Abstract
Introduction
Lymph node metastasis (LNM) from primary tumors of the central nervous system (CNS) is an infrequent condition, and classically it was thought that CNS tumors could not spread via the lymphatic route. Recent discoveries about this route of dissemination make its knowledge necessary for surgeons and pathologists to avoid delays in diagnosis and unnecessary treatments. The aim of this paper is to review the literature and to discuss the relevant pathogenetic mechanism and the cytologic features along with recommendations for surgical treatment of these cervical LNM.
Materials and Methods
Using PRISMA guidelines, we conducted a systematic review of the literature published from 1944 to 2021, updating the comprehensive review published in 2010 by our group.
Results
Our review includes data of 143 articles obtaining 174 patients with LNM from a primary CNS tumor. The mean age of the patients was 31.9 years (range, 0.1–87) and there were 61 females (35.1%) and 103 males (59.2%), and in 10 cases (5.7%) the gender was not specified. The more frequent sites of distant metastasis were bones (23%), lungs (11.5%) and non-cervical lymph nodes (11%).
Conclusion
Cervical LNM from CNS tumors is infrequent. Pathologic diagnosis can be obtained by fine-needle aspiration cytology in most cases, giving surgeons the option to plan the appropriate surgical treatment. Given the poor prognosis of these cases, the most conservative possible cervical dissection is usually the treatment of choice.
Introduction
Cervical lymph node metastases typically originate from primary carcinomas arising from mucosa of the head and neck, skin, salivary glands, or thyroid gland. In a 2–5% of the cases, cervical lymph node metastasis (LNM) may be the first clinical manifestation of an occult primary tumor.Citation1 While originating from a head and neck cancer in the vast majority of cases, rarely, a cervical LNM may occur from non-head and neck sites, such as lung, breast, digestive and reproductive systems, typically located in lower neck levels.Citation2
The occurrence of cervical LNM arising from primary malignant tumors of the central nervous system (CNS) has been reported infrequently in case reports yet should be taken into consideration in diagnostic work-up of an unknown primary tumor. Despite this rare occurrence, clinical cases of cervical LNM of primary malignant CNS tumors are well documented in the literature.Citation3 The anatomical features of the cervical lymph nodes networks and its connections with intracranial lymphatic structures through the jugular foramen represent an important possible route for the spread of cancers to and from the CNS. Based on the basic research findings on the dural lymphatic vessels transporting fluid into deep cervical lymph nodes,Citation4–Citation6 investigation of the existing clinical literature on this phenomenon has clinical importance. In addition, better awareness of this rare presentation could help in diagnosing occult primary tumor in patients presenting with cervical LNM from a clinically hidden primary tumor.
Primary malignant tumors of the CNS represent 2% of all cancers.Citation7 The blood–brain barrier and the absence or paucity of lymphatic vessels are presumed to be the cause of the low incidence of metastasis outside the CNS.Citation8 Historically, the presence of cervical metastasis was explained by surgical disruption of the dura mater during the primary surgery through which tumor cells could seed outside the limits of the CNS.Citation9 However, the recent discovery of a dural lymphatic networkCitation4 undermines theses historical assumptions, and may better explain cases of cervical metastasis without previous surgery nor dural invasion.Citation10 Novel therapeutic approaches have extended the life of cancer patients and increased the risk of extracranial dissemination of CNS tumors.Citation3 Histological types of primary CNS tumors that have reported to present with cervical LNM are glioblastoma (the most common), oligodendroglioma, ependymoma, metastasizing meningioma, medulloblastoma (most common in children), and pituitary carcinoma. In addition to the neck LNM, the most frequent extracranial sites of distant metastases are the lungs, pleura, liver and bones.Citation3 Although cervical metastases have been described in a wide variety of CNS tumors, yet the reports are confined to case reports and limited case series.
The aim of this paper is to review the literature on the presence of metastasis in lymph nodes of the neck from CNS primary tumors and to discuss the relevant pathogenetic mechanism and the cytologic features along with recommendations for surgical treatment of these cervical LNM. For this purpose, we updated the comprehensive review published in 2010 by our group.Citation3
Materials and Methods
The Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) method was utilized to evaluate the literature.Citation11 The search strategy included full-text articles with CNS tumors that metastasized to the cervical lymph nodes. We performed a PubMed search updated to July 29, 2021 for English language publications between 2010 and 2021 including these search criteria: “extracranial metastases from CNS tumors” or “cervical lymph node” or “neck lymph node” coupled with “glioblastoma”, “medulloblastoma”, “oligodendroglioma”, “ependymoma”, “metastasizing meningioma”, “intracranial neuroblastoma”, “pituitary carcinoma”, “astrocytoma”, “germ cell tumors”, “yolk sac tumor” and “primitive neuroectodermal tumor”. The search results were reviewed for eligible studies. When there was information in the abstract addressing cervical metastasis, the full-text article was searched. This was supplemented with a hand search of the references in relevant review articles and in all retrieved full-text articles (). Studies were selected if they met the following inclusion criteria: (a) patients with a CNS tumor with histological confirmation and (b) histological or cytological confirmation of cervical LNM. Studies without adequate evidence of the presence of a cervical LNM were excluded. Case reports were included. We considered the cases with metastases in the parotid gland as cervical LNM cases, although when compiling the data and presenting them in , the location in the parotid was specified within the rest of the metastatic sites presented by the patients. We include data from the previous review conducted by our group from 1944 to 2010 in order to complete the revision. No ethical approval was required.
Table 1 Literature Review of Studies Reporting Cervical LNM from CNS Tumors
Results
We identified 4182 papers but after applying our inclusion criteria, 143 were selected as summarized in .Citation8,Citation12–Citation152 Most papers did not show cervical LNM, so they were excluded. Our review includes a period of 78 years (1944–2021) with 172 cases and we included two additional cases of LNM of glioblastoma (GBM) from one of the coauthors [Gebrim EMMS] as personal communications. There were 75 (43.1%) GBM, 27 (15.5%) medulloblastoma, 21 (12.1%) meningioma (including anaplastic, metastasizing, sarcomatous and rhabdoid meningioma), 13 (7.5%) pituitary carcinoma, 12 (6.9%) oligodendroglioma (including anaplastic oligodendroglioma), 10 (5.7%) ependymoma (including anaplastic ependymoma), 7 (4%) astrocytoma (including malignant, anaplastic, and grade III–IV astrocytoma), 3 (1.7%) intracranial hemangiopericytoma and 6 (3.4%) other CNS tumors (including primitive neuroectodermal tumor, germ cell tumor, yolk sac tumor, germinoma and neuroblastoma). All cases had a previous history of CNS tumor.
If all reviewed cases were considered (174 cases), the mean age of the patients with cervical LNM from a CNS tumor was 31.9 years (range, 0.1–87) and there were 61 females (35.1%) and 103 males (59.2%), and in 10 cases (5.7%) the gender was not specified. The more frequent sites of distant metastasis were bones (23%), lungs (11.5%) and non-cervical lymph nodes (11%). In 15 cases (8.6%), metastases in the parotid gland lymph nodes were described, and in only one of them the metastasis was found in the glandular tissues too.Citation103
In the studied period, we have detected a large increase in the number of published cases, since in the previous review there were found 128 cases in 67 years (1944–2010), and in the current one there were 46 cases reported in 12 years (2010–2021).
