Abstract
Hepatocellular carcinoma (HCC) is a severe complication of advanced liver disease with a worldwide incidence of more than 600,000 patients per year. Liver function, clinical performance status, and tumor size are considered in the Barcelona Clinic Liver Cancer (BCLC) system. While curative treatment options are available for early stages, most patients present with intermediate- or advanced-stage HCC, burdened with a poor prognosis, substantially influenced by the degree of liver-function impairment. Hypervascularization is a major characteristic of HCC, and antiangiogenic treatments are the basis of treatment in noncurative stages, including interventional and pharmacological treatments. Currently, the tyrosine-kinase inhibitor sorafenib is still the only approved drug for HCC. Further improvements in survival in patients with intermediate- and advanced-stage HCC may be anticipated by both multimodal approaches, such as combination of interventional and systemic treatments, and new systemic treatment options. Until now, the Phase III development of other tyrosine-kinase inhibitors in patients with advanced HCC has failed due to minor efficacy and/or increased toxicity compared to sorafenib. However, promising Phase II data have been reported with MET inhibitors in this hard-to-treat population. This review gives a critical overview of antiangiogenic drugs and strategies in intermediate- and advanced-stage HCC, with a special focus on safety.
Keywords:
Introduction
The worldwide incidence of hepatocellular carcinoma (HCC) exceeds 600,000 patients per year, and is still rising.Citation1 An important characteristic of HCC is the predominant occurrence in liver cirrhosis and advanced chronic liver disease.Citation1 This explains why overall prognosis remains poor, as survival may depend on impaired liver function rather than tumor progression in some patients, and therapeutic options often are limited by potential hepatotoxicity.Citation1,Citation2
The Barcelona Clinic Liver Cancer (BCLC) therapeutic algorithm takes this into account by combining tumor stage, clinical performance status, and liver function to stratify prognosis and treatment.Citation3,Citation4 Early stages (BCLC 0 and BCLC A) are characterized by limited tumor size and preserved liver function, while intermediate- (BCLC B), advanced- (BCLC C), and end-stage (BCLCD) cancer are defined by extended tumor size and decreased liver function. Consequently, surgical (resection or transplantation) or percutaneous thermal therapies (radiofrequency or microwave ablation) are mainly considered suitable for the early stage, while interventional therapies (transarterial chemo- or radioembolization) are applied in patients with intermediate-stage HCC. Systemic treatment with the tyrosine-kinase inhibitor sorafenib is considered the treatment of choice for patients with advanced-stage HCC. Patients with BCLC stage D do not benefit from cancer treatment, and thus are being considered for best supportive care only. Thus, recent strategies have focused on the establishment of new drugs for patients with advanced-stage HCC. Moreover, selected current trials focus on adjuvant pharmacological treatment options in early stage HCC or combination of interventional therapies and sorafenib in intermediate-stage HCC.
The development of efficient new drugs in HCC is challenged by the need for a safety profile, defined by low or absent hepatotoxicity and nephrotoxicity. Moreover, putative accumulation of the agent and its metabolites in patients with impaired liver and/or kidney function has to be taken into account and must be avoided.
Theoretically, HCC should be prone to inhibition of angiogenesis because it is a highly vascular tumor, and hypervascularization is an essential characteristic of HCC, closely linked to carcinogenesis and progression.Citation5–Citation7 Indeed, antiangiogenic treatment of HCC, either by mechanical destruction of arterial tumor vessels after transarterial chemoembolization (TACE) or by pharmacological inhibition with the dual-kinase inhibitor sorafenib, which is still the only systemic agent approved for HCC, is the current basis of noncurative approaches in HCC.Citation8–Citation12 So far, antiangiogenic tyrosine-kinase inhibitors other than sorafenib have failed in randomized placebo-controlled pivotal trials, due to either minor efficacy or unacceptable toxicity profiles. This review gives a critical overview of established antiangiogenic drugs and those currently being developed, and strategies with special focus on safety in intermediate- and advanced-stage HCC.
Angiogenesis in liver cirrhosis and HCC
Angiogenesis is closely related to chronic hepatitis and hepatic fibrogenesis, which in turn may lead to liver cirrhosis and HCC. The vascular endothelial growth-factor (VEGF) pathway was identified as the major driver in tumor angiogenesis. However, activation and/or upregulation of abundant proangiogenic signaling pathways may lead to resistance to VEGF-based antiangiogenic therapy, reinducing tumor angiogenesis and subsequently resulting in tumor progression.Citation5 VEGF is crucially involved in angiogenesis, as well as in fibrogenesis in chronic liver disease, but other cytokines, growth factors, and metalloproteinases are additionally involved in these processes.Citation13 HCC nodules larger than 2 cm typically show early arterial enhancement, a surrogate of hypervascularization, which is pathognomonic for HCC.Citation6,Citation7 In patients with HCC, higher VEGF serum levels were associated with poor outcome in the majority of but not all studies addressing this issue.Citation14–Citation19 Moreover, increased expression of angiopoietin 1/2 messenger RNA in tumor tissue, another proangiogenic factor, has been reported in patients with HCC.Citation20 Therefore, it may be concluded that angiogenesis in HCC is a complex process and most likely heterogeneous.
