Abstract
Purpose
Endometrial carcinoma (EC) is a common gynecological malignancy. Vaginal cuff brachytherapy (VBT) is an adjuvant treatment for EC. Since a single-channel cylinder sometimes delivers inadequate dose coverage to the vaginal apex, three-dimensional (3D) printing technology can be used to achieve satisfactory dose distribution. Here, we report the first case of an EC patient with Herlyn-Werner-Wunderlich syndrome (HWWS) treated with VBT using 3D-printed applicators.
Case Presentation
Here, we present a case study of an endometrial cancer patient with HWWS who underwent surgery. During adjuvant radiotherapy, 3D-printed applicators were used in VBT. To accomplish the reconstruction of the source pathways on magnetic resonance imaging, catheters with copper sulfate were placed in two 3D-printed applicators. The early tolerance of this treatment was positive. During the 6-month follow-up, locoregional recurrence was not detected.
Conclusion
Our findings strongly indicate that VBT with 3D-printed applicators may be a reasonable treatment option for EC with HWWS.
Introduction
Endometrial carcinoma (EC) is the seventh most common cancer in women, with a high mortality rate.Citation1 Vaginal cuff brachytherapy (VBT) has become the standard adjuvant treatment for EC, with a high risk of recurrence after resection.Citation2 Vaginal applicators are tools for performing VBT and are the most commonly used single-channel cylinders (SCC).Citation3 However, SCC sometimes delivers inadequate dose coverage to the vaginal apex, owing to source anisotropy.Citation4 Creating individualized applicators for different patients using three-dimensional (3D) printing technology achieves satisfactory dose distribution.Citation5,Citation6 Herlyn-Werner-Wunderlich syndrome (HWWS) is a rare disease often discovered at menarche and consists of anomalies of the female reproductive tract. It comprises the three most common forms of anomalies, including uterus didelphys, unilateral blind hemivagina, and ipsilateral renal agenesis.Citation7 This case report describes a patient with HWWS who was admitted to our hospital with postoperative EC and provides insights into a personalized treatment approach that has resulted in satisfactory clinical outcomes.
Case Presentation
A middle-aged woman with irregular vaginal bleeding was admitted to another hospital. Routine blood test results, liver function, kidney function, and electrolyte levels were within normal ranges. The patient’s serum cancer antigen (CA) 125 (CA125) level was elevated to 182.0 IU/mL (0–35.0 IU/mL), and CA199 and carcinoembryonic antigen levels were within the normal range. Pelvic magnetic resonance imaging (MRI) revealed that the uterus was poorly formed and showed bi-cavitary changes. The left uterine volume was significantly increased, the myometrial signal was significantly heterogeneous, multiple nodules were observed, and some protruded from the uterine contour; the largest size was 7.4×7.3 cm. Sagittal T2-weighted imaging of the right uterus showed a normal structure and signal intensity in the three layers of the uterus. Chest and abdominal computed tomography (CT) revealed no tumor metastasis. Subsequently, hysteroscopic bilateral endometrial biopsy was performed. Pathology, in combination with immunohistochemistry, revealed that the left uterus was an adenocarcinoma, which is considered a high-grade serous carcinoma. Total laparoscopic hysterectomy, bilateral salpingo-oophorectomy, pelvic lymph node dissection, para-aortic lymph node dissection, omentectomy, abdominopelvic adhesiolysis, and peritoneal brush cytology were performed. Postoperative histology revealed that the left uterus and ovary had mixed high-grade adenocarcinomas, including some serous carcinomas and some endometrial carcinomas, with a focal invasion of the myometrium. Multiple uterine myomas with adenomyosis were also observed in the left uterus. Tumor invasion of uterine blood vessels was also observed. Adenomyosis with some epithelial dysplasia was observed in the right uterus, but no carcinoma was observed in the parametrium. One of the 16 lymph nodes in the left pelvic cavity was positive, while 10 lymph nodes in the right pelvic cavity and six para-aortic lymph nodes showed no lymph node metastasis. No malignant tumor cells were found on peritoneal cytology. Using the International Federation of Gynecology and Obstetrics (FIGO) Staging System, version 2018, the tumor was classified as Stage IIIC1. Using the American Joint Committee on Cancer TNM Staging System version 8, the tumor was classified as T3aN1M0 (Stage IIIC1). Immunohistochemical examination of the tumor revealed positivity for Vimentin, P53, P16, ER, and CD34 were positive. However, the napsin A, WT-1, and HNF-1B tests were negative. The Ki-67 proliferation index was ~70%. A definitive diagnosis of EC was confirmed based on the histopathological and immunohistochemical results. Genetic testing revealed a TP53 exon7 mutation with an abundance of 41.14%. Molecular typing was classified as having a high copy number.