The reported GBM patients had a mean age at diagnosis of the primary CNS tumor of 37.2 years (range, 4–75), there were 24 females (32%) and 49 males (65.3%). The most common extracranial site of metastases, other than cervical lymph nodes, was bones (22.7%), lungs (16%), parotid (12%), non-cervical lymph nodes of the body (12%) and liver (6.7%). Medulloblastoma patients had a mean age of 8.9 years (range, 0.6–31), there were 10 females (37%) and 16 males (59.3%). Other sites of metastases were more frequently observed in bones (37%), lungs (11.1%) and non-cervical lymph nodes (11.1%). Meningioma patients were more frequently male (66.7%). The mean age of the patients was 45.7 years (range, 5–87), and the more common other sites of metastasis were lungs (14.3%), parotid (9.5%) and spine (14.3%). Pituitary carcinomas were seen with more frequency in females (53.8%). Mean age at diagnosis was 48 years (range, 16–69) and the more common other site of metastasis was the liver (15.4%). Cervical LNM from oligodendrogliomas was described in 6 females (50%) and 3 men (25%), with a mean age of 37.8 years (range, 12–54). Other metastases were described in bones (66.7%), non-cervical lymph nodes (33.3%) and scalp (25%). Ependymoma patients comprised 6 females (60%) and 4 males (40%), with a mean age at diagnosis of 16.8 years (range, 6.7–30). Metastases were present at bones (30%), scalp (30%) and non-cervical lymph nodes, vertebrae and skull (10% each one). Astrocytoma patients had a mean age of 18.5 years (range, 3–42) and were predominantly men (71.4%). Other sites of metastases were more commonly observed in pleura and non-cervical lymph nodes (33.3% each one). Three cases of cervical LNM from an intracranial hemangiopericytoma were reported, but only one of them provided patient characteristics. The remaining 6 cases included different histologies and were grouped under the denomination “others”.
Discussion
Pathophysiology
When a patient presents with an adenopathy in the neck suspicious of metastasis, diagnostic efforts are focused on the most frequent locations of the primary tumor, among which are the upper aerodigestive tract and the thyroid gland. Rarely is the origin thought to be in more distant locations, ie, below clavicles and almost never in the primary CNS tumors. LNM from a CNS tumor should be suspected when the patient had history of such tumor, craniotomy and/or cranial irradiation.Citation31 Formation of hematogenous metastases have historically been explained by disruption of the normal anatomic barriers of spread (craniotomy or tumor invasion) allowing the tumor cells to enter the lumen of vascular vessels, dissemination using the Batson’s plexus, local recurrence in the craniotomy flap with posterior lymphovascular spread and by shunting procedures commonly used in this type of surgeries. The final location of metastasis after using these pathways is usually the lungs, bones and liver.Citation3 However, the mechanism by which CNS tumors spread via the lymphatic route is not known given the presumed absence of lymphatic vessels at the central level. In 2015, Aspelund et alCitation4 reported the finding of a lymphatic vessel network in the dura mater of the mouse brain. They explained that dural lymphatic vessels absorb cerebrospinal fluid from the adjacent subarachnoid space and brain interstitial fluid via the lymphatic system. They demonstrated that dural lymphatic vessels transport fluid into deep cervical lymph nodes via foramina at the base of the skull. These findings may explain why primary brain tumors can metastasize into cervical lymph nodes. More recently, Yağmurlu et alCitation6 described in humans the connection of the deep cervical lymph nodes and lymphatic tributaries with the intracranial space through the jugular foramen. In 2020, Song et alCitation5 using a mouse model of GBM, were able to demonstrate a limited CD8 T-cell immunity against GBM antigen when the tumor is confined to the CNS, leading to uncontrolled tumor growth. They observed that expression of VEGF-C promotes enhanced CD8 T-cell priming in deep neck lymph nodes, CD8 T-cell migration to the tumor and rapid clearance of the GBM.
Main Histologic Types
Primary tumors of the CNS can be classified as gliomas or nongliomas. The glioma group includes glioblastoma, oligodendrogliomas and ependymomas. The non-glioma group includes benign and malignant tumors: meningiomas, pituitary adenomas, medulloblastomas.Citation7
GBM is the most common and aggressive tumor in adults located in the brain.Citation153 GBM does not usually spread extracranially, and metastasis is rare.Citation154 We have found 75 cases of cervical LNM of GBM (), the most common other sites of GBM metastases were bones, lungs, parotid, and non-cervical lymph nodes. Although metastases at the parotid gland level were considered as LNM in the neck, parotid gland LNM was identified to provide insights on that location particularly. In the table, we have not included rare locations of distant metastasis showed in some articles to keep the table clear. Moreover, we considered that infrequent locations are important as a clinical case but not for the purposes of this review. Pietschmann et alCitation155 in their meta-analysis suggested that extraneural metastasis from GBM and gliosarcoma occurs more often in younger patients (median age in their cohort was 42 years), most probably due to longer expectancy. This is in concordance with our observation since the mean age of GBM patients in our series was 37.2 years.
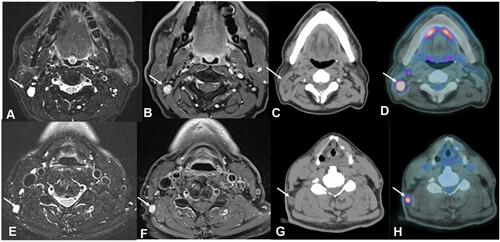
Figure 2 Male, 60 years old, cervical lymph node metastases (arrows) from a GBM. MRI axial T2-weighted sequences (A and E), MRI axial contrast-enhanced T1-weighted sequences (B and F), CT scans axial plane (C and G) and Fused Positron Emission Tomography- Computed Tomography axial plane (D and H). Observe the lymph nodes (arrows) in the right IIB (A–C) and V right level (E–G). Also, notice the increased uptake of fluorodeoxyglucose (FDG) in those lymph nodes (arrows in (D) and (H)).
Medulloblastoma represents the most common malignant brain tumor in the pediatric population.Citation156 Spread beyond the primary tumor is seen at the time of the diagnosis in more than 40% of cases. Extraneural metastasis affects more frequently bones, bone marrow, lungs, liver, and lymph nodes.Citation135
Meningioma is the most frequent primary CNS tumor.Citation157 These tumors are histologically divided into three grades.Citation158 The malignant transformation is possible producing a significant decrease in survival.Citation159 Although cervical node metastases from meningiomas are uncommon, 21 cases were reported according to our review.
Pituitary tumors are frequent, however, pituitary carcinoma is a rare histologic entity.Citation160 Diagnosis of pituitary carcinoma requires the presence of metastasis. Their low incidence makes it even more difficult to standardize diagnostic algorithm and treatment guidelines.Citation25
Oligodendroglioma is a diffuse glial tumor. Extracranial metastasis is infrequent, and it seems that metastasis is essentially linked to a prior neurosurgical resection.Citation161 Oligodendrogliomas are characterized by multiple recurrences; however, extraneural spread is unusual with the most frequent metastatic site being bones and bone marrow, followed by lymph nodes, liver and scalp,Citation32–Citation34 as was confirmed by our review.
