Abstract
Purpose
We aimed to investigate the clinicopathological characteristics and survival risk factors in small cell lung cancer (SCLC) patients with preexisting type 2 diabetes mellitus (preDM).
Patients and Methods
All patients with SCLC admitted to our hospital between January 2013 and August 2018 were followed up until August 2020 and retrospectively analyzed. Clinical characteristics of SCLC patients with and without preDM were extracted. Cox proportional hazards models were conducted to identify potential independent prognostic factors.
Results
Of 628 eligible individuals, 88 individuals had preDM. preDM was independently significantly associated with distant metastasis in all SCLC patients (p =0.016, OR=1.80, 95% CI 1.11–2.91), while preDM did not affect the outcome of SCLC patients (p=0.803, HR=1.04, 95% CI 0.79–1.36) by multivariate analysis. In the preDM group, the median overall survival (OS) was shorter in the insulin group than in the non insulin group (13.93 months versus 21.77 months, p=0.024). Multivariate analysis identified that insulin treatment was an independent unfavorable factor associated with OS (p =0.009, HR=2.10, 95% CI 1.19–3.64). In addition, poorer performance status (PS) and liver metastasis were also independent unfavorable prognostic factors (all p<0.01), while thoracic therapy significantly improved OS and decreased mortality risk in diabetic patients with SCLC (p<0.05).
Conclusion
preDM may promote distant metastasis of SCLC while it is insulin therapy and not preDM which adversely affects the prognosis of SCLC patients. These findings indicate that enhancing blood glucose control and reducing insulin analog use may be essential to the improvement of the long-term survival of the diabetic population with SCLC.
Introduction
Both cancer and diabetes mellitus are multimechanism and systemic diseases that affect human health worldwide. Type 2 diabetes mellitus (T2DM), which is one of the most common endocrine diseases, has risen surprisingly in the past decade and has been an expanding global health problem. T2DM is characterized by hyperglycemia, insulin resistance and relative impairment in insulin secretion.Citation1 Lung cancer is the leading cause of cancer-related deaths worldwide.Citation2 Small-cell lung cancer (SCLC), which accounts for approximately 15% of all lung cancers, is a high-grade neuroendocrine neoplasm.Citation3 SCLC has an exceptionally high proliferative rate, with completely different pathological, clinical, and molecular features from non-small-cell lung cancer (NSCLC). Although it is sensitive to chemotherapy, SCLC still has a poor prognosis due to early metastasis and frequent relapse.Citation4 In recent years, immune checkpoint inhibitors have been an important breakthrough for SCLC treatment,Citation5–Citation7 however, the improvement in survival has been limited.
Accumulating epidemiologic evidence suggests innumerable links between diabetes mellitus, related medication and cancer,Citation8–Citation10 while reports that T2DM is connected to the risk or mortality of lung cancer had inconsistent conclusions. It has been reported that diabetes increases the risk of lung cancerCitation11 and adversely affects lung cancer outcome,Citation12 while other reports have shown that diabetes is not related toCitation13 or even prolongs the survival of lung cancer patients.Citation14 Therefore, it is necessary to provide more evidence about the association of T2DM and lung cancer. In addition, there are few data that have examined the significance of T2DM and its medication on the prevalence, progression and prognosis of SCLC.
To explore whether features of diabetes or diabetes medication would have an impact on different clinicopathological characteristics at first diagnosis and affect the prognosis of SCLC patients, we retrospectively investigated the clinicopathological characteristics and survival risk factors in SCLC patients with preexisting T2DM (preDM).
Materials and Methods
Study Population
All patients with SCLC admitted to Sichuan Cancer Hospital & Institution, Sichuan Cancer Center, University of Electronic Science and Technology of China between January 2013 and August 2018 were followed up until August 2020 and retrospectively analyzed. The inclusion criteria were as follows: (a) pathological confirmation of SCLC; (b) preDM before the diagnosis of SCLC or without T2DM who should have their morning fasting plasma glucose (FPG) lower than 7.0 mmol/L. The following patients were excluded: (a) those who had other concurrent malignancies or SCLC mixed with NSCLC; (b) those who had impaired FPG or diabetes diagnosed after SCLC; and (c) those who lacked basic clinical data or accurate TNM staging. T2DM was diagnosed due to a history of diabetes, current or previous use of antidiabetic drugs, an elevated FPG level >7.0 mmol/L, or elevated random plasma glucose >11.0 mmol/L, or glycated hemoglobin (HbA1c) ≥6.5%. This retrospective study was conducted with approval of the appropriate institutional review board.