Sorafenib in advanced hepatocellular carcinoma
The proof of concept that pharmacological inhibition of angiogenesis is clinically meaningful in HCC was provided by four clinical trials showing consistently a survival benefit of approximately 3 months in patients with advanced HCC and preserved liver function treated with sorafenib, which is still the only systemic agent approved for advanced HCC.Citation21–Citation24 Sorafenib is a multikinase inhibitor with activity against VEGF receptor (VEGFR)-2, platelet-derived growth-factor receptor (PDGFR), receptor of the tyrosine kinase c-Kit, rapidly accelerated fibrosarcoma B kinase, and mitogen-activated protein kinase p38 signal-transduction pathways, which seem to be involved in the pathogenesis of HCC.Citation8 The main effect of sorafenib is disease stabilization, and sorafenib can be used with an acceptable safety profile under daily practice conditions.Citation25,Citation26 However, adverse effects – mainly fatigue, diarrhea, and hand–foot syndrome – may significantly alter quality of life and may lead to dose reduction of sorafenib.Citation21–Citation26 Within a recent Phase II study, dose escalation of sorafenib was not superior to best supportive care in patients with advanced HCC and disease progression during sorafenib 400 mg twice daily, while adverse events (diarrhea 80%, weight loss 75%, fatigue 67%, hand–foot skin reaction 49%, abdominal pain 37%, stomatitis 26%) were common.Citation27
Antiangiogenic drugs in clinical development
A consequent step of antiangiogenic drug development was to investigate tyrosine-kinase inhibitors with other or additional targets than sorafenib in HCC. Sunitinib, a tyrosine-kinase inhibitor targeting the tyrosine kinase Kit, PDGFR-α and -β, and VEGFR1, -2, and -3, was compared to sorafenib as first-line treatment of advanced HCC in the SUN1170 trial.Citation28 This trial was terminated early because of a higher rate of drug-related adverse events in the sunitinib arm, including fatal outcomes. Overall survival in patients taking sunitinib was 7.9 months compared to 10.2 months in the sorafenib arm. Linifanib, a selective VEGFR and PDGFR tyrosine-kinase inhibitor, was also investigated in first-line treatment of advanced HCC compared to sorafenib.Citation29 Linifanib was less effective than sorafenib, with a median overall survival of 9.1 months compared to 9.8 months in the sorafenib arm. A comparison of overall survival in current head-to-head Phase III studies investigating sorafenib, sunitinib, brivanib, linifanib, and erlotinib is given in .
Figure 1 Overall survival in patients with advanced hepatocellular carcinoma treated with sorafenib (all studies, including the pivotal SHARP trial),Citation21 sunitinib (SUN1170 trial),Citation28 brivanib (BRISK-FL trial), linifanib, (LIGHT trial),Citation29 and sorafenib plus erlotinib (SEARCH trial),Citation135 according to current head-to-head Phase III studies.
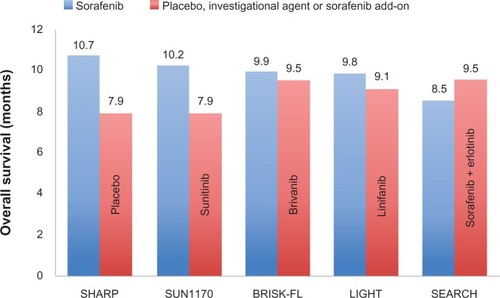
Recently, it was shown that inhibition of the fibroblast growth-factor receptor (FGFR)-4 pathway is involved in HCC development in a mouse model.Citation30 Brivanib, a selective dual inhibitor of VEGFR and FGFR,Citation31 was shown to have antitumor activity in patients with advanced HCC in two open-label Phase II studies.Citation32,Citation33 Unfortunately, brivanib was not superior compared to placebo in patients after sorafenib-treatment failure or intolerance to sorafenib in a Phase III study.Citation34 In another Phase III trial comparing brivanib and sorafenib as first-line treatment in advanced HCC, brivanib failed to prove noninferiority in comparison to sorafenib. Moreover, serious adverse events were common in both the brivanib (59%) and sorafenib (52%) treatment arms.Citation35 Therefore, the development of brivanib in HCC was stopped. Of note, the combination of sorafenib and the EGFR inhibitor erlotinib was not superior to sorafenib alone in terms of progression-free or overall survival.Citation1 Moreover, the toxicity profile of this combination was worse than that of sorafenib alone. The results of recent clinical trials in advanced HCC are summarized in .
Table 1 Efficacy of systemic targeted monotherapy in hepatocellular carcinoma according to current Phase I–III studies
Toxic effects of antiangiogenic therapy in HCC
Based on the clinical trial experience of the last few years with antiangiogenic agents in HCC, certain “class” toxicity profiles have emerged. In HCC, as in other malignancies, these include hypertension, bleeding, thromboembolic events, and proteinuria. Some toxic effects are more specific for tyrosine-kinase inhibitors, eg, hand–foot skin reaction, rash, and diarrhea. In addition, a general problem of anti-angiogenic agents in HCC is the risk of worsening liver function, which might result in liver-enzyme elevation and fatigue, and more importantly in jaundice, hepatic encephalopathy, and ascites.
With sorafenib, these side effects are manageable.Citation21–Citation24 However, especially in “dirty” kinase inhibitors, such as sunitinib, liver-specific toxicity seems to be even more prominent.Citation28 Therefore, a goal of future development of antiangiogenic agents in HCC is a manageable side-effect profile with a low incidence of liver-related toxicity.