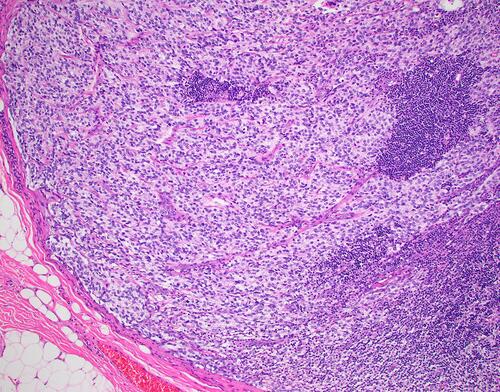
Ependymomas can arise in the spinal cord (adults) or intracranial (pediatrics).Citation162 The most aggressive is the anaplastic type () having an infiltrating behavior and the propensity to disseminate by seeding. Extraneural metastases are rare, but they affect more commonly the bones, parotid and scalp,Citation41–Citation44 as was confirmed also by our study.
Figure 3 Microphotograph demonstrating an anaplastic ependymoma within a cervical lymph node. (Hematoxylin and Eosin, X200).
Astrocytoma, intracranial hemangiopericytoma and the subgroup classified as “others”, rarely metastasize to cervical LNM. In fact, to our knowledge, there has been no published cases of cervical LNM of astrocytoma since 1988.
Diagnostic Work-Up and Treatment Considerations
When a cervical LNM of CNS tumor is suspected, due to the patient’s history for example, fine needle aspiration cytology (FNAC) can clarify the diagnosis.Citation18,Citation23,Citation33,Citation93,Citation106 Yet, without a precedent of a brain tumor, the underlying diagnosis can be difficult, due to the fact that the tumor may be similar to a sarcoma, carcinoma, or a hematopoietic neoplasm.Citation3 In dubious cases, histologic examination of tissue obtained with core biopsy of suspicious lymph node is sometimes necessary to obtain a reliable diagnosis. Several authors have stated that FNAC may be the method of choice to detect a lymph node metastasis in the neck from a CNS tumor, since it is a safe and minimally invasive procedure that can confirm the diagnosis and eliminates the need for a more aggressive core biopsy.Citation18,Citation23,Citation33,Citation93,Citation106 In the case of GBM, Gestrich et alCitation106 and Romero Rojas et alCitation93 made the diagnosis with a FNAC showing their cytomorphology as highly cellular, with pleomorphic cells arranged in small, loosely cohesive clusters and single cells. The nuclei are described as high nuclear to cytoplasmic ratios, coarsely clumped hyperchromatic chromatin, irregular nuclear membranes and prominent single or multiple nucleoli in the background of necrosis. Intranuclear and cytoplasmic inclusions and rare mitoses may be seen. The differential diagnosis includes melanoma, squamous cell carcinoma and poorly differentiated carcinoma.Citation106 For oligodendrogliomas, the differential diagnosis of primary neuroectodermal tumor and other small round cell tumors can be done when the cytopathologist is aware of the previous history of oligodendroglioma.Citation33 The tumor cells show round nuclei with prominent nucleoli. Fibrillary background varied and can have a cloudy appearance. Microcalcifications and a rich capillary network can help in the diagnosis, as well as necrosis, nuclear atypia, high cellularity, and mitosis in the case of anaplastic tumors.Citation32–Citation34 The cytologic differential diagnosis of the pituitary carcinoma includes hematopoietic malignancies and metastatic carcinomas.Citation23 The FNAC characteristics of these tumors include dispersed cells in small groups with a round to oval shape. Cells have a moderate amount of cytoplasm. Nuclear features range from minimal to marked nuclear pleomorphism with irregular nuclear contours, variable chromatin, and brisk mitoses.Citation18,Citation23 If the diagnosis cannot be made based on FNAC, a core needle biopsy or excisional biopsy is advised. Diagnosing metastatic CNS tumor is extremely challenging for pathologists. It is essential to have the clinical information of a previous CNS tumor, including the histologic type and immunophenotype. Without this information, a correct diagnosis is extremely difficult or even impossible to make. To confirm the diagnosis of a metastatic CNS tumor, immunohistochemistry must be used, for example, for GFAP, OLIG2, SOX2, etc. depending on immunophenotype of the primary tumor. However, one must keep in mind that patients with a history of a primary CNS tumor may suffer from other tumors. Moreover, markers believed to be characteristic for primary CNS tumors can be expressed in other tumor types, for example, GFAP or OLIG2 can be expressed in myoepithelial tumors, carcinomas and melanomas. Similarly, brain tumors can show aberrant expression of epithelial and other markers. A close cooperation between pathologists and clinicians, including all essential information is therefore needed to confirm the diagnosis of a metastatic CNS tumor.
Since cases of cervical lymphatic metastases from CNS tumors are rare, it is difficult to standardize treatment. Surgery is generally performed if the patient’s condition is suitable and neck mass(es) operable. However, other factors to be considered are operability of primary tumor, presence of distant metastases at other sites and cyto/histological confirmation. In the reports included in this review, the treatment performed spanned from open biopsy of the neck lesion to radical neck dissection, revealing enormous variability in the treatment of these lesions that prevents its standardization. Based on our results, we believe that the most reasonable approach to these lesions is get a diagnosis first, preferentially by FNAC of the node being suspicious based on the patient’s history; and secondly, if the patient condition allows, and in cases with isolated neck LNM without distant metastases (the use of positron emission tomography will be mandatory) and with primary tumor under control, the surgeon can remove all the affected lymph nodes with the least possible morbidity. Therefore, a selective or modified radical dissection would be the most appropriate. Open cervical biopsies should be avoided.
The increased number of cases in the last years (2010–2021) compared with the previous period (1944–2010) may be due to the advances in the treatment of these tumors, giving to the patients a higher chance to survive longer with a consequent increase in the risk to develop distant metastases. The low mean age of the patients in our review may also influence the life expectancy and the risk of distant relapse.
Conclusions
Cervical LNM from CNS tumors is rare. However, when the patient has a history of CNS primary tumor, this option should be considered when presented with a cervical mass. Pathologic diagnosis can be obtained by FNAC in most cases, giving surgeons the option to plan the appropriate surgical treatment. Given the poor prognosis of these cases, the most conservative possible cervical dissection is usually the treatment of choice.
Acknowledgments
This article was written by members and invitees of the International Head and Neck Scientific Group (www.IHNSG.com).
Disclosure
The authors report no conflicts of interest in this work.