Data Collection
Baseline characteristics of SCLC patients were extracted from hospital records, including age, sex, smoking status, BMI, history of hypertension, history of coronary heart disease, ECOG PS, TNM stage, and metastasis sites. In the population of SCLC patients with diabetes, data on blood glucose level, diabetes duration at cancer diagnosis, diabetes medication (insulin analogs, metformin, and others), liver function index and kidney function index were also analyzed. OS was calculated from the date of pathological confirmation of SCLC to the date of death from any cause or censoring at the last follow-up.
Statistical Analysis
Pearson’s chi-square test and Fisher’s test were used to examine differences between dichotomous variables. A t-test was employed to compare measurement data, such as age and BMI, between the two groups. Logistic regression was carried out to explore the association between clinical characteristics and distant metastasis, such as age, sex, smoking status, BMI, hypertension, coronary heart disease (CHD), and preDM. OS for the two groups was compared using the Kaplan–Meier method and the unstratified Log rank test. Both univariate and multivariate Cox proportional hazards models were conducted to identify potential independent prognostic factors. A p-value was less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS 25.0.
Result
Patients Features
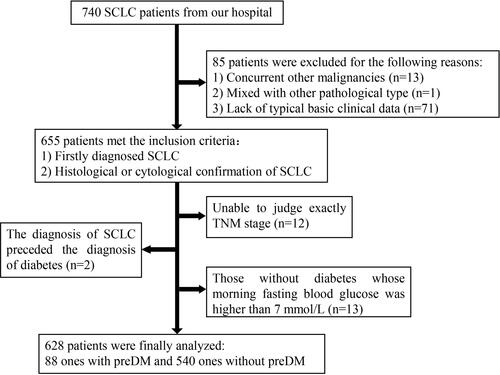
A total of 740 patients who had pathological confirmation of SCLC in Sichuan Cancer Hospital were analyzed. A total of 655 SCLC patients met the inclusion criteria, including 97 patients with preDM, which showed that the rate of preDM occurrence was 14.81% (97/655) of the SCLC population. Among them, 9 patients with preDM and 18 patients without preDM were excluded for the following reasons: (a) pTNM stage could not be judged exactly; (b) those without diabetes whose morning fasting blood glucose was higher than 7 mmol/L. The final population analyzed was limited to 628 patients, which included 88 with preDM and 540 without preDM ().
General Clinical Characteristics of SCLC Patients with and without preDM
We compared the differences in clinical characteristics between SCLC patients with and without preDM. Compared to the NDM group, patients in the DM group were significantly older on average (58.98±9.74 vs 62.85±8.93, p=0.000). Patients with preDM had higher BMI values and more cardiovascular comorbidities, such as hypertension and CAD, which were statistically significant (p<0.050). Patients with preDM had poorer PS than those without DM(p=0.000). A total of 60.2% of patients had distant metastasis in the DM group, which was significantly different from that in the NDM group (45.6%, p=0.011). More patients had pleural metastasis in the DM group than in the NDM group (13.6% vs 6.3%, p=0.014). No difference was found in sex, smoking status, occurrence of liver metastasis, brain metastasis or bone metastasis between the two groups ().
Table 1 The Comparison of Clinical Characteristics Between SCLC Patients with and without preDM
Distant Metastasis
To explore the risk factors for distant metastasis in SCLC patients, we carried out further analysis of the association between clinical characteristics and distant metastasis in SCLC patients using logistic regression, and found that age, smoking history, BMI value and preDM were significantly associated with distant metastasis in SCLC patients (p=0.016, OR=1.50, 95% CI 1.08–2.10) (p=0.000, OR=1.96, 95% CI 1.38–2.80) (p=0.004, OR=0.60, 95% CI 0.42–0.85) (p=0.016, OR=1.80, 95% CI 1.11–2.91) (). This result suggested that preDM may increase the risk of distant metastasis in SCLC patients.
