Abstract
5-HT3 antagonists have been available as oral and intravenous preparations for decades. The availability more recently of transdermal granisetron and the anticipated availability of a subcutaneous granisetron preparation have provided helpful alternatives to patients, and these preparations have been shown to have less potential to prolong QT than other drugs in the class.
Introduction
Problem of chemotherapy-induced nausea and vomiting
Chemotherapy-induced nausea and vomiting (CINV) occurs in 10%–90% of patients, depending on the emetic risk of the chemotherapeutic agent.Citation1,Citation2 There is a considerable cost involved, not only for the expense of hospitalization and the direct treatment of CINV, but also because of the loss of workdays and the consequences of delayed chemotherapy administration.Citation3–Citation7 There is consistent evidence that 5-HT3 antagonist antiemetic therapy has improved this problem without increasing the cost.Citation3–Citation7
Unmet needs in CINV treatment
Delayed nausea and vomiting occurring hours or days after chemotherapy, either as a result of reduced plasma concentrations of prophylactic antiemetics administered at the time of chemotherapy, or inefficacy of ongoing oral antiemetic medications, is a significant management problem.Citation3,Citation8 Palonosetron, due to its long half-life, provided a potential solution to this problem,Citation9 yet despite its introduction, the problem continues to adversely affect the quality of life of chemotherapy recipients.Citation10 An important objective of the granisetron transdermal (TD) delivery system is maintenance of effective plasma concentration of granisetron during and for several days after administration of chemotherapy.
Oral and intravenous (IV) administration of granisetron and other antiemetics is problematic in some patients. Because the oral route is complicated by variable bioavailability and by noncompliance, while the IV route may be too inconvenient, painful or expensive for some patients, other modes of administration have been sought.Citation11
Purpose of this report
The purpose of this report is to review and assess the contribution of TD granisetron to the control of acute and delayed CINV, and to delineate its place in the management of CINV. Because of the recent safety concerns related to QT prolongation by 5-HT3 antagonists, leading to multiple label changesCitation12 and a product recall,Citation13 the potential for arrhythmo-genesis through TD granisetron is discussed at length.
Methods
This report is based on a review of the medical literature, available US Food and Drug Administration (FDA) communications and product labels, and a P&T Product ProfilerCitation14 provided by ProStrakan (Bridgewater, NJ, USA). In addition, pharmacokinetic and cardiac safety data regarding APF530, a sustained release subcutaneous (SC) preparation of granisetron, was provided by AP Pharma, Inc (Redwood City, CA, USA).
Preparations, pharmacology, and pharmacokinetics of granisetron
Currently available preparations of granisetron
Granisetron is available as an oral tablet (1 mg or 2 mg), and an IV infusion (1 mg in 1 mL and 3 mg in 3 mL ampules), both marketed under the brand name Kytril (Roche, Basel, Switzerland) and as a generic compound. Granisetron in a TD patch (34.3 mg) was approved in 2008 and marketed under the brand name Sancuso (ProStrakan, Inc). A sustained-release SC injection (APF530, AP Pharma, Inc) may become available before or soon after publication of this report, as the US FDA has responded to a new drug application submission for this preparation, and approval during 2013 was established by the sponsor as its objective.
Pharmacology of granisetron
Granisetron and other selective 5-HT3 antagonists block receptors in afferent vagal nerve termini in the gut and in the chemoreceptor trigger zone (CTZ). Vagal receptors in the gut, when stimulated by serotonin release by the intestinal mucosa as a result of chemotherapy, send a signal to the vomiting center and the CTZ that induces nausea and vomiting. Direct stimulation of the CTZ by chemotherapeutic agents may also induce emesis. Granisetron prohibits the emesis reflex by blocking these receptors.
Pharmacokinetics of IV and oral granisetron
As indicated in the package insert,Citation15 the elimination half-life (t1/2) of IV granisetron (40 μg per kg) depends on both age and health status. While the maximum plasma concentration (Cmax) is similar among young, elderly, and normal volunteers and patients, terminal elimination is considerably slower in patients with cancer, and nearly as slow in elderly normals compared to young normals ().
Table 1 Pharmacokinetics of granisetron
Oral dosing with granisetron 2 mg qd (once a day) in normal volunteers resulted in a mean Cmax of 5.5 nanogram/ milliliter (ng/mL) and t1/2 of 7.9 hours on day 5,Citation16 while a dose of 1 mg bid (twice a day) in patients with cancer resulted in a Cmax of 5.9 ng/mL, according to the package insert.Citation16 t1/2 in patients is not reported in the package insert or in the published literature.