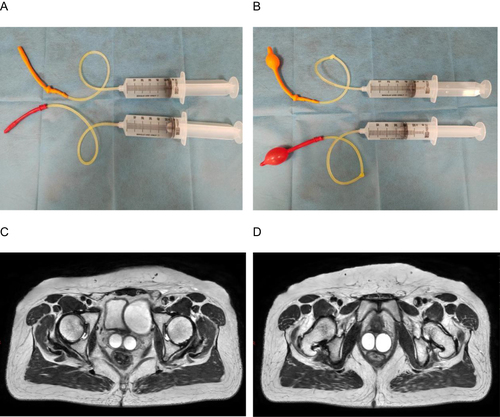
The patient received three cycles of chemotherapy with paclitaxel and carboplatin. The patient was admitted to our hospital for adjuvant radiotherapy. External beam radiotherapy (EBRT) and intracavitary brachytherapy (ICBT) were performed. For EBRT, the clinical target volume (CTV) included the lower half of the vaginal, parapodium, and pelvic lymphatic drainage areas. The planning target volume was defined as a 6‒10 mm margin added to the CTV in all directions. The prescribed dose was 50.4 Gy to at least 95% of the planning clinical target volume in 28 fractions. High-dose-rate ICBT generally begins after intensity-modulated radiation therapy with 192 Ir. For ICBT, to show the shape of the vagina, two inflated balloons with sterile physiological salt solution filled the double vagina for MRI ( and ). As shown in and , MRI was used to create a 3D-printed ICBT applicator.
Figure 1 (A and B) Images of two inflated balloons with a sterile physiological salt solution. (C and D) T2-weighted magnetic resonance images were obtained after two inflated balloons with sterile physiological salt solution filled the double vagina.
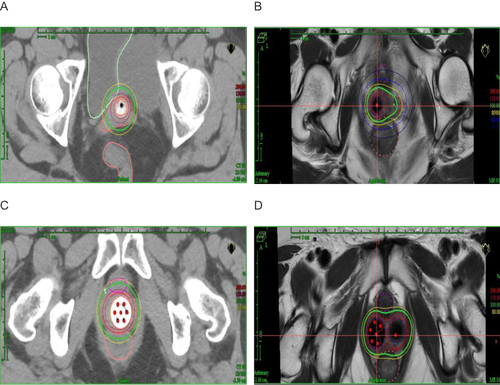
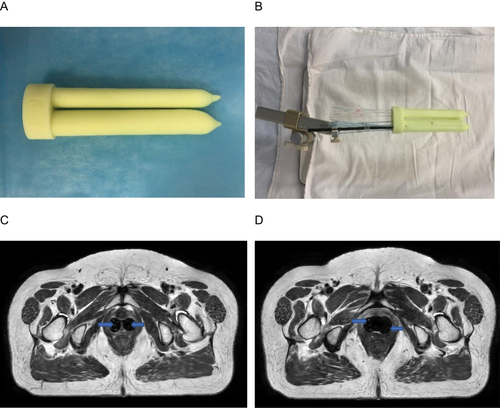
A 3D-printed applicator was designed and sent to a 3D printer (Eden260VS; Stratasys, Inc, Gilbert, AZ, USA). Examples are shown in . Applicators are available for clinical use after quality assurance. As shown in , catheters with copper sulfate were placed in two 3D-printed applicators to show the needle paths in the applicators. As shown in and , a planning MRI scan was performed. CTV included the lower half of the vaginal wall. The dose calculations were performed using the Oncentra Brachy Treatment Planning System (Elekta, Stockholm, Sweden). A prescribed dose of 10 Gy was delivered in two fractions. The CTV and organs at risk (OARs) doses were converted to an equivalent dose of 2 Gy (EQD2). The final goal was a cumulative dose to the CTV of 65.89 Gy in the 3D-printed plan. The cumulative doses of the D2cc of the bladder, rectum, and sigmoid were 4858, 4362, and 5293 cGy, respectively. A multichannel cylinder (MCC) simulation plan is developed. As shown in , the dosimetric indices were superior in the 3D-printed plan than in the MCC plan. In and , when evaluating the CTV from the vaginal apex, the isodose curve of 100% cover was better than that in the MCC plan. and show a 100% isodose curve covering a larger volume range of the urethra and rectum, respectively. Comparing the two groups of plans, these results favor the 3D-printed plan. The patient’s follow-up time was 6 months, with an Eastern Cooperative Oncology Group score of 0 and no reported acute urinary or lower digestive tract events.
