately the patient’s underlying tumor immune response in a serial manner, then appropriately and accurately synchronize therapy with immune fluctuation.Citation15,Citation30–Citation34 This time-dependent dynamic aspect of the immune response in the cancer patient has been largely overlooked in the past, and has prevented us from being able to observe how our in vivo therapeutic approaches are influencing the immune response to produce the observed clinical effects. Indeed, this logic might also be expected to apply to administration of other immunotherapeutic agents, such as CTLA4 antibodies or vaccines, to improve their clinical efficacy.Citation16,Citation32,Citation36 In addition, the notion of timing and immune responsiveness may apply more widely to many other therapies.Citation35
Specifically, the pattern of the intrinsic fluctuation in the patient’s individual immune response can be determined by measuring serum inflammatory markers, eg, C-reactive protein daily or near daily over a 2–3-week time period. Once these fluctuations are known, IL2 therapy can then be carefully delivered in a synchronized pulse, and at the predicted time when T effector cells are maximally expressing the IL2 receptor and T regulatory cells are not ( and ). The aim of this discrete approach is to avoid the T regulatory phase in order to engineer an extended, predominant T effector response in the cancer patient to achieve maximum clinical benefit. This would effectively counter the repetitive homeostatic attenuation of the immune response that arises from chronic persistent antigen load and stimulation. For autoimmune disease control and transplantation/graft acceptance, the opposite approach with careful enhancement of T regulatory function might well apply.
Conclusion
Since the cloning of IL2 about 30 years ago, and its clinical introduction and regulatory approval as an immune stimulatory agent some 20 years ago, the complete response rate in two different advanced cancers in both small and large clinical trials has remained remarkably constant at about 7%.Citation2,Citation16 Over that time, our understanding of the apparent paradoxical bimodal/opposing proinflammatory and immune regulatory influence of IL2 on the immune response has emerged. This bimodal role of IL2, together with considerable clinical experience with IL2 therapy, potentially explains why some patients respond and others do not.
We propose that a way forward to resolve this longstanding randomly “locked” low clinical efficacy is by serially monitoring each patient, then synchronizing the administration of IL2 in time, to match the homeostatic fluctuations in the late-stage cancer patient’s immune response.Citation15,Citation35 In doing this, we also propose that the bimodal activity of IL2 can be harnessed and modulated towards bulk activation of antitumor effector cells to produce a substantial increase in the complete response rate. Thus, generation of far more predictable, reliable clinical responses and improved long-term survival for patients can be engineered.
Acknowledgments
We wish to acknowledge the enormously valuable contribution of numerous scientists and clinicians who conducted and published previous IL2 research, and their patients, without whom our collective understanding would not have been possible.
Disclosure
The authors report no conflicts of interest in this work.
References
- SmithKAThe structure of IL2 bound to the three chains of the IL2 receptor and how signaling occursMed Immunol200653816907989
- GrivasPDRedmanBGImmunotherapy of kidney cancerCurr Clin Pharmacol2011615116321827390
- MurphyWJCommunity corner – a delicate balance: tweaking IL-2 immunotherapyNat Med20121820820922310686
- KorethJMatsuokaKKimHTInterleukin-2 and regulatory T cells in graft-versus-host diseaseN Engl J Med20113652055206622129252
- SaadounDRosenzwajgMJolyFRegulatory T cell responses to low-dose interleukin-2 in HCV-induced vasculitisN Engl J Med20113652067207722129253
- AtkinsMBLotzeMTDutcherJPHigh-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993J Clin Oncol1999172105211610561265
- FisherRIRosenbergSAFyfeGLong-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinomaCancer J Sci Am20006 Suppl 1S55S5710685660
- KlapperJADowneySGSmithFOHigh-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006Cancer200811329330118457330
- SojkaDKHughsonASukiennickiTLFowellDJEarly kinetic window of target T cell susceptibility to CD25+ regulatory T cell activityJ Immunol20051757274728016301632
- KnoechelBLohrJKahnEBluestoneJAAbbasAKSequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigenJ Exp Med20052021375138616287710
- AbbasAKLichtmanAHCellular and Molecular Immunology5th edPhiladelphia, PAElsevier-Saunders2005
- McNallyAHillGRSparwasserTThomasRSteptoeRJCD4+ CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasisProc Natl Acad Sci U S A20111087529753421502514
- BoymanOSprentJThe role of interleukin-2 during homeostasis and activation of the immune systemNat Rev Immunol20121218019022343569
- AhmadzadehMRosenbergSAIL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patientsBlood20061072409241416304057
- CoventryBJAshdownMLMarkovicSNImmune therapies for cancer: bimodality – the blind spot to clinical efficacy – lost in translationJ Immunother201134717
- PrietoPAYangJCSherryRMCTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanomaClin Cancer Res2012182039204722271879
- QuanWDJrQuanFMPerezMJohnsonEOutpatient intravenous interleukin-2 with famotidine has activity in metastatic melanomaCancer Biother Radiopharm6252012 [Epub ahead of print.]