Transarterial chemoembolization as antiangiogenic treatment
Hepatic tissue hypoxemia, amongst others, seems to be a relevant trigger for angiogenesis in chronic liver disease via induction of VEGF.Citation36 TACE was introduced into treatment algorithms for intermediate-stage HCC years before the approval of sorafenib. TACE may lead to reduction of tumor vascularization and viable tumor volume in HCC,Citation37,Citation38 and response to TACE is higher in patients with lower baseline VEGF serum levels.Citation39 Increased expression of VEGF after TACE has been reported, and development of satellite HCC nodules adjacent to TACE-treated lesions is a known clinical problem.Citation40–Citation43 TACE-induced hypoxemia may therefore trigger the expression of angiogenic factors, ultimately resulting in tumor progression.Citation40–Citation44 These observations form the rationale for combining TACE – or other trans-arterial treatments – with sorafenib, in order to prevent upregulation of VEGF. Several trials using a combination of sorafenib with lipiodol-based TACE, doxorubicin-eluting beads (DEB)-TACE, and selective internal radiation therapy (SIRT) have been reported (). The combination of sorafenib and TACE seems favorable in a subgroup of patients, but current data are controversial.Citation45–Citation54 In a recent meta-analysis, the efficacy of DEB-TACE was reported to be comparable to lipiodol-based TACE.Citation55 The combination of sorafenib with DEB-TACE showed promising results in a Phase II trial.Citation56 However, in the SPACE trial, [A Phase II Randomized, Double-blind, Placebo-controlled Study of Sorafenib or Placebo in Combination With Transarterial Chemoembolization (TACE) Performed With DC Bead and Doxorubicin for Intermediate Stage Hepatocellular Carcinoma (HCC)], a randomized Phase II trial, the combination of sorafenib with DEB-TACE in intermediate-stage HCC was not meaningfully superior to DEB-TACE alone in terms of time to tumor progression and overall survival.Citation57 Moreover, the combination treatment was associated with an increased rate of toxicity, especially in Caucasian patients.Citation57 In contrast, a recent cohort study showed that DEB-TACE alone was safe and associated with a median survival of 48.6 months. Therefore DEB-TACE – and also lipiodol-based TACE – seems to be an alternative treatment in patients with BCLC A-stage HCC not feasible for resection, ablation, or liver transplantation.Citation58 Further studies still have to establish the role of sorafenib in combination with TACE.
Table 2 Efficacy of sorafenib and TACE or SIRT in hepatocellular carcinoma (sequential therapy not included), according to current Phase I and II studies
Strategies to overcome resistance to antiangiogenic treatment
Since tumor angiogenesis is a complex process based not only on VEGF, but on a subtle interplay of intricately interweaved pathways, targeting different drivers of tumor angiogenesis might overcome antiangiogenic resistance. VEGFR2 is the critical receptor involved in tumor angiogenesis, with its activation inducing a number of other cellular modifications, resulting in tumor growth and metastases. Ramucirumab (IMC-1121B) is a fully human monoclonal antibody developed to specifically inhibit VEGFR2. Ramucirumab is currently being investigated in multiple clinical trials across a variety of tumor types, including a placebo-controlled Phase III trial in patients with HCC after failure of sorafenib. Results of this trial are expected early next year (http://clinicaltrials.gov/show/NCT01140347).
Another important regulator of vessel remodeling and maturation is the angiopoietin/Tie ligand/receptor system, which is an attractive therapeutic target in cancer.Citation59 In theory, angiopoietin inhibitors could inhibit tumor angiogenesis effi-ciently, but may lack typical tyrosine-kinase receptor inhibitor-associated toxicity. Currently, the selective angiopoietin 1/2-neutralizing peptibody AMG 386 is being investigated in combination with sorafenib in a Phase II trial in advanced or inoperable HCC (http://www.clinicaltrials.gov/ct2/show/NCT00872014). Completion of this study is also expected in the near future.
The most promising target in HCC is currently MET, a proto-oncogene that encodes a protein known as hepatocyte growth-factor receptor.Citation60,Citation61 Activation of MET signaling leads to tumor-cell growth, tumor-cell migration and invasion, and angiogenesis.Citation62 In HCC, aberrant MET signaling is frequently found, and MET overexpression is associated with advanced tumor stage and poor prognosis.Citation63–Citation65 Tivantinib (ARQ 197) is an oral, selective MET tyrosine-kinase inhibitor that is developed in non-small-cell lung cancer, colorectal cancer, and HCC.Citation62,Citation66 Recent data from a randomized placebo-controlled Phase II study in advanced HCC after sorafenib failure demonstrated a benefit of patients with MET-high HCC only.Citation65 In this study, the median time to progression was 2.7 months in the tivantinib arm and 1.4 months in the placebo arm, and median overall survival was 7.2 months and 3.8 months, respectively, in the small group of patients with MET-high tumors. Of note, severe neutropenia developed in a substantial proportion of patients, and the dose of tivantinib was reduced from 360 mg to 240 mg for the further development of tivantinib in HCC. Recently, a randomized Phase III trial with tivantinib vs placebo in advanced MET-high HCC after failure of sorafenib was started (http://clinicaltrials.gov/show/NCT01755767). Cabozantinib, an oral inhibitor of RET (“rearranged during transfection”), VEGFR2, and MET is currently also being developed in a randomized Phase III trial in advanced HCC after sorafenib failure.