References
- Dorobisz K, Wlodarska-Polinska I, Pazdro-Zastawny K, et al. The impact of the patient’s condition, diagnostic procedures and treatment on the survival of carcinoma of unknown primary site patients. Cancer Manag Res. 2019;11:6603–6614. doi:10.2147/CMAR.S204346
- López F, Rodrigo JP, Silver CE, et al. Cervical lymph node metastases from remote primary tumor sites. Head Neck. 2016;38(Suppl 1):E2374–E2385. doi:10.1002/hed.24344
- Mondin V, Ferlito A, Devaney KO, Woolgar JA, Rinaldo A. A survey of metastatic central nervous system tumors to cervical lymph nodes. Eur Arch Otorhinolaryngol. 2010;267(11):1657–1666. doi:10.1007/s00405-010-1357-1
- Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–999. doi:10.1084/jem.20142290
- Song E, Mao T, Dong H, et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020;577(7792):689–694. doi:10.1038/s41586-019-1912-x
- Yağmurlu K, Sokolowski JD, Çırak M, et al. Anatomical features of the deep cervical lymphatic system and intrajugular lymphatic vessels in humans. Brain Sci. 2020;10(12):953. doi:10.3390/brainsci10120953
- Buckner JC, Brown PD, O’Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82(10):1271–1286. doi:10.4065/82.10.1271
- Varan A, Sari N, Akalan N, et al. Extraneural metastasis in intracranial tumors in children: the experience of a single center. J Neurooncol. 2006;79(2):187–190. doi:10.1007/s11060-006-9123-3
- Giordana MT, Ghimenti C, Leonardo E, Balteri I, Iudicello M, Duò D. Molecular genetic study of a metastatic oligodendroglioma. J Neurooncol. 2004;66(3):265–271. doi:10.1023/B:NEON.0000014519.61604.da
- Anzil AP. Glioblastoma multiforme with extracranial metastases in the absence of previous craniotomy. Case report. J Neurosurg. 1970;33(1):88–94. doi:10.3171/jns.1970.33.1.0088
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi:10.1136/bmj.g7647
- Nowotny K, Kraus H, Zeitlhofer J. Zur Frage der extracranielien Metastasierung von Gliomen. [On the question of extracranial metastasis of gliomas]. Wien Z Nervenheilk. 1951;4:120–124. German.
- Komatsu K, Inaba Y, Hiratsuka H, Takahashi S, Kamisasa A. Extensive extra-cranial metastases of glioblastoma multiforme—a case report and clinicopathological observation on the cases in the literature. No to Shinkei. 1972;14:339–351.
- Scholz DA, Gastineau CF, Harrison EG. Cushing’s syndrome with malignant chromophobe tumor of the pituitary and extracranial metastasis: report of a case. Proc Staff Meet Mayo Clin. 1962;37:31–42.
- D’Abrera VS, Burke WJ, Bleasel KF, Bader L. Carcinoma of the pituitary gland. J Pathol. 1973;109:335–343. doi:10.1002/path.1711090408
- Luzi P, Miracco C, Lio R, et al. Endocrine inactive pituitary carcinoma metastasizing to cervical lymph nodes: a case report. Hum Pathol. 1987;18:90–92. doi:10.1016/S0046-8177(87)80200-9
- Mountcastle RB, Roof BS, Mayfield RK, et al. Pituitary adenocarcinoma in an acromegalic patient: response to bromocriptine and pituitary testing: a review of the literature on 36 cases of pituitary carcinoma. Am J Med Sci. 1989;298:109–118. doi:10.1097/00000441-198908000-00007
- Cartwright DM, Miller TR, Nasr AJ. Fine-needle aspiration biopsy of pituitary carcinoma with cervical lymph node metastases: a report of two cases and review of the literature. Diagn Cytopathol. 1994;11:68–73. doi:10.1002/dc.2840110116
- Jamjoom A, Moss T, Coakham H, Jamjoom ZAB, Anthony P. Cervical lymph nodes metastases from a pituitary carcinoma. Br J Neurosurg. 1994;8:87–92. doi:10.3109/02688699409002399
- Sreenan S, Sengupta E, Tormey W, Landau R. Metastatic pituitary carcinoma in a patient with acromegaly: a case report. J Med Case Rep. 2012;6:322. doi:10.1186/1752-1947-6-322
- Kovács GL, Góth M, Rotondo F, et al. ACTH-secreting Crooke cell carcinoma of the pituitary. Eur J Clin Invest. 2013;43(1):20–26. doi:10.1111/eci.12010
- Zemmoura I, Wierinckx A, Vasiljevic A, Jan M, Trouillas J, François P. Aggressive and malignant prolactin pituitary tumors: pathological diagnosis and patient management. Pituitary. 2013;16(4):515–522. doi:10.1007/s11102-012-0448-y
- Wang H, Liang J, Yong WH, Sullivan P. Metastatic pituitary carcinoma to cervical lymph node: diagnosis by fine needle aspiration and review of the literature. Acta Cytol. 2017;61(3):242–246. doi:10.1159/000467384
- el-Gindi S, Salama M, el-Henawy M, Farag S. Metastases of glioblastoma multiforme to cervical lymph nodes. Report of two cases. J Neurosurg. 1973;38(5):631–634. doi:10.3171/jns.1973.38.5.0631
- Yoo F, Kuan EC, Heaney AP, Bergsneider M, Wang MB. Corticotrophic pituitary carcinoma with cervical metastases: case series and literature review. Pituitary. 2018;21(3):290–301. doi:10.1007/s11102-018-0872-8
- James TGI, Pagel W. Oligodendroglioma with extracranial metastases. Br J Surg. 1951;39:56–65. doi:10.1002/bjs.18003915312
- Ordóñez NG, Ayala AG, Leavens ME. Extracranial metastases of oligodendroglioma: report of a case and review of the literature. NeuroSurgery. 1981;8:391–396. doi:10.1227/00006123-198103000-00012
- Macdonald DR, O’Brien RA, Gilbert JJ, Cairncross JG. Metastatic anaplastic oligodendroglioma. Neurology. 1989;39:1593–1596. doi:10.1212/WNL.39.12.1593
- Steininger H, von Streitberg U. Oligodendroglioma with cervical lymph node metastasis. Pathologe. 1993;14:386–390.
- Williams HK, Brown AM, Barber PC. Parotid gland metastases of two uncommon neoplasms with low metastatic potential. J Oral Pathol Med. 1997;26:388–392. doi:10.1111/j.1600-0714.1997.tb00237.x
- Volavsek M, Lamovec J, Popović M. Extraneural metastases of anaplastic oligodendroglial tumors. Pathol Res Pract. 2009;205(7):502–507. doi:10.1016/j.prp.2008.11.003
- Noshita N, Mashiyama S, Fukawa O, Asano S, Watanabe M, Tominaga T. Extracranial metastasis of anaplastic oligodendroglioma with 1p19q loss of heterozygosity—case report. Neurol Med Chir. 2010;50:161–164. doi:10.2176/nmc.50.161
- Can B, Akpolat I, Meydan D, Üner A, Kandemir B, Söylemezoğlu F. Fine-needle aspiration cytology of metastatic oligodendroglioma: case report and literature review. Acta Cytol. 2012;56(1):97–103. doi:10.1159/000331769
- Li G, Zhang Z, Zhang J, et al. Occipital anaplastic oligodendroglioma with multiple organ metastases after a short clinical course: a case report and literature review. Diagn Pathol. 2014;9:17. doi:10.1186/1746-1596-9-17
- Dolman CL. Lymph node metastasis as first manifestation of glioblastoma. Case report. J Neurosurg. 1974;41:607–609. doi:10.3171/jns.1974.41.5.0607
- Ferracini R, Manetto V, Poletti V, Giangasparo F. A cerebral ependymoma with extracranial metastases. Tumori. 1984;70:389–392. doi:10.1177/030089168407000416
- Wakabayashi T, Yoshida J, Kuchiwaki H, Kobayashi T, Kageyama N, Hashizume Y. Extraneural metastases of malignant ependymoma inducing atelectasis and superior vena cava syndrome–a case report and review of the literature. No Shinkei Geka. 1986;14:59–65.