Transdermal granisetron
Product description
The granisetron TD patch uses a drug-in-adhesive matrix diffusion method to deliver a predictable dose of granisetron for up to 7 days. The patch is a 52 cm2 rectangle that contains 34.3 mg of granisetron, and delivers it at a rate of 3.1 mg per 24 hours. Transdermal drug delivery is possible because the skin can absorb small, lipophilic molecules by diffusion. The size of the transdermal patch, specific characteristics of the matrix material in which the drug is suspended, properties of the drug itself, and the status of the skin to which the patch is applied determine the rate and extent of drug delivery. While transdermal delivery is generally slow, requiring several hours or days to achieve steady state, the plasma concentrations achieved are predictable and are not subject to rapid change, maximizing the potential for efficacy without toxicities that result from excessive plasma concentration.
Pharmacokinetics of transdermal granisetron
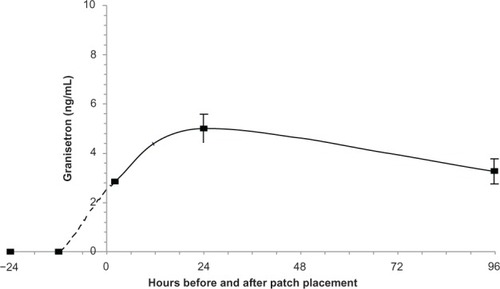
In a Phase 1 crossover study of twelve normal volunteers, Howell et alCitation17 compared the pharmacokinetics of the 52 cm2 patch to that of oral granisetron 2 mg daily (). Several differences are evident. The time at which maximum plasma concentration was achieved (Tmax) was 1.5 hours to 2 hours during oral dosing, and approximately the same Cmax was achieved each day. Tmax for the patch was 48 hours, and Cmax varied substantially from day to day. While Cmax was greater after oral dosing compared to patch administration, mean plasma concentration through 5 days was similar. The t1/2 of oral granisetron varied between 6.4 and 7.9 hours, while the apparent t1/2 during patch administration was 35.9 hours. shows clearly that the within-day variation in granisetron concentration was much greater during oral administration. It also shows that plasma concentration reached its mean value at 24 hours and its Cmax 24 hours later. Thus, the patch must be applied 24 hours to 48 hours before chemotherapy is started.
Figure 1 Plasma concentrations of granisetron resulting from repeated oral dosing (2 mg daily) and the Sancuso 52 cm2 TD patchCitation17 in normal volunteers.
Note: The patch was removed at the end of day 6 (144 hours).
Abbreviations: TD, transdermal; ng/mL, nanogram/milliliter.
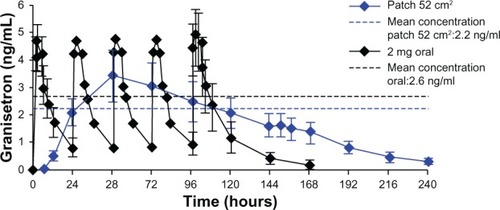
In a Phase 3 study that included 90 recipients of TD granisetron (Straken Pharmaceutical Ltd, 2007), the plasma concentration curve during patch treatment was very similar to the curve observed in normal volunteers ().
Efficacy of transdermal granisetron
TD granisetron was found to be noninferior to oral granisetron, 2 mg, in premarketing clinical trials in patients receiving a single dose (Straken Pharmaceutical Ltd, 2007) of chemotherapy and repeated dosesCitation19 of chemotherapy over 3 to 5 days, in controlling both acute (first 24 hours) and late (24–120 hours) CINV. In the single-day chemotherapy study,Citation18 210 patients were randomized in a 1:1 ratio to receive either oral granisetron or the TD patch, both administered once. Total control of CINV (no nausea and no use of rescue medication) was achieved in 52.4% of patients in the former and 43.7% in the latter group (not statistically or clinically significantly different).
In the multiple-day study,Citation19 in which 323 patients were randomized to receive daily oral granisetron given when chemotherapy was administered, and 318 to receive the TD patch applied once 24 hours before chemotherapy and removed on day 7, complete control of CINV (no more than mild nausea) was reported by 64.8% in the former group, and 60.2% in the latter (not significantly different) during the acute phase. Rescue medications were used equally in the two groups during the late phase, when the chemotherapy regimen was of 3 days’ duration, and more frequently in the TD patch group when chemotherapy was administered for 4 days or 5 days.