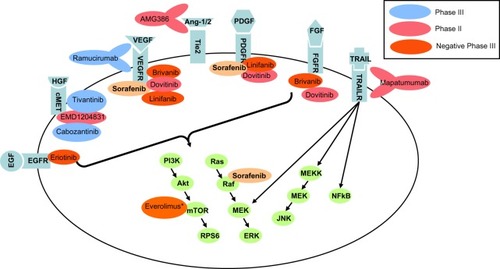
Further promising drugs that are under development for advanced HCC are the multiple tyrosine-kinase inhibitor dovitinib,Citation134 the oral histone-deacetylase inhibitor resminostat (http://clinicaltrials.gov/show/NCT00943449), and RO5137382 (GC33), a humanized anti-glypican-3 monoclonal antibody (http://www.clinicaltrials.gov/ct2/show/study/ NCT01507168). An overview of current molecular targets and targeted drugs in HCC is given in . Another approach to overcome resistance to antiangiogenic therapy is combination of targeted therapy with other systemic agents (). Currently, the efficacy and safety of these combination therapies cannot comprehensively rated, since only data from Phase I and II studies have been reported.
Table 3 Efficacy of combination therapy with systemic acting agents and targeted therapy in hepatocellular carcinoma, according to current Phase I–II studies.
Figure 2 Molecular targets in hepatocellular carcinoma and antiangiogenic drugs according to current Phase II and Phase III studies in advanced hepatocellular carcinoma. Most agents in clinical development are antiangiogenic agents targeting angiogenesis and include different tyrosine-kinase inhibitors as well as antibodies to different cell-growth receptors. *press release (http://www.novartis.com/newsroom/media-releases/en/2013/1721562.shtml)
Abbreviations: Ang-1/2, angiopoietin-1/2; EGF(R), epidermal growth factor (receptor); ERK, extracellular-signal-regulated kinase; FGF(R), fibroblast growth factor (receptor); HGF, hepatocyte growth factor; JNK, c-Jun N-terminal kinases; mTOR, mammalian target of rapamycin; NFkB, nuclear factor kappa-light-chain-enhancer of activated B cells; PDGF(R), platelet-derived growth factor (receptor); PI3K, phosphatidylinositide 3-kinases; RPS6, ribosomal protein S6; TRAIL, TNF-related apoptosis-inducing ligand; VEGF(R), vascular endothelial growth factor (receptor).
Summary
The multikinase inhibitor sorafenib is still the only approved drug for advanced HCC. Data concerning the combination of sorafenib with locoregional therapies are still controversial. Multiple clinical trials are currently investigating new antiangiogenic drugs, especially in patients after failure of sorafenib. Inhibition of VEGFR2, MET, or angiopoietin, either alone or in combination with sorafenib, are promising approaches that might ultimately improve the prognosis of advanced HCC.
Disclosure
MW Welker received honoraria from Bayer Health Care. J Trojan received honoraria from Bayer Health Care and served on the advisory boards for Bayer Health Care, Daiichi Sankyo, Lilly Imclone, Novartis, Bristol-Myers Squib, and Roche. The authors report no other conflicts of interest in this work.
References
- El SeragHBRudolphKLHepatocellular carcinoma: epidemiology and molecular carcinogenesisGastroenterology20071322557257617570226
- BruixJLlovetJMPrognostic prediction and treatment strategy in hepatocellular carcinomaHepatology20023551952411870363
- LlovetJMBurroughsABruixJHepatocellular carcinomaLancet20033621907191714667750
- LlovetJMBruCBruixJPrognosis of hepatocellular carcinoma: the BCLC staging classificationSemin Liver Dis19991932933810518312
- Bottsford-MillerJNColemanRLSoodAKResistance and escape from antiangiogenesis therapy: clinical implications and future strategiesJ Clin Oncol2012304026403423008289
- FornerAVilanaRAyusoCDiagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinomaHepatology2008479710418069697
- BruixJShermanMManagement of hepatocellular carcinomaHepatology2005421208123616250051
- WilhelmSCarterCLynchMDiscovery and development of sorafenib: a multikinase inhibitor for treating cancerNat Rev Drug Discov2006583584417016424
- AvilaMABerasainCSangroBPrietoJNew therapies for hepatocellular carcinomaOncogene2006253866388416799628
- BruixJSalaMLlovetJMChemoembolization for hepatocellular carcinomaGastroenterology2004127S179S18815508083
- LlovetJMBruixJUnresectable hepatocellular carcinoma: meta-analysis of arterial embolizationRadiology200423030030114695404
- De LopeCRTremosiniSFornerAReigMBruixJManagement of HCCJ Hepatol201256SupplS75S8722300468
- FernandezMSemelaDBruixJColleIPinzaniMBoschJAngiogenesis in liver diseaseJ Hepatol20095060462019157625
- WangBXuHGaoZQNingHFSunYQCaoGWIncreased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolizationActa Radiol20084952352918568538
- MoonWSRhyuKHKangMJOverexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma?Mod Pathol20031655255712808060
- YamaguchiRYanoHIemuraAOgasawaraSHaramakiMKojiroMExpression of vascular endothelial growth factor in human hepatocellular carcinomaHepatology19982868779657098
- ChaoYLiCPChauGYPrognostic significance of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin in patients with resectable hepatocellular carcinoma after surgeryAnn Surg Oncol20031035536212734082
- PoonRTLauCPHoJWYuWCFanSTWongJTissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinomaClin Cancer Res200395339534514614019