- Dunst J, Klinger M, Thierauf P. Ependymoma with cervical lymph node metastases. Klin Padiatr. 1987;199:19–21. doi:10.1055/s-2008-1026752
- Kinoshita M, Izumoto S, Kagawa N, Hashimoto N, Maruno M, Yoshimine T. Long-term control of recurrent anaplastic ependymoma with extracranial metastasis: importance of multiple surgery and stereotactic radiosurgery procedures—case report. Neurol Med Chir. 2004;44:669–673. doi:10.2176/nmc.44.669
- Kumar P, Rastogi N, Jain M, Chhabra P. Extraneural metastases in anaplastic ependymoma. J Cancer Res Ther. 2007;3:102–104. doi:10.4103/0973-1482.34689
- Chao MM, Packer RJ, Myseros JS, Rood BR. Isolated extracranial recurrence of anaplastic ependymoma. Pediatr Blood Cancer. 2011;56:317–318. doi:10.1002/pbc.22764
- Davis MJ, Hasan F, Weinreb I, Wallace MC, Kiehl TR. Extraventricular anaplastic ependymoma with metastasis to scalp and neck. J Neurooncol. 2011;104(2):599–604. doi:10.1007/s11060-010-0525-x
- Pachella LA, Kamiya-Matsuoka C, Lee ELT, Olar A, Yung WKA. Supratentorial extraventricular anaplastic ependymoma with extracranial metastasis. J Clin Neurosci. 2015;22(3):605–607. doi:10.1016/j.jocn.2014.09.006
- Umbach G, El Ahmadieh TY, Plitt AR, et al. Extraneural metastatic anaplastic ependymoma: a systematic review and a report of metastases to bilateral parotid glands. Neurooncol Pract. 2020;7(2):218–227.
- Coffey JH. Cerebral astrocytoma metastatic to cervical lymph nodes. A case report. J Lancet. 1964;84:227–229.
- Nikkanen V, Törmä T, Kalimo H, Ojala A, Aärimaa T. Extracranial metastasis of glioblastoma multiforme in a child. Duodecim. 1974;90:1744–1748.
- Brust JMC, Moiel RH, Rosenberg RH. Glial tumor metastases through a ventriculopleural shunt. Resultant massive pleural effusion. Arch Neurol. 1968;18:649–653. doi:10.1001/archneur.1968.00470360071007
- Dietz R, Burger L, Merkel KSK, Schimrigk K. Malignant gliomas—glioblastoma multiforme and astrocytoma III–IV with extracranial metastases. Acta Neurochir (Wien). 1981;57:99–105. doi:10.1007/BF01665120
- Hoffman HJ, Becker LE, Jenkin D, Chuang SH, Munro IR. Extraneural metastases of a cerebral astrocytoma. Can J Neurol Sci. 1981;8:115–119. doi:10.1017/S0317167100043006
- Rosemberg S, Lopes MB, Elks L, Teixeira MJ, Serrano VA. Extraneural metastasis of a brainstem astrocytoma in a child: clinicopathological report. Clin Neuropathol. 1988;7:131–133.
- Gill SS, Bharadwaj R. Cytomorphologic findings of hemangiopericytoma of the meninges: a case report. Indian J Pathol Microbiol. 2007;50:422–425.
- Chamberlain MC, Glantz MJ. Sequential salvage chemotherapy for recurrent intracranial hemangiopericytoma. Neurosurgery. 2008;63:720–726. doi:10.1227/01.NEU.0000325494.69836.51
- Henriquez AS, Robertson DM, Marshall WJ. Primary neuroblastoma of the central nervous system with spontaneous extracranial metastases. Case report. J Neurosurg. 1973;38:226–231. doi:10.3171/jns.1973.38.2.0226
- Takeuchi J, Handa H. Spontaneous extracranial metastasis of cerebral neuroblastoma. Surg Neurol. 1979;12:337–339.
- Rubery ED, Wheeler TK. Metastases outside the central nervous system from a presumed pineal germinoma. Case report. J Neurosurg. 1980;53:562–565. doi:10.3171/jns.1980.53.4.0562
- Yao YT, Lin WH, Hung CC, Liu KN. A case of glioblastoma multiforme with extracranial metastasis. Taiwan Yi Xue Hui Za Zhi. 1975;74(3):220–228.
- Campbell AN, Chan HS, Becker LE, Daneman A, Park TS, Hoffman HJ. Extracranial metastases in childhood primary intracranial tumors. A report of 21 cases and review of the literature. Cancer. 1984;53:974–981. doi:10.1002/1097-0142(19840215)53:4<974::AID-CNCR2820530426>3.0.CO;2-C
- Yu IT, Ho DM, Wong TT, Liu HC. Congenital cerebral primitive neuroectodermal tumor with astrocytic differentiation and extracranial metastases. Childs Nerv Syst. 1990;6:179–182. doi:10.1007/BF00308497
- Shen WC, Ho YJ, Lee SK, Lee KR. Intracranial germ cell tumors. Zhonghua Yi Xue Za Zhi (Taipei). 1992;49:354–364.
- Montaut J, Metaizeau JP, Gerbaux A, Renard M. Extracranial metastasis of primary brain tumours. Neurochirurgie. 1976;22:653–669.
- Schuster H, Jellinger K, Gund A, Regele H. Extracranial metastases of anaplastic cerebral gliomas. Acta Neurochir (Wien). 1976;35:247–259. doi:10.1007/BF01406121
- O’Conner W, Challa V, Nelson O, Gotlieb S. Extracranial metastases of glioblastoma multiforme confirmed by electron microscopy. Surg Neurol. 1977;8:347–349.
- Konovalov OV, Petrova SM. Brain glioblastoma with metastases into the cervical and lumbar lymph nodes. Arkh Patol. 1979;41:68–70.
- Pasquier B, Pasquier D, N’Golet A, Panh MH, Couderc P. Extraneural metastases of astrocytomas and glioblastomas: clinicopathological study of two cases and review of literature. Cancer. 1980;45:112–125. doi:10.1002/1097-0142(19800101)45:1<112::AID-CNCR2820450121>3.0.CO;2-9
- Zeitlhofer J, Kranus H. Extracranial metastases of glioma. Zentralbl Neurochir. 1952;12:347–356.
- Potter CR, Kaufman R, Page RB, Chung C. Glioblastoma multiforme metastatic to the neck. Am J Otolaryngol. 1983;4:74–76. doi:10.1016/S0196-0709(83)80007-6
- Shinmura F, Chen M, Itoh T, Ariwa R. An autopsy case of extraneural metastases of giant cell glioblastoma with intracerebral hemorrhage. No Shinkei Geka. 1985;13:1245–1250.
