Abstract
Dasatinib is a potent oral tyrosine kinase inhibitor which targets several kinases, including the SRC family kinases. SRC family kinases have been implicated in androgen therapy resistance that often develops in metastatic castration-resistant prostate cancer (mCRPC), which drives the need for non-androgen targeting therapies. This article describes the preclinical rationale for the use of combination dasatinib and docetaxel therapy in mCRPC, and highlights the results of a phase I–II trial in which 46 patients with mCRPC, treated with a regimen of dasatinib and docetaxel, demonstrated improvements in bone scans, high rates of soft tissue responses, and modulation of markers of bone turnover. This brief report discusses in detail follow-up data on two patients who remain alive after >2.5 years on dasatinib single-agent therapy after discontinuing docetaxel treatment.
Introduction
Prostate cancer is the second most common malignancy worldwide, representing the sixth leading cause of cancer-related deaths in men.Citation1 In advanced disease, androgen deprivation therapy results in decreased serum prostate-specific antigen (PSA) and tumor regression in the majority of patients.Citation2 However, while prostate cancer is an androgen-dependent malignancy, over time resistance occurs through androgen-independent processes, one of which is the SRC family kinase signaling pathway. Targeting androgen receptor (AR)-independent processes and resensitizing the AR to alternate therapy are potential therapeutic approaches for metastatic castration-resistant prostate cancer (mCRPC). Resistance is thought to derive from many mechanisms: the AR can become transactivated in a low-androgen environment through amplification and mutation of the receptor, crosstalk with other signaling pathways, and altered regulation by coregulatory proteins.Citation3 Importantly, SRC kinase has been implicated in AR crosstalk. Upon ligand binding, the AR binds and quickly activates SRC kinase and downstream events. In turn, SRC kinase can tyrosine phosphorylate the AR, augmenting its transcriptional activity.Citation4 Additionally, SRC kinase activity has been found to be upregulated in a number of preclinical models of CRPC;Citation5,Citation6 as such, a potentially effective strategy to inhibit prostate cancer growth and metastasis may be to target SRC kinase.
Dasatinib (SPRYCEL®, Bristol-Myers Squibb, Princeton, NJ, USA) is a potent oral tyrosine kinase inhibitor that has been approved for the treatment of Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML).Citation7 Dasatinib inhibits several tyrosine kinases, including SRC kinase, which has been shown in preclinical studies to have synergistic activity with docetaxel,Citation8 antimetastatic properties,Citation9,Citation10 and potent inhibition of osteoclast function.Citation11
In an open-label, phase I–II study, 46 patients with mCRPC treated with a combination of dasatinib and docetaxel experienced a 60% Response Evaluation Criteria In Solid Tumors (RECIST) response rate, 77% disease control rate, as well as stabilization of bone metastases accompanied by decreases in bone marker turnoverCitation12 (). In addition, 80% of patients experienced a PSA decrease, including 57% with a confirmed PSA response (sustained ≥50% decline for ≥6 weeks).Citation12 These findings provided the basis for the initiation of the phase III READY trial, a randomized, double-blind, placebo-controlled study comparing the efficacy and safety of dasatinib and docetaxel/prednisone combination versus the current standard of care of docetaxel/prednisone plus placebo in men with mCRPC (Clinicaltrials.gov NCT00744497).
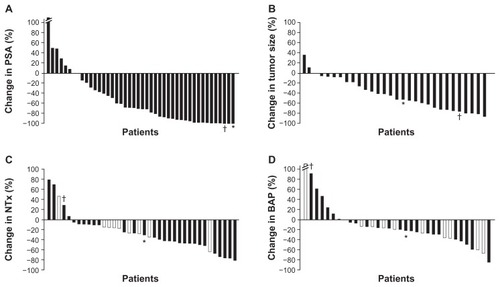
Figure 1 Waterfall plots show maximal percentage changes from baseline in individual patients with mCRPC treated with combination dasatinib and docetaxel in an open-label, phase I–II study: (A) PSA;a (B) Tumor size; (C) NTx; (D) BAP.b
Notes: In panels C and D, patients who were receiving ongoing bisphosphonate therapy are shown in white bars. Values for changes in PSA, tumor size, NTx, and BAP that correlate to patient 1 have been denoted with an asterisk (*), while those for patient 2 have been denoted with a dagger (†). Both patients 1 and 2 had striking PSA decreases, indicating that the combination of dasatinib and docetaxel affected the prostate cancer epithelial cells (A). Compared to the entire population of patients, they fell on the greatest decreases in PSA. This was also reflected in a decrease in tumor size and volume of disease (B). Patient 1, whose disease was predominately in bone, had a decrease in bone turnover markers (NTx and BAP, C and D, respectively), whereas patient 2, whose disease was solely detected in lymph nodes, had an increase in both bone markers. When comparing bone markers alone, as compared to other patients within the study, patient 1 fell within bone marker turnover median as indicated in the waterfall plots. He demonstrated one of the most durable responses, and, therefore, a decrease in PSA combined with decrease in bone turnover markers in patients with metastatic bone disease might portend a better outcome. On the other hand, in patients with soft tissue disease (lymph nodes alone, patient 2), a decrease in PSA combined with increase in these markers may lead to an improved outcome and survival advantage, which needs to be investigated further. aThe best percent change from baseline for subject 86035 (left-most whisker) was 205.7 and has been truncated on the graph shown in panel A; bthe best percent change from baseline for subject 86037 (left-most whisker) was 426.540, and has been truncated on the graph shown in panel D.