- PoonRTHoJWTongCSLauCNgIOFanSTPrognostic significance of serum vascular endothelial growth factor and endostatin in patients with hepatocellular carcinomaBr J Surg2004911354136015376182
- TorimuraTUenoTKinMOverexpression of angiopoietin-1 and angiopoietin-2 in hepatocellular carcinomaJ Hepatol20044079980715094228
- LlovetJMRicciSMazzaferroVSorafenib in advanced hepatocellular carcinomaN Engl J Med200835937839018650514
- Abou-AlfaGKSchwartzLRicciSPhase II study of sorafenib in patients with advanced hepatocellular carcinomaJ Clin Oncol2006244293430016908937
- FuruseJIshiiHNakachiKSuzukiEShimizuSNakajimaKPhase I study of sorafenib in Japanese patients with hepatocellular carcinomaCancer Sci20089915916517953709
- YauTChanPNgKKPhase 2 open-label study of single-agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B-endemic Asian population: presence of lung metastasis predicts poor responseCancer200911542843619107763
- WornsMAWeinmannAPfingstKSafety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma in consideration of concomitant stage of liver cirrhosisJ Clin Gastroenterol20094348949519247201
- WelkerMWLubomierskiNGogCEfficacy and safety of sorafenib in advanced hepatocellular carcinoma under daily practice conditionsJ Chemother20102220521120566428
- RimassaLPressianiTBoniCA phase II randomized dose escalation trial of sorafenib in patients with advanced hepatocellular carcinomaOncologist20131837938023580239
- ChengAKangYLinDPhase III trial of sunitinib (Su) versus sorafenib (So) in advanced hepatocellular carcinoma (HCC)J Clin Oncol201129Suppl4000
- CainapCQinSHuangWTPhase III trial of linifanib versus sorafenib in patients with advanced hepatocellular carcinoma (HCC)J Clin Oncol201331Suppl249
- FrenchDMLinBCWangMTargeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse modelsPloS One20127e3671322615798
- CaiZZhangYBorzilleriRMDiscovery of brivanib alaninate ((S)-((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f] [1,2,4]triazin-6-yloxy)propan-2-yl)2-aminopropanoate), a novel prodrug of dual vascular endothelial growth factor receptor-2 and fibroblast growth faJ Med Chem2008511976198018288793
- FinnRSKangYKMulcahyMPhase II, open-label study of brivanib as second-line therapy in patients with advanced hepatocellular carcinomaClin Cancer Res2012182090209822238246
- ParkJWFinnRSKimJSPhase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinomaClin Cancer Res2011171973198321349999
- LlovetJMDecaensTRaoulJLBrivanib versus placebo in patients with advanced hepatocellular carcinoma (HCC) who failed or were intolerant to sorafenib: results from the phase 3 BRISK-PS studyJ Hepatol201256SupplS549
- JohnsonPQinSParkJWBrivanib (BRI) versus sorafenib (SOR) as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma (HCC): results from the phase 3 BRISK-FL studyHepatology201256Suppl15191520
- RossMASanderCMKleebTBWatkinsSCStolzDBSpatiotemporal expression of angiogenesis growth factor receptors during the revascularization of regenerating rat liverHepatology2001341135114811732003
- VoglTJNaguibNNNour-EldinNEReview on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indicationsEur J Radiol20097250551618835117
- LlovetJMRealMIMontanaXArterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trialLancet20023591734173912049862
- PoonRTLauCYuWCFanSTWongJHigh serum levels of vascular endothelial growth factor predict poor response to transarterial chemoembolization in hepatocellular carcinoma: a prospective studyOncol Rep2004111077108415069550
- LiXFengGSZhengCSZhuoCKLiuXExpression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor levelWorld J Gastroenterol2004102878288215334691
- ShimJHParkJWKimJHAssociation between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patientsCancer Sci2008992037204419016764
- LeelawatKLaisupasinPKiatdilokrutAThe effect of doxorubicin on the changes of serum vascular endothelial growth factor (VEGF) in patients with hepatocellular carcinoma after tran-scatheter arterial chemoembolization (TACE)J Med Assoc Thai2008911539154318972897
- XiongZPYangSRLiangZYAssociation between vascular endothelial growth factor and metastasis after transcatheter arterial chemoembolization in patients with hepatocellular carcinomaHepatobiliary Pancreat Dis Int2004338639015313674
- SekiTTamaiTIkedaKRapid progression of hepatocellular carcinoma after transcatheter arterial chemoembolization and percu-taneous radiofrequency ablation in the primary tumour regionEur J Gastroenterol Hepatol20011329129411293452
- ErhardtAKolligsFTDollingerMFirst-in-men demonstration of sorafenib plus TACE for the treatment of advanced hepatocellular carcinoma – interim analysis of the SOCRATES trialHepatology200950SupplA1080
- ParkJWKohYHKimHBPhase II study of concurrent transar-terial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinomaJ Hepatol2012561336134222314421
- DuanFWangMQLiuFYWangZJSongPWangYSorafenib in combination with transarterial chemoembolization and bronchial arterial chemoinfusion in the treatment of hepatocellular carcinoma with pulmonary metastasisAsia Pac J Clin Oncol2012815616322524574