Table 2 Correlation of Clinicopathological Factors and Distant Metastasis in SCLC Patients
Survival of SCLC Patients
The median follow-up for all patients was 50.17 months (range: 0.03–87.73 months), and the median OS was 18.70 months, with a 1-year OS rate of 62.74%, a 2-year OS rate of 33.76%, and a 3-year OS rate of 14.33%. The Kaplan–Meier curve for OS did not display a statistically significant association with survival and preDM with a median OS of 19.17 months versus 16.10 months in patients with and without preDM, respectively (log-rank χ2 1.510, p=0.219). The 1-, 2-, and 3-year OS rates for patients with and without preDM were 61.36% versus 62.96%, 29.55% versus 34.44%, and 12.50% versus 14.63%, respectively. According to the multivariate Cox proportional hazards model, age, distant metastasis and liver metastasis were unfavorable prognostic factors in all SCLC patients (p=0.025, HR=1.24, 95% CI 1.03–1.49; p=0.013, HR=1.34, 95% CI 1.06–1.69; p=0.000, HR=1.62, 95% CI 1.24–2.11, respectively). Additionally, poorer PS, brain metastasis and pleural metastasis tended to be unfavorable prognostic factors, but there was no significant difference. Multivariate analysis revealed that preDM was not an independent factor associated with OS in SCLC patients (p=0.803, HR=1.04, 95% CI 0.79–1.36) (), which indicated that it was not preDM that directly affected the prognosis of SCLC patients.
Table 3 Multivariable Proportional Hazard Regression Model on Overall Survival in SCLC Patients
Insulin Treatment and Overall Survival of Diabetic Patients
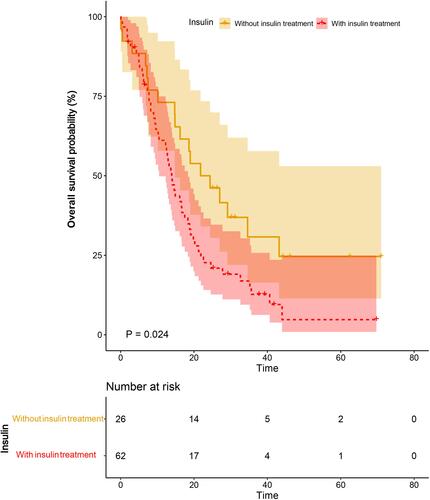
The clinical factors were compared in patients with and without insulin treatment. More patients had FPG higher than 7 mmol/L and accepted chemotherapy in the group treated with insulin analogs (P=0.040, P=0.032, respectively). No difference was found in age, sex, smoking status, ECOG PS, hypertension history, CHD history, occurrence and lesion of distant metastasis, or thoracic radiotherapy between the two groups (). The Kaplan–Meier curve for OS displayed a statistically significant association between survival and insulin treatment, with a median OS of 13.93 months versus 21.77 months in patients treated with and without insulin analogs, respectively (log-rank χ2 1.51, p=0.024) (). The 1-, 2-, and 3-year OS rates for patients treated with insulin analogs were 56.45%, 20.97%, and 9.68%, respectively, and were significantly lower than those treated without insulin treatment (1-, 2-, and 3-year OS: 73.08%, 50.00%, 19.23%). This result implied that insulin treatment may increase the mortality risk in diabetic patients with SCLC.
Table 4 The Comparison of Clinical Characteristics Between Diabetic Patients with and without Insulin Treatment
Univariate and Multivariate Survival Analysis of Diabetic Patients
We conducted univariate and multivariate analyses with a Cox proportional hazards model to identify factors associated with survival in SCLC patients with preDM. Univariate analysis showed the following factors associated with OS: poorer PS, distant metastasis, liver metastasis, insulin treatment, AST value over the normal range (>40.00 U/L), creatinine value over the normal range (>106.00 µmol/L), uric acid value over the normal range (>440. 00 µmol/L), chemotherapy and thoracic therapy, p<0.05. Further multivariate analysis identified that insulin treatment was an independent unfavorable factor associated with OS (p=0.009, HR=2.10, 95% CI 1.19–3.64). In addition, poorer PS and liver metastasis were also independent unfavorable prognostic factors (p=0.005, HR=2.05, 95% CI 1.24–3.39; p=0.008, HR=2.32, 95% CI 1.25–4.31, respectively), while thoracic therapy significantly improved OS and decreased mortality risk in diabetic patients with SCLC (p=0.017, HR=0.53, 95% CI 0.31–0.89) ().