Safety and tolerability of transdermal granisetron
TD drug delivery, in general, has advantages and disadvantages, as summarized by Patel et al.Citation20 Adhesion is a major issue for all transdermally delivered medications.Citation21 Loss of adhesion results in reduced drug delivery and can result in inefficacy. Among 308 patients receiving Sancuso in a Phase 3 study (392MD/15/C):Citation22 64% had ≥90% adhesion; 90% had ≥75% adhesion; and two patients (1%) had complete patch detachment. In another Phase 3 study (392MD/8/C),Citation18 one of 88 subjects had complete patch detachment. Though considerable work has been done to develop methods to assess completeness of chronic patch adhesion,Citation23 there is no organized data in the medical literature against which to compare the adhesion success of Sancuso. The low rate of complete patch detachment in these two Phase 3 studies is impressive, but detachment of at least 10% of the patch surface in 36% of patients in the two Phase 3 studies noted is a drawback.
In the same two studies, two of 308 subjects in 392/ MD/15/C and three of 88 in study 392MD/8/C, or an average of 1.3%, developed skin irritation.Citation18,Citation22 Skin tolerance of the Sancuso patch was very good.
The incidence of the most common adverse events reported in the Kytril label for 2 mg oral dosingCitation16 and the incidence associated with oral granisetron and TD patch granisetron from studies 392MD/15/C and 392MD/8/C are summarized in , which was drawn from Table 6 in the P&T Profiler.Citation14 Patch delivery produces a side effect profile similar to that of oral delivery of granisetron.
Table 2 IC50 for hERG cardiac K+ channel inhibition
Table 3 Incidence of most common adverse events
Other granisetron preparations and other drug treatment options
Fourteen specific alternatives to oral and IV granisetron are listed in . Four are not yet approved for marketing; two are not available in the US; and one is approved only for veterinary use. APF530 is a formulation of granisetron for sustained release after SC injection. It consists of granisetron in a bioerodible poly(ortho ester) polymer that results in slow delivery of granisetron from the injection site into the circulation for as long as a week.
Table 4 Other 5-HT3 antagonist preparations and other drug therapies for CINV in current use or under development
QT prolongation associated with granisetron preparations, other 5-HT3 antagonists
HERG potassium channel (IKr) block appears to be a 5-HT3 antagonist class effect. shows the drug concentration causing 50% inhibition (IC50) for IKr tail current inhibition by the four members of the class for which data are available. The clinical safety margin for each drug administered at a recommended oral dose was calculated from known physical characteristics of the drugs and published mean Cmax levels.
In December 2010, the US FDA prohibited use of IV dolasetron for CINV because of excessive QTc prolongation.Citation24 Its use for control of postoperative nausea and vomiting was not discontinued because of the lower recommended dose, but new warnings were applied to the oral formulation. In 2011, IV dolasetron for any indication was withdrawn from the Canadian market.Citation25 The low safety margin during oral dosing, lowest among the four co-classified drugs shown in , is consistent with these outcomes.
Ondansetron is the most potent IKr blocker on a molar basis among the four 5-HT3 antagonists (), and its safety margin after oral doses of 8 mg is small. The US FDA recently required the drug’s manufacturer to perform a thorough QT study of high IV doses used for control of CINV. In June 2012, the US FDA prohibited use of IV ondansetron at doses above 16 mg because of marked QT prolongation noted in the thorough QT study (TQTS) and anecdotal reports of ondansetron-related arrhythmias.Citation26 Subsequently, 32 mg ondansetron ampules were withdrawn from the market.Citation13 The recommended oral dose of 24 mg administered as three 8 mg tablets over 30 minutes, and IV doses of 16 mg or less are still permitted.
Current labeling of palonosetron no longer includes a warning regarding QT prolongation. Removal of the previous warning in 2008 was based on a TQTS that showed virtually no effect of palonosetron on the QT interval at IV doses as high as 2.25 mg.Citation27 Note that palonosetron has the largest safety margin among the four drugs in . This results from the fact that it has the highest IC50 concentration and nearly the lowest effective plasma concentration.
Granisetron also has a very high safety margin (). However, it acquired a new safety warning in October 2009:Citation28
An adequate QT assessment has not been conducted, but QT prolongation has been reported with KYTRIL. Therefore, Kytril should be used with caution in patients with pre-existing arrhythmias or cardiac conduction disorders, as this might lead to clinical consequences.