- TanWFQiuZQYuYSorafenib extends the survival time of patients with multiple recurrences of hepatocellular carcinoma after liver transplantationActa Pharmacol Sin2010311643164821102481
- QuXDChenCSWangJHThe efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinomaBMC Cancer20121226322721173
- ChungYHHanGYoonJHInterim analysis of START: study in Asia of the combination of TACE (transcatheter arterial chemoem-bolization) with sorafenib in patients with hepatocellular carcinoma trialInt J Cancer20131322448245823129123
- BaiWWangYJZhaoYSorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching studyJ Dig Dis20131418119023324079
- HanGYangJShaoGSorafenib in combination with transarterial chemoembolization in Chinese patients with hepatocellular carcinoma: a subgroup interim analysis of the START trialFuture Oncol2013940341023469975
- ZhaoYWangWJGuanSSorafenib combined with transarte-rial chemoembolization for the treatment of advanced hepatocellular carcinoma: a large-scale multicenter study of 222 patientsAnn Oncol2013241786179223508822
- DufourJFHoppeHHeimMHContinuous administration of sorafenib in combination with transarterial chemoembolization in patients with hepatocellular carcinoma: results of a phase I studyOncologist2010151198120421036880
- GaoSYangZZhengZDoxorubicin-eluting bead versus conventional TACE for unresectable hepatocellular carcinoma: a meta-analysisHepatogastroenterology201360
- ReyesDKAzadNKamelIRPhase II trial of sorafenib combined with doxorubicin eluting bead-transarterial chemoembolization (DEB-TACE) for patients with hepatocellular carcinoma (HCC): interim safety and efficacy analysisHepatology200950Suppl6A7A19437494
- LencioniRLlovetJHanGSorafenib or placebo in combination with transarterial chemoembolization (TACE) with doxorubicin-eluting beads (DEBDOX) for intermediate-stage hepatocellular carcinoma (HCC): phase II, randomized, double-blind SPACE trialJ Clin Oncol201230SupplLBA154
- BurrelMReigMFornerASurvival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial designJ Hepatol2012561330133522314428
- GeraldDChintharlapalliSAugustinHBenjaminLAngiopoietin-2: an attractive target for improved antiangiogenic tumor therapyCancer Res2013731649165723467610
- ChristensenJGBurrowsJSalgiaRc-Met as a target for human cancer and characterization of inhibitors for therapeutic interventionCancer Lett200522512615922853
- Danilkovitch-MiagkovaAZbarBDysregulation of Met receptor tyrosine kinase activity in invasive tumorsJ Clin Invest200210986386711927612
- GherardiEBirchmeierWBirchmeierCVande WoudeGTargeting MET in cancer: rationale and progressNat Rev Cancer2012128910322270953
- KondoSOjimaHTsudaHClinical impact of c-Met expression and its gene amplification in hepatocellular carcinomaInt J Clin Oncol20131820721322218908
- Kaposi-NovakPLeeJSGòmez-QuirozLCoulouarnCFactorVMThorgeirssonSSMet-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotypeJ Clin Invest20061161582159516710476
- UekiTFujimotoJSuzukiTYamamotoHOkamotoEExpression of hepatocyte growth factor and its receptor, the c-met proto-oncogene, in hepatocellular carcinomaHepatology1997256196239049208
- WhittakerSMaraisRZhuAXThe role of signaling pathways in the development and treatment of hepatocellular carcinomaOncogene2010294989500520639898
- WelkerMWTrojanJAnti-angiogenesis in hepatocellular carcinoma treatment: current evidence and future perspectivesWord J Gastroenterol20111730753081
- O’NeilBHWilliams-GoffLWKauhJA phase II study of AZD6244 in advanced or metastatic hepatocellular carcinomaJ Clin Oncol200927Supple15574
- SchwartzJDSchwartzMLehrerDBevacizumab in unresectable hepatocellular carcinoma (HCC) for patients without metastasis and without invasion of the portal veinJ Clin Oncol2006244144
- SiegelABCohenEIOceanAPhase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinomaJ Clin Oncol2008262992299818565886
- BoigeVMalkaDBourredjemAEfficacy, safety, and biomark-ers of single-agent bevacizumab therapy in patients with advanced hepatocellular carcinomaOncologist2012171063107222707516
- KimGPMahoneyMRSzydloDAn international, multicenter phase II trial of bortezomib in patients with hepatocellular carcinomaInvest New Drugs20123038739420839030
- GruenwaldVWilkensLGebelMA phase II open-label study of cetuximab in unresectable hepatocellular carcinoma: final resultsJ Clin Oncol200725Suppl4598
- ZhuAXStuartKBlaszkowskyLSPhase 2 study of cetux-imab in patients with advanced hepatocellular carcinomaCancer200711058158917583545
- PhilipPAMahoneyMRAllmerCPhase II study of erlotinib (OSI-774) in patients with advanced hepatocellular cancerJ Clin Oncol2005236657666316170173
- ThomasMBChadhaRGloverKPhase 2 study of erlotinib in patients with unresectable hepatocellular carcinomaCancer20071101059106717623837
- ShiahHSChenCYDaiCYRandomised clinical trial: comparison of two everolimus dosing schedules in patients with advanced hepatocellular carcinomaAliment Pharmacol Ther201337627323134470