- Steinbok P, Dolman CL, Goldie JH. Variation in response to CCNU of glioblastoma multiforme in brain and cervical lymph node. Case report. J Neurosurg. 1985;62:918–921. doi:10.3171/jns.1985.62.6.0918
- Trattnig S, Schindler E, Ungersböck K, et al. Extra-CNS metastases of glioblastoma: CT and MR studies. J Comput Assist Tomogr. 1990;14:294–296. doi:10.1097/00004728-199003000-00026
- Zappia JJ, Wolf GT. Cervical metastatic glioblastoma multiforme. Arch Otolaryngol Head Neck Surg. 1992;118:755–756. doi:10.1001/archotol.1992.01880070085016
- González Cámpora R, Otal Salaverri C, Vázquez Ramirez F, Salguero Villadiego M, Galera Davidson H. Metastatic glioblastoma multiforme in cervical lymph nodes report of a case with diagnosis by fine needle aspiration. Acta Cytol. 1993;37:938–942.
- Gezen F, Baysefer A, Elçi H, Can C, Çanakçi Z, Seber N. Extraneural metastasis of glioblastoma multiforme. Case report. Turk Neurosurg. 1994;4:132–135.
- Chrétien F, Gray F, Funalot B, et al. Extracerebral metastases of a glioblastoma, in the absence of surgery. Arch Anat Cytol Pathol. 1995;43:342–349.
- Vural G, Hagmar B, Walaas L. Extracranial metastasis of glioblastoma multiforme diagnosed by fine-needle aspiration: a report of two cases and a review of the literature. Diagn Cytopathol. 1996;15:60–65. doi:10.1002/(SICI)1097-0339(199607)15:1<60::AID-DC12>3.0.CO;2-A
- Wallace CJ, Forsyth PA, Edwards DR. Lymph node metastases from glioblastoma multiforme. AJNR Am J Neuroradiol. 1996;17:1929–1931.
- Garret R. Glioblastoma and fibrosarcoma of the brain with extracranial metastases. Cancer. 1958;11:888–894. doi:10.1002/1097-0142(195809/10)11:5<888::AID-CNCR2820110504>3.0.CO;2-T
- Jamjoom AB, Jamjoom ZAB, Ur-Rahman N, Al-Rikabi AC. Cervical lymph node metastasis from a glioblastoma multiforme in a child: report of a case and a review of the literature. Ann Saudi Med. 1997;17:340–343. doi:10.5144/0256-4947.1997.340
- Datta CK, Weinstein JD, Bland JE, Brager PM, Stewart MA. A case of cervical lymph node metastasis resulting from glioblastoma multiforme. W V Med J. 1998;94:276–278.
- Park CC, Hartmann C, Folkerth R, et al. Systemic metastasis in glioblastoma may represent the emergence of neoplastic subclones. J Neuropathol Exp Neurol. 2000;59:1044–1050. doi:10.1093/jnen/59.12.1044
- Hübner F, Braun V, Richter HP. Case reports of symptomatic metastases in four patients with primary intracranial gliomas. Acta Neurochir (Wien). 2001;143:25–29. doi:10.1007/s007010170134
- Ates LE, Bayindir C, Bilgic B, Karasu A. Glioblastoma with lymph node metastases. Neuropathology. 2003;23:146–149. doi:10.1046/j.1440-1789.2003.00490.x
- Ueda S, Mineta T, Suzuyama K, Furuta M, Shiraishi T, Tabuchi K. Biologic characterization of a secondary glioblastoma with extracranial progression and systemic metastasis. Neuro Oncol. 2003;5:14–18. doi:10.1093/neuonc/5.1.14
- Moon KS, Jung S, Lee MC, et al. Metastatic glioblastoma in cervical lymph node after repeated craniotomies: report of a case with diagnosis by fine needle aspiration. J Korean Med Sci. 2004;19:911–914. doi:10.3346/jkms.2004.19.6.911
- Taha M, Almad A, Wharton S, Jellinek D. Extra-cranial metastasis of glioblastoma multiforme presenting as acute parotitis. Br J Neurosurg. 2005;19:348–351. doi:10.1080/02688690500305506
- Piccirilli M, Brunetto GM, Rocchi G, Giangaspero F, Salvati M. Extra central nervous system metastases from cerebral glioblastoma multiforme in elderly patients. Clinico-pathological remarks on our series of seven cases and critical review of the literature. Tumori. 2008;94:40–51. doi:10.1177/030089160809400109
- Frank S, Kuhn SA, Brodhun M, et al. Metastatic glioblastoma cells use common pathways via blood and lymphatic vessels. Neurol Neurochir Pol. 2009;43:183–190.
- Brodskaia IA. A case of extracranial metastasis of glioblastoma. Arkh Patol. 1960;22:78–80.
- Ulitin AI, Zabrodskaia IM, Oliushin VE, et al. Metastatic glioblastoma in a submandibular lymph node (a rare case). Vopr Onkol. 2009;55:230–236.
- Zhen L, Yufeng C, Zhenyu S, Lei X. Multiple extracranial metastases from secondary glioblastoma multiforme: a case report and review of the literature. J Neurooncol. 2010;97:451–457. doi:10.1007/s11060-009-0044-9
- Seo YJ, Cho WH, Kang DW, Cha SH. Extraneural metastasis of glioblastoma multiforme presenting as an unusual neck mass. J Korean Neurosurg Soc. 2012;51(3):147–150. doi:10.3340/jkns.2012.51.3.147
- Snopkowska-Wiaderna D, Zieliński KW, Radek M, Papierz W. Extracerebral metastases of glioblastoma have a different vasculature than primary tumour. A case report of glioblastoma extracranial metastases. Folia Neuropathol. 2012;50(4):413–416. doi:10.5114/fn.2012.32376
- Mujtaba SS, Haroon S, Faridi N. Cervical metastatic glioblastoma multiforme. J Coll Physicians Surg Pak. 2013;23(2):160–161.
- Romero-Rojas AE, Diaz-Perez JA, Amaro D, Lozano-Castillo A, Chinchilla-Olaya SI. Glioblastoma metastasis to parotid gland and neck lymph nodes: fine-needle aspiration cytology with histopathologic correlation. Head Neck Pathol. 2013;7(4):409–415. doi:10.1007/s12105-013-0448-x
- Taskapilioglu MO, Aktas U, Eser P, Tolunay S, Bekar A. Multiple extracranial metastases from secondary glioblastoma: a case report and review of the literature. Turk Neurosurg. 2013;23(6):824–827. doi:10.5137/1019-5149.JTN.6497-12.1
- Anghileri E, Elena A, Castiglione M, et al. Extraneural metastases in glioblastoma patients: two cases with YKL-40-positive glioblastomas and a meta-analysis of the literature. Neurosurg Rev. 2016;39(1):37–45;discussion 45–6. doi:10.1007/s10143-015-0656-9
- Wassati H, Loo SW, Low HL. Lymphatic metastasis due to glioblastoma. Neurosci (Riyadh). 2016;21(2):168–169.