While a warning of this sort might be appropriate for any drug with a significant interaction with the IKr channel, it is not clear why granisetron is so labeled while palonosetron is not. In fact, I V, TD, and SC preparations of granisetron have been evaluated in thorough QT studies and found to have minimal effect on the QT interval. ProStrakan, Inc, sponsored a TQTS of IV (10 μg per kg over 30 seconds) and TD (34.3 mg in a 52 cm2 patch) granisetron,Citation12 while AP Pharma, Inc, sponsored a study of IV granisetron 50 μg per kg over 3 minutes and its sustained-released, SC preparation of granisetron (APF530, 20 mg) (AP Pharma Inc, 2012).
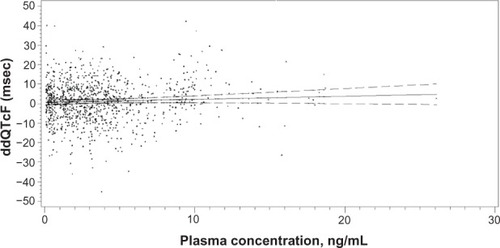
In the ProStrakan study, 240 subjects were randomized to four parallel treatment arms, including the two granisetron preparations, placebo, and oral moxifloxacin, 400 mg. In the primary analysis, none of the placebo-corrected changes from baseline at any time point for either granisetron preparation exceeded 1.9 milliseconds (msec), and the maximum observed 90% upper confidence boundary was 6.88 msec, well below the threshold of 10 msec for regulatory concern. In addition, the linear ddQTcF-plasma concentration model () showed a very shallow positive slope of 0.157 ms/ (ng/mL) for the relationship, which predicted a ddQTcF of only 4.79 msec for the maximum individual plasma concentration observed in the study (26.1 ng/mL). The ddQTcF refers to baseline and placebo-correct QTcF.
Figure 3 Linear regression of granisetron plasma concentration and associated placebo-corrected change in baseline-subtracted QTcF in the TQTS sponsored by ProStrakan, Inc.Citation12
Notes: The slope of the relationship was 0.157 msec/9 ng/mL. The model predicted a ddQTcF at the maximum plasma concentration (26.1 ng/mL observed in the study of only 4.79 msec).
Abbreviations: QTcF, QT corrected by the Fridericia formula; TQTS, thorough QT study; ddQTcF, baseline and placebo subtracted QTcF; msec, millisecond; ng/ mL, nanogram/milliliter.
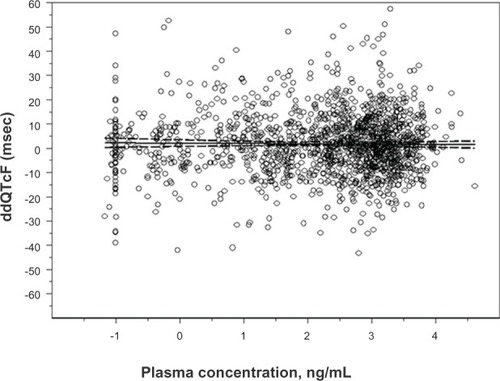
In the AP Pharma study, a crossover trial involving 51 completers, the largest change in ddQTcF in the linear mixed-effects model for either granisetron preparation was 3.75 msec, and the largest 90% upper boundary was 6.89 msec. Many ddQTcF values were negative. The log- linear pharmacokinetic–pharmacodynamic model () predicted a very shallow negative slope of −0.1326, and it predicted a ddQTcF value of 1.37 msec for the highest observed plasma concentration (82.1 ng/mL).
Figure 4 Linear regression the natural log of granisetron plasma concentration and associated placebo-corrected change in baseline-subtracted QTcF in the TQTS sponsored by AP Pharma Inc (2012).
Notes: The slope of the relationship was −0.1326 msec/9 ng/mL. The model predicted a ddQTcF at the maximum plasma concentration (82.1 ng/mL observed in the study of only 1.37 msec).
Abbreviations: QTcF, QT corrected by the Fridericia formula; ddQTcF, baseline and placebo subtracted QTcF; TQTS, thorough QT study; msec, millisecond; ng/ mL, nanogram/milliliter.