Table 5 Univariate and Multivariate Analysis for Overall Survival in SCLC Patients with preDM
Discussion
In the current study, we provided new evidence on the association of diabetes and SCLC. We found that preDM was an independent impact factor associated with distant metastasis of SCLC, however, preDM was not a prognostic factor for SCLC patients. Hence, we performed further multivariate survival analysis in the subgroup with preDM, interestingly we found that insulin treatment decreased the OS of SCLC patients with preDM, with a median survival time of 13.93 months (versus 21.77 months in patients without insulin treatment), illuminating that insulin analogs may adversely affect the progression and outcome of SCLC.
Epidemiological data show that T2DM is a risk factor for the occurrence, development and prognosis of various tumors, and it has a significant impact on the prognosis of breast cancer, colorectal cancer and gastric cancer.Citation15,Citation16 The mechanisms are complex, possibly including excessive ROS-formation, oxidative stress, destruction of several types of essential molecules, chronic inflammation, impaired healing phenomena, and multiple abnormalities in the levels of DNA, RNA, metabolites, and proteins, jointly resulting in carcinogenesis under diabetic conditions.Citation9 However, there is a paucity of data on the relationship between SCLC and diabetes. Given the poor prognosis of SCLC, there is an imperative need to explore new biomarkers to optimize the treatment strategy of SCLC. Our study showed that preDM may promote the occurrence of distant metastasis of SCLC at first diagnosis. Similarly, Overbeek’s study reported that women with diabetes had a higher risk of developing breast cancer with more advanced tumor stages.Citation17 Takasumi’ team showed that diabetes intensified liver metastasis of colon cancer associated with angiotensin activation.Citation18 Although the mechanism of the interaction between diabetes and cancer is not explicit, it is believed that diabetes-related hyperinsulinemia, hyperglycemia, and chronic inflammation may play crucial roles in the initiation and progression of neoplastic lesions which may involve several pathways, such as phosphorylation of TET2 at serine 99 by AMP-activated kinase, leading to the destabilization of TET2 and dysregulation of the tumor suppressive function of TET2,Citation19 and upregulating Glut1/MMP2/MMP9 axis expression.Citation20 However, reports on the relationship between preDM and lung cancer survival have mixed conclusions. For example, a prospective study showed that among women with lung cancer, preDM predicted poor prognosis.Citation21 Another review reported that preDM prolonged the survival of patients with lung cancer while the level of blood glucose decreased progression-free survival.Citation14 In our study, it was not preDM, but rather insulin therapy that had an adverse impact on SCLC survival.
Preclinical evidence has demonstrated that cancer cells remain insulin-sensitive, resulting in substantially increased signaling downstream of receptors substantially and promoting neoplastic growth.Citation22 The insulin-like growth factor/insulin receptor (IGF/IR) signaling pathway plays a vital role in tumor development. Insulin activates downstream signals not only through insulin receptors, but also through insulin-like growth factor receptor (IGFR). Activation of IGFR1 stimulates multiple intracellular downstream signal cascades, resulting in a variety of biological effects in tissues and cells. Moreover, activation of IGFR1 is essential for the growth of cancers.Citation23 We have previously found that the IGF/IR signaling pathway plays an important role in the development of colorectal,Citation24 non-small-cell lung cancerCitation25 and breast cancersCitation26 in diabetic conditions. The use of insulin analogs in SCLC patients with T2DM partly increases the exposure to high levels of circulating insulin. There is evidence that insulin therapy for cancer patients with T2DM may worsen the cancer prognosis and increase the cancer mortality rates.Citation27 A retrospective cohort study also showed that breast cancer patients with preDM given insulin analogs had higher mortality rates than those taking other oral hypoglycemic drugs.Citation28 Furthermore, it is plausible that synthetic insulin by subcutaneous injection may lead to altered pharmacokinetic profiles and different affinities for receptor-binding, such as insulin receptors, IGFR1 and hybrid receptors, bringing about a stronger cancer-promoting effect than endogenous insulin.Citation29–Citation31 In addition, in our study, those who had received insulin treatment usually had higher fasting glucose levels, suggesting that they had poor glycemic control. It has been reported that high glucose levels have a negative effect on the clinical outcome of cancer patients.Citation32,Citation33 After anticancer treatment, blood glucose levels worsen, while poor glycemic control may affect the progression and recurrence of the tumor, and thus affect the overall survival of patients.Citation34 Therefore, enhancing blood glucose control and reducing insulin analogs use may be essential to the improvement of the long-term survival of the diabetic population with SCLC. In addition, it is worth to mentioning that our study found that radiotherapy to chest lesions improved overall survival for any stage of SCLC patients with preDM, indicating that diabetic patients might be more suitable for chest radiotherapy and this result needs to be further explored.