- Xu M, Wang Y, Xu J, Yao Y, Yu WX, Zhong P. Extensive therapies for extraneural metastases from glioblastoma, as confirmed with the OncoScan assay. World Neurosurg. 2016;90:698.e7–698.e11. doi:10.1016/j.wneu.2016.01.074
- Ley A, Campillo D, Oliveras C. Extracranial metastases of glioblastoma multiforme. J Neurosurg. 1961;18:313–330. doi:10.3171/jns.1961.18.3.0313
- Sun Q, Xu R, Xu H, Wang G, Shen X, Jiang H. Extracranial metastases of high-grade glioma: the clinical characteristics and mechanism. World J Surg Oncol. 2017;15(1):181. doi:10.1186/s12957-017-1249-6
- Wu W, Zhong D, Zhao Z, Wang W, Li J, Zhang W. Postoperative extracranial metastasis from glioblastoma: a case report and review of the literature. World J Surg Oncol. 2017;15(1):231. doi:10.1186/s12957-017-1300-7
- Hori YS, Fukuhara T, Aoi M, Oda K, Shinno Y. Extracranial glioblastoma diagnosed by examination of pleural effusion using the cell block technique: case report. Neurosurg Focus. 2018;44(6):E8. doi:10.3171/2017.8.FOCUS17403
- Ilangovan VS, Kumar VRR, Sankaran V, Bapu KRS, Kapilavayi S. Aggressive brainstem glioblastoma in a 9-year-old child with neck node metastases: a case report and review of literature. J Pediatr Neurosci. 2018;13(2):234–236. doi:10.4103/JPN.JPN_160_17
- Jie W, Bai J, Li B. An extracranial metastasis of glioblastoma mimicking mucoepidermoid carcinoma. World Neurosurg. 2018;116:352–356. doi:10.1016/j.wneu.2018.05.132
- Swinnen J, Gelin G, Fransis S, Vandevenne J, Van Cauter S. Glioblastoma with extracranial parotid, lymph node, and pulmonary metastases: a case report. Radiol Case Reports. 2019;14(11):1334–1347. doi:10.1016/j.radcr.2019.08.011
- Alhoulaiby S, Abdulrahman A, Alouni G, Mahfoud M, Shihabi Z. Extra-CNS metastasis of glioblastoma multiforme to cervical lymph nodes and parotid gland: a case report. Clin Case Rep. 2020;8(9):1672–1677. doi:10.1002/ccr3.2985
- Gestrich C, Cowden D, Harbhajanka A. Cytomorphology of glioblastoma metastic to a cervical lymph node diagnosed by fine needle aspiration (FNA): a case report and review of literature. Diagn Cytopathol. 2020;48(6):567–570. doi:10.1002/dc.24412
- Rodrigues L, Camacho A, Spohr C. Secondary glioblastoma metastasis outside the central nervous system in a young HIV-infected patient. Ther Adv Med Oncol. 2020;12:1758835920923432. doi:10.1177/1758835920923432
- Rossi J, Giaccherini L, Cavallieri F, et al. Extracranial metastases in secondary glioblastoma multiforme: a case report. BMC Neurol. 2020;20(1):382. doi:10.1186/s12883-020-01959-y
- Labitzke HG. Glioblastoma multiforme with remote extracranial metastases. Arch Pathol. 1962;73:223–229.
- Eftekhar Javadi A, Moradi Tabriz H, Zandnejadi A. Postoperative extra-cranial metastasis of glioblastoma: a case report. Iran J Pathol. 2021;16(1):90–94. doi:10.30699/ijp.2020.122780.2349
- Noch EK, Sait SF, Farooq S, Trippett TM, Miller AM. A case series of extraneural metastatic glioblastoma at Memorial Sloan Kettering Cancer Center. Neurooncol Pr. 2021;8(3):325–336.
- Oktay K, Yildirim DC, Acikalin A, Ozsoy KM, Cetinalp NE, Erman T. Extensive extraneural metastases of cerebral glioblastoma in a pediatric patient: an extreme case report and comprehensive review of the literature. Pediatr Neurosurg. 2021;56(3):300–305. doi:10.1159/000515348
- Schwock J, Mirham L, Ghorab Z. Cytology of extraneural metastases of nonhematolymphoid primary central nervous system tumors: six cases with histopathological correlation and literature update. Acta Cytol. 2021;65:529–540. doi:10.1159/000517480
- Buchmann E, Zuelch KJ. On the problem of the postoperative survival period and remote metastases in cerebellar medulloblastoma. Acta Neurochir (Wien). 1959;7:263–273. doi:10.1007/BF01405891
- Rubinstein LJ. Extracranial metastases in cerebellar medulloblastoma. Am J Pathol Bacteriol. 1959;78:187–195. doi:10.1002/path.1700780120
- Paterson E. Distant metastases from medulloblastoma of the cerebellum. Brain. 1961;84:301–309. doi:10.1093/brain/84.2.301
- Oberman HA, Hewitt WC, Kalivoda J. Medulloblastomas with distant metastases. Am J Clin Pathol. 1963;39:148–160. doi:10.1093/ajcp/39.2.148
- Rubinstein LJ, Northfield DW. Medulloblastoma and the so called “arachnoidal cerebellar sarcoma”: critical re-examination of a nosological problem. Brain. 1964;87:379–412. doi:10.1093/brain/87.2.379
- Gyepes MT, D’Angio GJ. Extracranial metastases from central nervous system tumors in children and adolescents. Radiology. 1966;86:55–63. doi:10.1148/87.1.55
- Schejbal V. Metastasizing glioma in childhood. Neoplasma (Bratisl). 1962;9:585–592.
- Van Epps RR, Samuelson DR, McCormick WF. Cerebral medulloepithelioma. Case report. J Neurosurg. 1967;27(6):568–573. doi:10.3171/jns.1967.27.6.0568
- Liwnicz BH, Rubinstein LJ. The pathways of extraneural spread in metastasizing gliomas: a report of three cases and critical review of the literature. Hum Pathol. 1979;10:453–467. doi:10.1016/S0046-8177(79)80051-9
- Naruse S, Horikawa Y, Yamaki T, Odake G, Tohyama M, Hirakawa K. Medulloblastoma with extracranial metastases–a case report (author’s transl). No Shinkei Geka. 1979;7:1005–1010.
- McComb JG, Davis RL, Isaacs HJ, Landing BH. Medulloblastoma presenting as neck tumors in 2 infants. Ann Neurol. 1980;7:113–117. doi:10.1002/ana.410070204
- Lowery GS, Kimball JC, Patterson RB, Raben M. Extraneural metastases from cerebellar medulloblastoma. Am J Pediatr Hematol Oncol. 1982;4:259–262.