- ZhuAXAbramsTAMiksadRPhase 1/2 study of everolimus in advanced hepatocellular carcinomaCancer20111175094510221538343
- O’DwyerPJGiantonioBJLevyDEFitzgeraldDBBensonABGefitinib in advanced unresectable hepatocellular carcinoma: results from the Eastern Cooperative Oncology Group’s study E1203J Clin Oncol200624Suppl414316896003
- LinAYFisherGASoSTangCLevittLPhase II study of ima-tinib in unresectable hepatocellular carcinomaAm J Clin Oncol200831848818376233
- Bekaii-SaabTMarkowitzJPrescottNA multi-institutional phase II study of the efficacy and tolerability of lapatinib in patients with advanced hepatocellular carcinomasClin Cancer Res2009155895590119737952
- RamanathanRKBelaniCPSinghDAA phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancerCancer Chemother Pharmacol20096477778319169683
- TohHCChenPJCarrBIPhase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinomaCancer201311938038722833179
- RizellMAnderssonMCahlinCHafstromLOlaussonMLindnerPEffects of the mTOR inhibitor sirolimus in patients with hepatocellular and cholangiocellular cancerInt J Clin Oncol200813667018307022
- ChengALKangYKChenZEfficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trialLancet Oncol200910253419095497
- KudoMImanakaKChidaNPhase III study of sorafenib after tran-sarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinomaEur J Cancer2011472117212721664811
- HodaDCatherineCStrosbergJPhase II study of sunitinib malate in adult pts (pts) with metastatic or surgically unresectable hepatocellular carcinoma (HCC)Presented at: 2008 Gastrointestinal Cancers SymposiumJanuary 25–27, 2008Orlando, FL
- ZhuAXSahaniDVDudaDGEfficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II studyJ Clin Oncol2009273027303519470923
- FaivreSRaymondEBoucherESafety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II studyLancet Oncol20091079480019586800
- KoeberleDMontemurroMSamarasPContinuous sunitinib treatment in patients with advanced hepatocellular carcinoma: a Swiss Group for Clinical Cancer Research (SAKK) and Swiss Association for the Study of the Liver (SASL) multicenter phase II trial (SAKK 77/06)Oncologist20101528529220203173
- WörnsMASchuchmannMDüberCOttoGGallePRWeinmannASunitinib in patients with advanced hepatocellular carcinoma after progression under sorafenib treatmentOncology201079859221071995
- BaroneCBassoMBiolatoMA phase II study of suni-tinib in advanced hepatocellular carcinomaDig Liver Dis Epub2112013
- PinterMWichlasMSchmidKThalidomide in advanced hepatocellular carcinoma as antiangiogenic treatment approach: a phase I/II trialEur J Gastroenterol Hepatol2008201012101918787470
- SantoroASimonelliMRodriguez-LopeCA phase-1b study of tivantinib (ARQ 197) in adult patients with hepatocellular carcinoma and cirrhosisBr J Cancer2013108212423287988
- SantoroARimassaLBorbathITivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 studyLancet Oncol201314556323182627
- KanaiFYoshidaHTateishiRA phase I/II trial of the oral antiangiogenic agent TSU-68 in patients with advanced hepatocellular carcinomaCancer Chemother Pharmacol20116731532420390419
- HsuCYangTSHuoTIVandetanib in patients with inoperable hepatocellular carcinoma: a phase II, randomized, double-blind, placebo-controlled studyJ Hepatol2012561097110322245891
- BrittenCDGomesASWainbergZATransarterial chemoem-bolization plus or minus intravenous bevacizumab in the treatment of hepatocellular cancer: a pilot studyBMC Cancer2012121622244160
- BuijsMReyesDKPawlikTMPhase 2 trial of concurrent beva-cizumab and transhepatic arterial chemoembolization in patients with unresectable hepatocellular carcinomaCancer20131191042104923132335
- PawlikTMReyesDKCosgroveDKamelIRBhagatNGeschwindJ-FHPhase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinomaJ Clin Oncol2011293960396721911714
- CabreraRPannuDSCaridiJThe combination of sorafenib with transarterial chemoembolisation for hepatocellular carcinomaAliment Pharmacol Ther20113420521321605146
- ChowPKPoonDWinKMMulticenter phase II study of SIR-sphere plus sorafenib as first-line treatment in patients with nonresectable hepatocellular carcinoma: the Asia-Pacific Hepatocellular Carcinoma Trials Group Protocol 05 (AHCC05)J Clin Oncol201028Suppl4072
- ErhardtAKolligsFTDollingerMSorafenib plus TACE for the treatment of advanced hepatocellular carcinoma – final results of the SOCRATES trialJ Clin Oncol201129Suppl4107
- WuJBXuGJLuYSEfficacy of transcatheter arterial chemoembolization (TACE) combined with sorafenib in the treatment of advanced hepatocellular carcinomaAfr J Pharm Pharmacol2012625152519
- SieghartWPinterMReiseggerMConventional transarte-rial chemoembolisation in combination with sorafenib for patients with hepatocellular carcinoma: a pilot studyEur Radiol2012221214122322215073
- HsuCHYangTSHsuCEfficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinomaBr J Cancer201010298198620160718