Table 1 Dosimetric Comparison of Organs at Risk Between Multichannel Cylinder (MCC) and Three-Dimensional (3D)-Printed Plan
Figure 2 (A) Photographs of the three-dimensional (3D)-printed applicators. (B) Catheters with copper sulfate were placed in two 3D-printed applicators. (C and D) T2-weighted magnetic resonance images were obtained after the catheters with copper sulfate were placed in two 3D-printed applicators. The catheters with copper sulfate are represented by blue arrows.
Discussion
To our knowledge, this is the first reported case of EC with HWWS that was treated with VBT. Moreover, this is the first study on VBT using 3D-printed applicators in patients with HWWS. In this case, copper sulfate was used as a photographic developer for the 3D-printed applicator reconstruction in MRI.
Abnormal development of the Müllerian and Wolffian ducts caused HWWS. It is a rare genital malformation, with a true incidence of approximately 0.1–3.8%.Citation8 The development of EC associated with HWWS is not yet clear. Research has shown that women with endometriosis and adenomyosis have an increased risk of developing EC.Citation9 In our case, postoperative pathology revealed adenomyosis in both uteri, suggesting that EC may be associated with HWWS.
According to European Society for Medical Oncology (ESMO) (Version 2022) guidelines, for the FIGO stage IIIC patients with EC who had regional lymph node involvement, the treatment methods included radical surgery with postoperative adjuvant treatment.Citation1 Adjuvant chemotherapy with or without postoperative radiotherapy is the treatment of choice for these patients. Pelvic EBRT with VBT and EBRT alone is a common radiotherapy treatments.Citation1 The vaginal vault is a common relapse site for patients with EC undergoing radical surgery.Citation10 Given the pattern of EC failure, VBT alone is regarded as the standard adjuvant therapy for high-intermediate-risk endometrial cancer.Citation10 However, the role and utilization of a VBT boost in EBRT are less clear in patients with stage III EC.Citation11 Bingham et al evaluated 12,988 patients with stage III EC using the National Cancer Database.Citation12 They found that EBRT plus VBT had a significantly improved 5-year survival rate compared to EBRT alone (69% vs 66%, respectively; P < 0.01) for patients with cervical stromal involvement. Rossi et al analyzed 611 women with stage IIIC EC.Citation13 They found that EBRT plus VBT had a significantly improved 5-year survival rate compared to EBRT alone (63% vs 47%, respectively; P < 0.001) for patients with direct local disease extension. For patients with stage III EC, the ESMO guidelines (Version 2022) list the optional inclusion of a VBT boost.Citation1 Uterine serous carcinoma is an aggressive histological subtype of endometrial cancer, with a high relapse rate.Citation14 Hong et al evaluated 5432 women with uterine serous carcinoma and found that VBT was associated with a significant survival advantage.Citation15 In this case, the patient was staged IIIC1 with uterine serous carcinoma, and a boost in VBT to EBRT was administered.
In this case, different treatments were considered. Interstitial brachytherapy is often used in specific situations, including large tumors, asymmetric tumors, organs at risk proximity, and patient anatomy considerations.Citation16 Owing to irregular dose distribution and special anatomy, interstitial brachytherapy may increase the risk of side effects such as bleeding, urethritis, and cystitis. In the case of postoperative endometrial cancer, the most commonly used brachytherapy is the intracavitary techniques.Citation17 The patient was diagnosed with complete vaginal septum and required removal of the entire vagina if surgical excision was necessary. It results in significant surgical trauma and affects the quality of life. In this case, a patient who received VBT for personalized applicators may be a suitable choice.