Compared to previous reports, our research provides a preliminary exploration of diabetes in the progression and survival of SCLC patients, and we reveal the first discovery of insulin therapy predicting poor prognosis in SCLC. However, this study also has some limitations. The retrospective nature and its small clinical sample size were drawbacks of this study, thus we were unable to obtain detailed data on glycemic control during treatment. Large prospective observational studies will be needed to further explore the results, and the mechanisms associated with insulin influencing the development of SCLC need to be further elucidated.
Conclusions
Our study performed a detailed analysis of the clinical characteristics and survival of SCLC patients with preDM, and found that diabetic patients are prone to develop distant metastasis at first diagnosis, while further prospective studies are needed to confirm the prognostic role of diabetes. In particular, insulin treatment was significantly correlated with a higher risk of mortality in SCLC patients with preDM, and the related mechanism needs to be clarified.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1995 Helsinki declaration. Meanwhile, this study was approved by the Ethics Committee of Sichuan Cancer Hospital (No.: SCCHEC-02-2021-071). We protect patient privacy because the data of this study is anonymous.
Consent to Participate
Because of the retrospective nature of this study, consent to participate for inclusion was waived.
Consent to Publish
Because of the retrospective nature of this study, consent to publish was waived.
Acknowledgments
This work was partly supported by the Foundation of Science and Technology Department of Sichuan Province (19YYJC1977) and National Natural Science Foundation of China (82002580).
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
- DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. doi:10.1038/nrdp.2015.19
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378(9804):1741–1755. doi:10.1016/S0140-6736(11)60165-7
- Sabari JK, Lok BH, Laird JH, Poirier JT, Rudin CM. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol. 2017;14(9):549–561. doi:10.1038/nrclinonc.2017.71
- Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. doi:10.1016/S1470-2045(20)30539-8
- Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi:10.1056/NEJMoa1809064
- Iams WT, Porter J, Horn L. Immunotherapeutic approaches for small-cell lung cancer. Nat Rev Clin Oncol. 2020;17(5):300–312. doi:10.1038/s41571-019-0316-z
- Suh S, Kim KW. Diabetes and cancer: cancer should be screened in routine diabetes assessment. Diabetes Metab J. 2019;43(6):733–743. doi:10.4093/dmj.2019.0177
- Srivastava SP, Goodwin JE. Cancer biology and prevention in diabetes. Cells. 2020;9(6):1380. doi:10.3390/cells9061380
- Wang M, Yang Y, Liao Z. Diabetes and cancer: epidemiological and biological links. World J Diabetes. 2020;11(6):227–238. doi:10.4239/wjd.v11.i6.227
- Luo J, Chlebowski R, Wactawski-Wende J, Schlecht NF, Tinker L, Margolis KL. Diabetes and lung cancer among postmenopausal women. Diabetes Care. 2012;35(7):1485–1491. doi:10.2337/dc11-2108
- Luo J, Hendryx M, Qi L, Ho GY, Margolis KL. Pre-existing diabetes and lung cancer prognosis. Br J Cancer. 2016;115(1):76–79. doi:10.1038/bjc.2016.141
- Karlin NJ, Amin SB, Buras MR, Kosiorek HE, Verona PM, Cook CB. Patient outcomes from lung cancer and diabetes mellitus: a matched case-control study. Future Sci OA. 2018;4(1):FSO248. doi:10.4155/fsoa-2017-0081
- Wang NF, Tang HM, Liu FL, Hong QY. Prolonged progression-free survival and overall survival are associated with diabetes mellitus but inversely associated with levels of blood glucose in patients with lung cancer. Chin Med J. 2020;133(7):786–791. doi:10.1097/CM9.0000000000000739
- Zhou Y, Liu S, Wang J, Yan X, Zhang L. Changes in blood glucose of elderly patients with gastric cancer combined with type 2 diabetes mellitus after radical operation and the effect of mediation adjustment for blood glucose on the recovery of gastric cancer. Oncol Lett. 2018;16(4):4303–4308. doi:10.3892/ol.2018.9197
- Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012;7(3):e33411. doi:10.1371/journal.pone.0033411
- Overbeek JA, van Herk-sukel MPP, Vissers PAJ, et al. Type 2 diabetes, but not insulin (analog) treatment, is associated with more advanced stages of breast cancer: a national linkage of cancer and pharmacy registries. Diabetes Care. 2019;42(3):434–442. doi:10.2337/dc18-2146
- Shimomoto T, Ohmori H, Luo Y, et al. Diabetes-associated angiotensin activation enhances liver metastasis of colon cancer. Clin Exp Metastasis. 2012;29(8):915–925. doi:10.1007/s10585-012-9480-6
- Wu D, Hu D, Chen H, et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature. 2018;559(7715):637–641. doi:10.1038/s41586-018-0350-5
- Sun XF, Shao YB, Liu MG, et al. High-concentration glucose enhances invasion in invasive ductal breast carcinoma by promoting Glut1/MMP2/MMP9 axis expression. Oncol Lett. 2017;13(5):2989–2995. doi:10.3892/ol.2017.5843
- Yi ZH, Luther Y, Xiong GH, et al. Association between diabetes mellitus and lung cancer: meta-analysis. Eur J Clin Invest. 2020;50(10):e13332. doi:10.1111/eci.13332
- Yee LD, Mortimer JE, Natarajan R, Dietze EC, Seewaldt VL. Metabolic health, insulin, and breast cancer: why oncologists should care about insulin. Front Endocrinol. 2020;11:58. doi:10.3389/fendo.2020.00058
- Janssen J. New Insights from IGF-IR stimulating activity analyses: pathological considerations. Cells. 2020;9(4):4. doi:10.3390/cells9040862
- Ding J, Li C, Tang J, Yi C, Liu JY, Qiu M. Higher expression of proteins in IGF/IR axes in colorectal cancer is associated with type 2 diabetes mellitus. Pathol Oncol Res. 2016;22(4):773–779. doi:10.1007/s12253-016-0065-6
- Ding J, Tang J, Chen X, et al. Expression characteristics of proteins of the insulin-like growth factor axis in non-small cell lung cancer patients with preexisting type 2 diabetes mellitus. Asian Pac J Cancer Prev. 2013;14(10):5675–5680. doi:10.7314/APJCP.2013.14.10.5675
- Xin C, Jing D, Jie T, Wu-Xia L, Meng Q, Ji-Yan L. The expression difference of insulin-like growth factor 1 receptor in breast cancers with or without diabetes. J Cancer Res Ther. 2015;11(2):295–299. doi:10.4103/0973-1482.138195
- Bowker SL, Yasui Y, Veugelers P, Johnson JA. Glucose-lowering agents and cancer mortality rates in type 2 diabetes: assessing effects of time-varying exposure. Diabetologia. 2010;53(8):1631–1637. doi:10.1007/s00125-010-1750-8
- Hosio M, Urpilainen E, Hautakoski A, et al. Survival after breast cancer in women with type 2 diabetes using antidiabetic medication and statins: a retrospective cohort study. Acta Oncol. 2020;59(9):1110–1117. doi:10.1080/0284186X.2020.1769858
- Kurtzhals P, Schäffer L, Sørensen A, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes. 2000;49(6):999–1005. doi:10.2337/diabetes.49.6.999
- Werner H, Chantelau EA. Differences in bioactivity between human insulin and insulin analogues approved for therapeutic use- compilation of reports from the past 20 years. Diabetol Metab Syndr. 2011;3(1):13. doi:10.1186/1758-5996-3-13
- Hansen BF, Kurtzhals P, Jensen AB, Dejgaard A, Russell-Jones D. Insulin X10 revisited: a super-mitogenic insulin analogue. Diabetologia. 2011;54(9):2226–2231. doi:10.1007/s00125-011-2203-8
- Supabphol S, Seubwai W, Wongkham S, Saengboonmee C. High glucose: an emerging association between diabetes mellitus and cancer progression. J Mol Med. 2021;99(9):1175–1193. doi:10.1007/s00109-021-02096-w
- Almehmadi MM. Association between random glucose level and leukocytes count in female cancer patients. Cureus. 2020;12(7):e8962. doi:10.7759/cureus.8962
- Pettit S, Cresta E, Winkley K, Purssell E, Armes J. Glycaemic control in people with type 2 diabetes mellitus during and after cancer treatment: a systematic review and meta-analysis. PLoS One. 2017;12(5):e0176941. doi:10.1371/journal.pone.0176941