- Morild I, Mørk S, Nyland H. Metastasizing neuroectodermal tumour. J Neurol. 1982;227:151–155. doi:10.1007/BF00313569
- Berger MS, Baumeister B, Geyer JR, Milstein J, Kanev PM, LeRoux PD. The risks of metastases from shunting in children with primary central nervous system tumors. J Neurosurg. 1991;74:872–877. doi:10.3171/jns.1991.74.6.0872
- Watterson J, Simonton SC, Rorke LB, et al. Fatal brain stem necrosis after standard posterior fossa radiation and aggressive chemotherapy for metastatic medulloblastoma. Cancer. 1993;71:4111–4117. doi:10.1002/1097-0142(19930615)71:12<4111::AID-CNCR2820711250>3.0.CO;2-4
- Sheikh B, Kanaan I. Lymph node metastasis in medulloblastoma. Pediatr Neurosurg. 1994;20:269–271. doi:10.1159/000120801
- Kochbati L, Bouaouina N, Hentati D, et al. Medulloblastoma with extracentral nervous system metastases: clinical presentation and risk factors. Cancer Radiother. 2006;10:107–111. doi:10.1016/j.canrad.2006.02.004
- Smith DR, Hardman JM, Earle KM. Metastasizing neuroectodermal tumors of the central nervous system. J Neurosurg. 1969;31:50–58. doi:10.3171/jns.1969.31.1.0050
- Frankel A, Lau Q, Olson S. Lymph node metastasis of medulloblastoma in a young adult. J Clin Neurosci. 2009;16:1485–1486. doi:10.1016/j.jocn.2009.01.012
- Matalka I, Alorjani M, Kanaan F, Al-Hussaini M. Medulloblastoma in an adult with cervical lymph node metastasis: a case report and review of the literature. Pathology. 2009;41:197–199. doi:10.1080/00313020802579326
- Mahajan A, Paul P, Sridhar E, et al. Extraneural metastases from desmoplastic medulloblastoma masquerading as lymphoma. Clin Nucl Med. 2017;42(5):354–357. doi:10.1097/RLU.0000000000001610
- Ataş E, Varan A, Akyüz C, Akalan N, Büyükpamukçu M. Cervical extraneural lymph node metastasis in a patient with medulloblastoma. Pediatr Neurosurg. 2019;54(1):71–73. doi:10.1159/000494930
- Globus JH, Levin S, Sheps JG. Primary sarcomatous meningioma (primary sarcoma of the brain). J Neuropathol Exp Neurol. 1944;3:311–343. doi:10.1097/00005072-194410000-00001
- Laymon CW, Becker FT. Massive metastasizing meningioma involving the scalp. Arch Derm Syphilol. 1949;59:626–635. doi:10.1001/archderm.1949.01520310033004
- Lima A. Metastase cervical de um meningioma parassaggital. Rev Esp Otoneurooftalmol. 1951;10:313–316.
- Paparo F, Pasetti A. Le metastasi extra-craniche da meningiomi. Lav Neuropsichiat. 1955;17:317–330.
- Opsahl RJ, Löken AC. Meningioma with metastases to cervical lymph nodes: case report. Acta Pathol Microbiol Scand. 1965;64:294–298. doi:10.1111/apm.1965.64.3.294
- Postma JU. A case of metastasizing meningioma? Psychiatr Neurol Neurochir. 1967;70:245–259.
- Dalmer J, Burgold H. Ungewoehnliche wachstumsform und metastasierung eines glioblasoms. Psychiatr Neurol Med Psychol. 1971;23:167–174.
- Guszowski K, Gruszka A, Sośnik H. Primary meningeal sarcoma with extracranial metastases. Neurol Neurochir Pol. 1971;5:753–757.
- Kollmannsberger A, Kazner E, Prechtel K, Stochdorph O. Extracranial metastasis of meningeal tumors. Malignant meningioma with regional lymph node metastasis. Zentralbl Neurochir. 1975;36:27–36.
- Leighton SE, Rees GL, McDonald B, Alun-Jones T. Metastatic meningioma in the neck. J Laryngol Otol. 1991;105:229–231. doi:10.1017/S0022215100115464
- Chamberlain MC, Glantz MJ. Cerebrospinal fluid-disseminated meningioma. Cancer. 2005;103:1427–1430. doi:10.1002/cncr.20926
- Doxtader EE, Butts SC, Holsapple JW, Fuller CE. Aggressive pediatric meningioma with soft tissue and lymph node metastases: a case report. Pediatr Dev Pathol. 2009;12:244–248. doi:10.2350/08-07-0501.1
- Omidvari S, Nasrolahi H, Daneshbod Y, et al. Cervical lymph node metastases from meningioma: report of two cases and treatment outcome. Middle East J Cancer. 2010;1(1):45–49. Available from: http://mejc.sums.ac.ir/article_41920.html%0Ahttp://mejc.sums.ac.ir/article_41920_20bedf18d9807ffd1383404446d481c2.pdf.
- Herbst A, Mueller-Vogt U, Brawanski A, Proescholdt M, Riemenschneider M, Schebesch KM. WHO grade III anaplastic meningioma metastasizing to the parotid gland and the lungs: case report and review of the literature. Cent Eur Neurosurg. 2011;72(S 01):e45.
- Moubayed SP, Guertin L, Lambert C, Desrochers P, Nehmé J, Coulombe G. Successful treatment of anaplastic meningioma metastatic to cervical lymph nodes. Head Neck. 2013;35(4):E115–E118. doi:10.1002/hed.21938
- Parameshwaran Nair R, Vinod SY, Nayal B, Kaur Dil S, Tripathi PK, Tripathi PK. Metastatic rhabdoid meningioma of the parotid - Mimicking primary salivary gland neoplasm. Int J Surg Case Rep. 2015;6:104–106. doi:10.1016/j.ijscr.2014.10.048
- Nguyen D, Duong N, Tran T. Rhabdoid meningioma metastases cervical lymph nodes: a rare clinical case report and treatment outcome. J Investig Med High Impact Case Rep. 2021;9:23247096211029788.
- Beauchesne P. Extra-neural metastases of malignant gliomas: myth or reality? Cancers (Basel). 2011;3(1):461–477. doi:10.3390/cancers3010461
- Lun M, Lok E, Gautam S, Wu E, Wong ET. The natural history of extracranial metastasis from glioblastoma multiforme. J Neurooncol. 2011;105(2):261–273. doi:10.1007/s11060-011-0575-8
- Pietschmann S, von Bueren AO, Kerber MJ, Baumert BG, Kortmann RD, Müller K. An individual patient data meta-analysis on characteristics, treatments and outcomes of glioblastoma/ gliosarcoma patients with metastases outside of the central nervous system. PLoS One. 2015;10(4):e0121592. doi:10.1371/journal.pone.0121592
- Rossi A, Caracciolo V, Russo G, Reiss K, Giordano A. Medulloblastoma: from molecular pathology to therapy. Clin Cancer Res. 2008;14(4):971–976. doi:10.1158/1078-0432.CCR-07-2072
- Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Suppl 5):v1–v100. doi:10.1093/neuonc/noz150
- Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James CD, Lukas RV. An overview of meningiomas. Future Oncol. 2018;14(21):2161–2177. doi:10.2217/fon-2018-0006
- Lim YS, Kim MK, Park BJ, Kim TS, Lim YJ. Long term clinical outcomes of malignant meningiomas. Brain Tumor Res Treat. 2013;1(2):85–90. doi:10.14791/btrt.2013.1.2.85
- Heaney AP. Clinical review: pituitary carcinoma: difficult diagnosis and treatment. J Clin Endocrinol Metab. 2011;96(12):3649–3660. doi:10.1210/jc.2011-2031
- Shaw EG, Scheithauer BW, O’Fallon JR, Tazelaar HD, Davis DH. Oligodendrogliomas: the Mayo Clinic experience. J Neurosurg. 1992;76(3):428–434. doi:10.3171/jns.1992.76.3.0428
- Gerstner ER, Pajtler KW. Ependymoma. Semin Neurol. 2018;38(1):104–111. doi:10.1055/s-0038-1636503
- Cooper PR, Budzilovich GN, Berczeller PH, Lieberman A, Battista A. Metastatic glioma associated with hypercalcemia. Report of two cases. J Neurosurg. 1974;40(2):255–259. doi:10.3171/jns.1974.40.2.0255