Plots redrawn with permission from John Wiley and Sons, Copyright 2011 American Cancer Society.Citation12
Abbreviations: BAP, bone alkaline phosphatase; mCRPC, metastatic castration-resistant prostate cancer; NTx, urinary N-telopeptide; PSA, prostate-specific antigen.
Of note in the phase I–II study, patients could continue to receive dasatinib after discontinuing docetaxel,Citation12 with the concept that the antimetastatic properties of dasatinib and its inhibition of osteoclasts would be able to further suppress the disease, and subsequently give prolonged responses by delaying progression. Overall, 61% of patients continued to receive dasatinib until progression.Citation12 Furthermore, after more than 2.5 years of discontinuing docetaxel, two patients with mCRPC continue to be treated with single-agent dasatinib with no evidence of progression by radiographic studies. In this article, we provide a detailed report of these two cases, examining their clinical results in an effort to learn more about the potential role of dasatinib in mCRPC.
Methods
The methodology of the open-label phase I–II study has been described previously.Citation12 Adult males with metastatic prostate cancer who had progressed despite castrate levels of serum testosterone (≤50 ng/dL) were eligible for enrollment.
Dosing
In phase I of the study, patients received escalating doses of docetaxel (administered intravenously every 21 days from day 1 of cycle 1 with oral prednisone 5 mg twice daily) and dasatinib (administered once daily beginning on day 3 of cycle 1) for a minimum of 6 cycles, except in cases of disease progression or significant toxicity. Doses of dasatinib (mg)/docetaxel (mg/m2) included the following, 50/60, 50/75, 70/75, 100/75, and 120/75. After 6 cycles of treatment, patients could discontinue docetaxel and continue to receive single-agent dasatinib until disease progression. In phase II of the study, patients received 100 mg of dasatinib and 75 mg/m2 of docetaxel and their regimen could be dose-reduced for either drug to 70 mg of dasatinib and 60 mg/m2 of docetaxel due to toxicity.
Assessments
Bone scans were performed within 28 days before treatment and every 6 weeks after initiating docetaxel. Computed tomography (CT) scans were also obtained at this time interval. Blood samples were assessed for PSA (every 3 weeks) and bone alkaline phosphatase (BAP), a marker of osteoblast differentiation (measured before treatment and at least once after cycle 1 while patients were receiving combination docetaxel and dasatinib treatment). Urine samples were also assessed for levels of urinary N-telopeptide (NTx), a marker of osteoclast activity, before treatment and at least once after cycle 1 while patients were receiving combination docetaxel and dasatinib treatment.
Results
Of the 46 patients enrolled in the study, 28 (61%) were treated with single-agent dasatinib for a median of 2.9 months up to data capture after discontinuing docetaxel.Citation12 Twenty-four of these patients continued dasatinib treatment for >42 days after discontinuation of concomitant docetaxel. Thirteen patients were treated with dasatinib for less than 6 months and eleven received treatment for more than 6 months. Of these later eleven patients, seven received treatment for more than 1 year, five patients for more than 2 years, and one patient for more than 3 years. At the time of data analysis, six of the 24 patients demonstrated no progression, with stable disease for a range of 3.4 to 11.7 months after the last docetaxel dose.Citation12
As of September 2012, two of the 24 patients remain alive after more than 2.5 years and 3 years of single-agent dasatinib. One of these two patients presented with multifocal bone disease after rapidly progressing through androgen therapy; the second patient presented with lymph node metastasis following progression on androgen therapy.