These two thorough QT studies indicate that granisetron has minimal effect, if any at all, on the QT interval at or below the highest plasma concentrations that was observed in either study (82.1 ng/mL) during treatment with IV granisetron administered at five-fold standard clinical doses of 10 μg per kg over 30 seconds or 50 μg per kg over 3 minutes, or with APF530 containing 20 mg of granisetron in a sustained-release polymer (twice the clinical dosage), or with TD-sustained release administration of 34.3 mg (the standard clinical dose).
Place of transdermal granisetron in CINV treatment
TD granisetron has an important role in the treatment of CINV. It is a unique preparation with a number of advantages and some disadvantages, related to its TD delivery (). Perhaps its greatest advantage is the ability to deliver an effective dose of granisetron over at least 5 days, without producing supratherapeutic concentrations that could cause adverse effects. This property makes Sancuso effective in preventing not only acute but also delayed CINV without repeated administration. It is the only antiemetic in its class that can be used by patients unable or unwilling to use the oral or parenteral route. Importantly, it has been shown to have a much lower potential for inducing QT prolongation in comparison to two of the drugs in its class (dolasetron and ondansetron).
Table 5 Advantages and disadvantages of transdermal delivery of granisetron
The most important drawback in the use of TD granisetron for control of CINV is the need to apply the patch 24 hours to 48 hours before chemotherapy is administered. This requirement is a source of unintended noncompliance, but, more importantly, it results in unnecessary exposure and increased expense in patients whose chemotherapy is unexpectedly delayed. Such delays, which may be required for a variety of disease or drug-related comorbidities, are commonplace and are usually initiated immediately before chemotherapy administration – well after the patch would have been applied. For example, Nagel et alCitation29 reported at least one delay in chemotherapy administration in 70 of 157 patients (45%) treated with a platinum and taxane-based regimen after cytoreductive surgery for ovarian cancer. A potential workaround to avoid unnecessary deployment of the TD patch is to administer oral or parenteral granisetron on the day of chemotherapy administration and apply the TD patch on the same day.
Conclusion
TD granisetron is a welcome addition to the available treatments for CINV. Though new preparations of existing drugs and new chemical entities are under development, Sancuso has a firm position in the prophylaxis of nausea and vomiting for the foreseeable future.
Disclosure
Both authors provided consultative support to AP Pharma, Inc, and to ProStrakan, Inc, that is unrelated to this report and its contents. The authors have no other conflicts of interest to disclose.
References
- GrallaRJOsobaDKrisMGRecommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical OncologyJ Clin Oncol19991792971299410561376
- RoilaFHeskethPJHerrstedtJPrevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus ConferenceAnn Oncol2006171202816314401
- Ihbe-HeffingerAEhlkenBBernardRThe impact of delayed chemotherapy-induced nausea and vomiting on patients, health resource utilization, and costs in German cancer centersAnn Oncol200415352653614998860
- StewartDJDahrougeSCoyleDEvansWKCosts of treating and preventing nausea and vomiting in patients receiving chemotherapyJ Clin Oncol199917134435110458253
- SykesAJKiltieAEStewartALOndansetron versus a chlorpromazine and dexamethasone combination for the prevention of nausea and vomiting: a prospective, randomized study to assess efficacy, cost effectiveness, and quality of life following single-fraction radiotherapySupport Care Cancer1997565005039406364
- Tina ShihYCXuYEltingLSCosts of uncontrolled chemotherapy-induced nausea and vomiting among working-age cancer patients receiving highly or moderately emetogenic chemotherapyCancer2007110367868517567835
- VanscoyGJFortnerBSmithRWeberRRihnTLPreventing chemotherapy-induced nausea and vomiting: the economic implications of choosing antiemeticsCommunity Oncology200522127132
- HeskethPJSanz-AltamiraPBusheyJHeskethAMProspective evaluation of the incidence of delayed nausea and vomiting in patients with colorectal cancer receiving oxaliplatin-based chemotherapySupport Care Cancer20122051043104721553313