MCC applicators have become more popular for VBT treatment because of their dose flexibility.Citation4 However, the vagina may not be cylindrical because of surgery and anatomic irregularities.Citation5 Furthermore, MCC applicators are limited to various applications owing to air gaps. Air spaces cause a 1–2 mm displacement of the vaginal wall, which can reduce the dose to the vaginal mucosa by more than 10%.Citation18 Personalized vaginal applicators are one choice to overcome the limitations of MCC applicators.Citation19 Yan et al showed that 3D-printed applicators obtained significant improvements in CTV-1 cm V100 (by 13%) and D90 (by 11%) compared to MCC; 3D-printed applicators demonstrated superiority over MCC in terms of OARs protection.Citation5 The advantages of 3D-printing technology include versatility, cost-effectiveness, and accuracy.Citation20 In this case, the doses to the bladder, rectum, urethra, and small intestine in the 3D-printed plan were lower than those in the MCC plan were. Meanwhile, the apex of the vagina was better covered by the CTV in the 3D-printed plan.
With the development of imaging technology, brachytherapy based on imaging modalities has increased the precision and accuracy of treatment.Citation21 MRI can provide better soft tissue contrast than CT and is already used for some gynecologic cancers.Citation22 The vaginal cuff can be visualized using MRI, which provides better images for defining the gross disease.Citation22 The introduction of brachytherapy by MRI has led to a new era of increasingly effective treatments.Citation23 One challenge of which is the reconstruction of the source pathways on MRI. Applicator reconstruction inaccuracies lead to dosimetric uncertainties in target volumes and OARs.Citation24 Schindel et al evaluated seven MRI marker agents and found that the CuSO4 marker was feasible for MRI-guided gynecological brachytherapy.Citation25 In this case, custom-made plastic catheters with CuSO4 solution were inserted into the source channels of MRI-compatible applicators. This method can provide accurate source pathway reconstruction.
In cases in which conventional treatments are complex; a personalized approach is essential for the patient to have the broadest range of medically reasonable options.
Currently, there is a report on EC with HWWS treated with VBT using 3D-printed applicators. Reconstruction of titanium applicators using MRI is complex. CuSO4 has been used as an MRI marker in brachytherapy, achieving relatively good dose distribution and no relevant acute toxicity reaction. This unique, personalized treatment approach has provided satisfactory outcomes for patients. Patients treated with personalized brachytherapy are expected to achieve long-term survival.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
Approval was obtained from the Institutional Review Board of Peking Union Medical College Hospital.
Consent Statements
The patient has provided informed consent for case details of this manuscript and accompanying images to be published.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Additional information
Funding
References
- Oaknin A, Bosse TJ, Creutzberg CL, et al. Endometrial cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(9):860–877. doi:10.1016/j.annonc.2022.05.009
- Zakem SJ, Robin TP, Smith DE, et al. Evolving trends in the management of high-intermediate risk endometrial cancer in the United States. Gynecol Oncol. 2019;152(3):522–527. doi:10.1016/j.ygyno.2018.12.010
- Harkenrider MM, Grover S, Erickson BA, et al. Vaginal brachytherapy for postoperative endometrial cancer: 2014 survey of the American brachytherapy society. Brachytherapy. 2016;15(1):23–29. doi:10.1016/j.brachy.2015.09.012
- Hou X, Liu A, Zhang F, Wong J, Chen YJ. Dosimetric advantages of using multichannel balloons compared to single-channel cylinders for high-dose-rate vaginal cuff brachytherapy. Brachytherapy. 2016;15(4):471–476. doi:10.1016/j.brachy.2016.03.002
- Yan J, Qin X, Zhang F, Hou X, Yu L, Qiu J. Comparing multichannel cylinder and 3D-printed applicators for vaginal cuff brachytherapy with preliminary exploration of post-hysterectomy vaginal morphology. J Contemp Brachytherapy. 2021;13(6):641–648. doi:10.5114/jcb.2021.112115
- Wiebe E, Easton H, Thomas G, Barbera L, D’Alimonte L, Ravi A. Customized vaginal vault brachytherapy with computed tomography imaging-derived applicator prototyping. Brachytherapy. 2015;14(3):380–384. doi:10.1016/j.brachy.2014.12.006
- Horst W, de Melo RC, Theilacker G, Schmitt B, de Melo RC. Herlyn-Werner-Wunderlich syndrome: clinical considerations and management. BMJ Case Rep. 2021;14(3):e239160. doi:10.1136/bcr-2020-239160
- Tong J, Zhu L, Lang J. Clinical characteristics of 70 patients with Herlyn-Werner-Wunderlich syndrome. Int J Gynaecol Obstet. 2013;121(2):173–175. doi:10.1016/j.ijgo.2012.11.023
- Hermens M, van Altena AM, Velthuis I, et al. Endometrial cancer incidence in endometriosis and adenomyosis. Cancers. 2021;13(18):4592. doi:10.3390/cancers13184592
- Nout, RA, Smit, V, Putter, H, et al Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet. 2010;375(9717):816-823. doi:10.1016/S0140-6736(09)62163-2.