- SunWSohalDHallerDGPhase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinomaCancer20111173187319221264839
- ThomasMBMorrisJSChadhaRPhase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinomaJ Clin Oncol20092784385019139433
- KasebAOGarrett-MayerEMorrisJSEfficacy of bevacizumab plus erlotinib for advanced hepatocellular carcinoma and predictors of outcome: final results of a phase II trialOncology201282677422327795
- YauTWongHChanPPhase II study of bevacizumab and erlotinib in the treatment of advanced hepatocellular carcinoma patients with sorafenib-refractory diseaseInvest New Drugs2012302384239022402942
- PhilipPAMahoneyMRHolenKDPhase 2 study of bevacizumab plus erlotinib in patients with advanced hepatocellular cancerCancer20121182424243021953248
- GovindarajanRSiegelEMakhoulIWilliamsonSBevacizumab and erlotinib in previously untreated inoperable and metastatic hepatocellular carcinomaAm J Clin Oncol20123625425722643560
- TreiberGWexTSchneiderGTreatment of advanced or metastatic hepatocellular cancer (HCC): final clinical results of a single-arm phase II study of bevacizumab and everolimusJ Clin Oncol201230Suppl4107
- ZhuAXBlaszkowskyLSRyanDPPhase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinomaJ Clin Oncol2006241898190316622265
- BerlinJDPowellMESuYBortezomib (B) and doxorubicin (dox) in patients (pts) with hepatocellular cancer (HCC): a phase II trial of the Eastern Cooperative Oncology Group (ECOG 6202) with laboratory correlatesJ Clin Oncol200826Suppl4592
- SanoffHBernardSGoldbergRPhase II study of capecitabine, oxaliplatin, and cetuximab for advanced hepatocellular carcinomaGastrointest Cancer Res20114788322043322
- LouafiSBoigeVDucreuxMGemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): results of a phase II studyCancer20071091384139017330837
- AsnaciosAFartouxLRomanoOGemcitabine plus oxaliplatin (GEMOX) combined with cetuximab in patients with progressive advanced stage hepatocellular carcinoma: results of a multicenter phase 2 studyCancer20081122733273918412149
- ChioreanEGRamasubbaiahRYuMPhase II trial of erlotinib and docetaxel in advanced and refractory hepatocellular and biliary cancers: Hoosier Oncology Group GI06-101Oncologist2012171322210086
- LuelmoSOsantoSWeijlNPhase II study of everolimus and capecitabine in patients with locally advanced or metastatic hepatocellular carcinoma (HCC). Results of the first 10 patients includedAnn Oncol201223Suppl1674
- KnoxJJChenXEFeldRA phase I–II study of oblimersen sodium (G3139, Genasense) in combination with doxorubicin in advanced hepatocellular carcinoma (NCI # 5798)Invest New Drugs20082619319418060598
- YauTChanPPangRNgKFanSTPoonRTPhase 1–2 trial of PTK787/ZK222584 combined with intravenous doxorubicin for treatment of patients with advanced hepatocellular carcinoma: implication for antiangiogenic approach to hepatocellular carcinomaCancer20101165022502920629034
- PetriniILencioniMRicasoliMPhase II trial of sorafenib in combination with 5-fluorouracil infusion in advanced hepatocellular carcinomaCancer Chemother Pharmacol20126977378022033636
- RichlyHSchultheisBAdamietzIACombination of sorafenib and doxorubicin in patients with advanced hepatocellular carcinoma: results from a phase I extension trialEur J Cancer20094557958719101137
- Abou-AlfaGKJohnsonPKnoxJJDoxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trialJAMA20103042154216021081728
- DimaGLuciaMLa GattutaGPerspective phase II study of combination sorafenib plus mitomycin-c in the treatment of advanced hepatocellular carcinoma (HCC)Ann Oncol200919Suppl49
- PreteSDMontellaLCaragliaMSorafenib plus octreotide is an effective and safe treatment in advanced hepatocellular carcinoma: multicenter phase II SoLAR studyCancer Chemother Pharmacol20106683784420041325
- Abou-AlfaGKChanSLLinCCPR-104 plus sorafenib in patients with advanced hepatocellular carcinomaCancer Chemother Pharmacol20116853954521594722
- BitzerMHorgerMGantenTEfficacy, safety, toler-ability, and PK of the HDAC inhibitor resminostat in sorafenib-refractory hepatocellular carcinoma (HCC)J Clin Oncol201230Suppl4115
- ShenYShaoYHsuCPhase II study of sorafenib plus tegafur/ uracil (UFT) in patients with advanced hepatocellular carcinoma (HCC)J Clin Oncol200826Suppl15664
- HsuCHShenYCLinZZPhase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinomaJ Hepatol20105312613120416968
- HsuCLinZLeeKA phase II trial of thalidomide plus tegafur/ uracil for patients with advanced/metastatic hepatocellular carcinoma (HCC): final reportJ Clin Oncol200927Suppl15533
- ZhuAXFuchsCSClarkJWA phase II study of epirubicin and thalidomide in unresectable or metastatic hepatocellular carcinomaOncologist20051039239815967833
- TaiWTChengALShiauCWDovitinib induces apoptosis and overcomes sorafenib resistance in hepatocellular carcinoma through SHP-1-mediated inhibition of STAT3Mol Cancer Ther20121145246322180308
- ZhuAXRosmorducOEvansJSEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with hepatocellular carcinoma (HCC)Ann Oncol201223SupplLBA2