- RubensteinEBPalonosetron: a unique 5-HT3 receptor antagonist indicated for the prevention of acute and delayed chemotherapy-induced nausea and vomitingClin Adv Hematol Oncol20042528428916163194
- Bloechl-DaumBDeusonRRMavrosPHansenMHerrstedtJDelayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatmentJ Clin Oncol200624274472447816983116
- KrautLFauserAAAntiemetics for cancer chemotherapy-induced emesis: potential of alternative delivery systemsDrugs200161111553156211577793
- MasonJ WSelnessDSMoonTEO’MahonyBDonachiePHowellJPharmacokinetics and repolarization effects of intravenous and transdermal granisetronClin Cancer Res201218102913292122452942
- Drug safety communication: updated information on 32 mg intravenous ondansetron (Zofran) dose and premixed ondansetron productsSilver Spring, MDUS Food and Drug Administration2012 [cited December 30, 2012] Available from: http://www.fda.gov/Drugs/DrugSafety/ucm330049.htmAccessed February 12, 2013
- GrossmanJCaspiASancuso® Granisetron transdermal delivery system: a formulation for chemotherapy-induced nausea and vomitingP&T Product Profiler2011362130
- Intravenous Kytril. [package insert]Basel, SwitzerlandRoche LaboratoriesInc1998–2005
- Oral Kytril. [package insert]Basel, SwitzerlandRoche Laboratories Inc2009
- HowellJSmeetsJDrenthHJGillDPharmacokinetics of a granisetron transdermal system for the treatment of chemotherapy-induced nausea and vomitingJ Oncol Pharm Pract200915422323119304880
- HowellJClarkGYellowleesAGutierrez-EsteinouREfficacy, safety, and tolerability of a transdermal granisetron patch for preventionof single-dose chemotherapy-induced nausea and vomiting: phase II trial resultsJ Oncol Pharm Pract200915Suppl 220
- HowellJYellowleesAGutierrez-EsteinouREfficacy, safety and tolerability of transdermal granisetron patch for prevention of multiday chemotherapy-induced nausea and vomiting: phase III trial resultsJ Support Oncol20086335 (abstract)
- PatelDChaudharySAParmarBBhuraNTransdermal drug delivery system: a reviewThe Pharma Innovation2012146675
- WokovichAMProdduturiSDoubWHHussainASBuhseLFTransdermal drug delivery system (TDDS) adhesion as a critical safety, efficacy, and quality attributeEur J Pharm Biopharm20066411816797171
- BocciaRVGordanLNClarkGHowellJDGrunbergSMEfficacy and tolerability of transdermal granisetron for the control of chemotherapy-induced nausea and vomiting associated with moderately and highly emetogenic multiday chemotherapy: a randomized, double-blind, phase III studySupport Care Cancer201119101609161720835873
- GutschkeEBrachtSNagelSWeitschiesWAdhesion testing of transdermal matrix patches with a probe tack test – in vitro and in vivo evaluationEur J Pharm Biopharm201075339940420350598
- Drug safety communication: Abnormal heart rhythms associated with use of Anzemet (dolasetron mesylate) [webpage on the Internet]Silver Spring, MDUS Food and Drug Administration2010 [cited Dec 17]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm237081.htm#safety_announcementAccessed February 12, 2013
- IV dolasetron withdrawal letter Available from: http://www.healthy-canadians.gc.ca/recall-alert-rappel-avis/hc-sc/2011/14633a-eng.phpAccessed February 12, 2013
- Drug safety communication: New information regarding QT prolongation with ondansetron (Zofran). [webpage on the Internet]Silver Spring, MDUS Food and Drug Administration6292012 Available from: http://www.fda.gov/Drugs/DrugSafety/ucm310190.htmAccessed February 12, 2013
- DailyMed. Palonsetron prescribing informationUS National Library of Medicine2008 Available from: http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=f4216772-6c37-4ff0-a575-f758390656e3Accessed February 12, 2013
- Kytril (granisetron hydrochloride) injection, tablets and oral solution [webpage on the Internet]Silver Spring, MDUS Food and Drug Administration2009 [cited October 21, 2009]. Available from: www.fda.gov/Safety/MedWatch/SafetyInformation/ucm187526.htmAccessed February 12, 2013
- NagelCIBackesFJHadeEMEffect of chemotherapy delays and dose reductions on progression free and overall survival in the treatment of epithelial ovarian cancerGynecol Oncol2012124222122422055764
- KuryshevYABrownAMWangLBenedictCRRampeDInteractions of the 5-hydroxytryptamine 3 antagonist class of antiemetic drugs with human cardiac ion channelsJ Pharmacol Exp Ther2000295261462011046096
- GurpideASadabaBMartin-AlgarraSRandomized crossover pharmacokinetic evaluation of subcutaneous versus intravenous granisetron in cancer patients treated with platinum-based chemotherapyOncologist20071291151115517914085