- RosenbergSAMuléJJSpiessPJReichertCMSchwarzSLRegression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2J Exp Med1985161116911883886826
- MaekawaRMatsumotoMKitagawaTHaradaMSatoKEffect of recombinant interleukin 2 (R-IL2) on in vivo growth of murine myeloma X5563Cancer Immunol Immunother19862325303490306
- ShrikantPMescherMFOpposing effects of IL-2 in tumor immunotherapy: promoting CD8 T cell growth and inducing apoptosisJ Immunol20021691753175912165496
- JackamanCBundellCSKinnearBFIL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2J Immunol20031715051506314607902
- FeinermanOJentschGTkachKESingle-cell quantification of IL-2 response by effector and regulatory T cells reveals critical plasticity in immune responseMol Syst Biol2010643721119631
- SabatinoMKim-SchulzeSPanelliMCSerum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapyJ Clin Oncol2009272645265219364969
- PanelliMCNagorsenDWangEMechanism of immune response during immunotherapyYonsei Med J200445 Suppl151715250044
- Rangel-CoronaRCorona-OrtegaTSoto-CruzIEvidence that cervical cancer cells secrete IL-2, which becomes an autocrine growth factorCytokine20105027327720227890
- PlaisanceSRubinsteinEAlilecheAHuman melanoma cells express a functional interleukin-2 receptorInt J Cancer1993551641708344747
- AlilecheAPlaisanceSHanDSHuman melanoma cell line M14 secretes a functional interleukin 2Oncogene19938179117968099724
- CoventryBJWeeksSCHeckfordSESykesPJBradleyJSkinnerJMLack of interleukin-2 (IL-2) cytokine expression despite IL-2 mRNA transcription in tumor infiltrating lymphocytes in primary human breast carcinoma: selective expression of early activation markersJ Immunol1996156348634928617977
- BarbourAHCoventryBJDendritic cell density and activation status of tumour-infiltrating lymphocytes in metastatic human melanoma: possible implications for sentinel node metastasesMelanoma Res20031326326912777981
- CoventryBJAshdownMLQuinnMAMarkovicSNYatomi-ClarkeSLRobinsonAPCRP identifies homeostatic immune oscillations in cancer patients: a potential treatment targeting tool?J Transl Med2009710219948067
- AshdownMLCoventryBJA matter of timeAustralasian Science20101820
- CoventryBJHerseyPHalliganA-MMicheleAImmuno-chemotherapy using repeated vaccine treatment can produce successful clinical responses in advanced metastatic melanomaJ Cancer Ther20101205213
- HoltanSGDroncaRSNevalaWKThe dynamic human immune response to cancer: it might just be rocket scienceImmunotherapy201131021102421913823
- LeontovichAADroncaRSSumanVJFluctuation of systemic immunity in melanoma and implications for timing of therapyFront Biosci (Elite Ed)2012495897522201928
- CoventryBJAshdownMLComplete clinical responses to cancer therapy caused by multiple divergent approaches: a repeating theme lost in translationCancer Manag Res2012413714922740774
- JainNNguyenHChambersCKangJDual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunityProc Natl Acad Sci U S A20111071524152820080649