- Wortman BG, Creutzberg CL, Putter H, et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: improving patient selection for adjuvant therapy. Br J Cancer. 2018;119(9):1067–1074. doi:10.1038/s41416-018-0310-8
- Bingham B, Orton A, Boothe D, et al. Brachytherapy improves survival in stage III endometrial cancer with cervical involvement. Int J Radiat Oncol Biol Phys. 2017;97(5):1040–1050. doi:10.1016/j.ijrobp.2016.12.035
- Rossi PJ, Jani AB, Horowitz IR, Johnstone PA. Adjuvant brachytherapy removes survival disadvantage of local disease extension in stage IIIC endometrial cancer: a SEER registry analysis. Int J Radiat Oncol Biol Phys. 2008;70(1):134–138. doi:10.1016/j.ijrobp.2007.05.048
- Bogani G, Ray-Coquard I, Concin N, et al. Uterine serous carcinoma. Gynecol Oncol. 2021;162(1):226–234. doi:10.1016/j.ygyno.2021.04.029
- Hong JC, Foote J, Broadwater G, Gaillard S, Havrilesky LJ, Chino JP. Impact of chemotherapy and radiotherapy on management of early stage clear cell and papillary serous carcinoma of the uterus. Int J Gynecol Cancer. 2017;27(4):720–729. doi:10.1097/IGC.0000000000000926
- Kamrava M, Alrashidi SM, Leung E. Interstitial brachytherapy for gynecologic malignancies: complications, toxicities, and management. Brachytherapy. 2021;20(5):995–1004. doi:10.1016/j.brachy.2020.12.008
- Prisciandaro JI, Zhao X, Dieterich S, Hasan Y, Jolly S, Al-Hallaq HA. Interstitial high-dose-rate gynecologic brachytherapy: clinical workflow experience from three academic institutions. Semin Radiat Oncol. 2020;30(1):29–38. doi:10.1016/j.semradonc.2019.08.001
- Hassouna A, Bahadur YA, Constantinescu C. Assessment of air pockets in high-dose-rate vaginal cuff brachytherapy using cylindrical applicators. J Contemp Brachytherapy. 2014;6(3):271–275. doi:10.5114/jcb.2014.45436
- Lindegaard JC, Madsen ML, Traberg A, et al. Individualised 3D printed vaginal template for MRI guided brachytherapy in locally advanced cervical cancer. Radiother Oncol. 2016;118(1):173–175. doi:10.1016/j.radonc.2015.12.012
- Mohammadi R, Siavashpour Z, Aghdam SRH, Fazli S, Major T, Rohani AA. Manufacturing and evaluation of multi-channel cylinder applicator with 3D printing technology. J Contemp Brachytherapy. 2021;13(1):80–90. doi:10.5114/jcb.2021.103590
- Fokdal L, Tanderup K, Nielsen SK, et al. Image and laparoscopic guided interstitial brachytherapy for locally advanced primary or recurrent gynaecological cancer using the adaptive GEC ESTRO target concept. Radiother Oncol. 2011;100(3):473–479. doi:10.1016/j.radonc.2011.08.016
- Chapman CH, Prisciandaro JI, Maturen KE, et al. MRI-based evaluation of the vaginal cuff in brachytherapy planning: are we missing the target? Int J Radiat Oncol Biol Phys. 2016;95(2):743–750. doi:10.1016/j.ijrobp.2016.01.042
- Westerveld H, Nesvacil N, Fokdal L, et al. Definitive radiotherapy with image-guided adaptive brachytherapy for primary vaginal cancer. Lancet Oncol. 2020;21(3):e157–e167. doi:10.1016/S1470-2045(19)30855-1
- Viswanathan AN, Dimopoulos J, Kirisits C, Berger D, Potter R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68(2):491–498. doi:10.1016/j.ijrobp.2006.12.021
- Schindel J, Muruganandham M, Pigge FC, Anderson J, Kim Y. Magnetic resonance imaging (MRI) markers for MRI-guided high-dose-rate brachytherapy: novel marker-flange for cervical cancer and marker catheters for prostate cancer. Int J Radiat Oncol Biol Phys. 2013;86(2):387–393. doi:10.1016/j.ijrobp.2012.12.026