Case study: patient 1
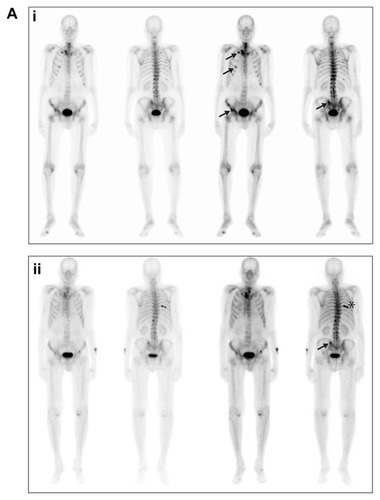
A male patient aged 74 years at the start of the trial, with a past medical history of chronic tobacco use and chronic obstructive pulmonary disease (COPD), presented with right flank pain and multifocal osseous metastasis in his ribs, spine, pelvic bones, right scapula, and right proximal femur in August 2007 at diagnosis. Prostate biopsies revealed a Gleason 8 (4 + 4) prostate adenocarcinoma with stage T2 disease and PSA level of 969 ng/mL. Complete androgen blockade of bicalutamide and a luteinizing hormone-releasing hormone analogue were initiated in September 2007, with PSA levels decreasing to a nadir of 1.5 ng/mL. In August 2008, the patient presented with a rising PSA. Bicalutamide was withdrawn and his PSA continued to rise in September 2008 and, though the patient was asymptomatic, progression of disease was revealed on bone scan (). Approximately 14 months after diagnosis, in October 2008, the patient was enrolled in the previously described open-label, phase I–II studyCitation12 and received 100 mg daily of dasatinib for 336 days and 75 mg/m2 of docetaxel, for a total of 16 cycles. Docetaxel was delayed 3 times during the course of treatment; in cycles 3, 5, and 7. The first delay was due to a COPD exacerbation in combination with a suspected upper respiratory infection (URI), delaying cycle 3 of treatment. A viral URI delayed cycle 5, and COPD exacerbation delayed cycle 7 of treatment. Each resulted in 1-week delays of docetaxel infusion. Of note, the patient was a long-time smoker (>120 pack-year history) and continued to smoke throughout the study. After 16 cycles, docetaxel was discontinued.
Figure 2A Bone scans from patient 1, taken in (i) September 2008 and (ii) June 2012.
Notes: Arrows in both panels indicate sites of metastases; the asterisk in the lower panel indicates a site of trauma.
Baseline study assessments prior to initiating dasatinib and docetaxel treatment (day 0), at docetaxel discontinuation (day 341), and at the last assessment on single-agent dasatinib therapy (day 1319) are summarized in .
Table 1 Assessments prior to docetaxel treatment, following docetaxel discontinuation, and at end of follow-up during single-agent dasatinib treatment
Case study: patient 2
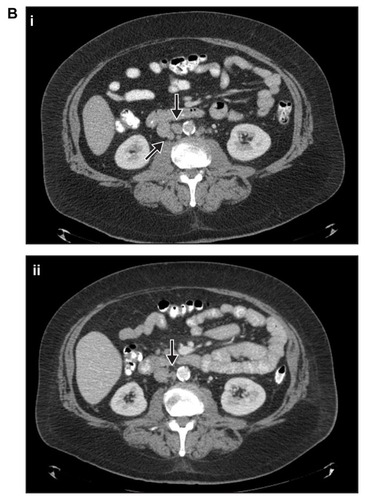
A male patient aged 53 years with a past medical history of hypertension, hypercholesterolemia, and coronary artery disease (with cardiac stents implanted in 2008), was diagnosed with a Gleason 7 (3 + 4) prostate adenocarcinoma with focal ductal features and stage T2 disease in April 2003. Six months postsurgery, the patient’s PSA was 1.0 ng/mL. Following an increase in the patient’s PSA level to 8.8 ng/mL in July 2004, he was treated with androgen ablation agents leuprolide and bicalutamide; his PSA had a nadir level of 0.1 ng/mL for <1 year. Bicalutamide was discontinued in March 2007 and the patient was treated with ketoconazole from April 2007 until June 2007, without PSA response but with subsequent radiographic progression in the aortocaval and para-aortic regions with lymph nodes of 2 cm and 1.7 cm, respectively, as well as smaller retroperitoneal lymph nodes, suggesting that the disease was becoming driven by AR-independent processes. In June 2007, the patient’s lymph nodes continued to enlarge on CT scan and his PSA level rose to 8.9 ng/mL, and he was continued on androgen ablation with leuprolide. In September 2008, the patient progressed, with increased lymph node size () and a PSA level of 24.2 ng/mL. Though asymptomatic and without any metastatic disease to bone, in October 2008, the patient was enrolled in the previously described open-label, phase I–II studyCitation12 and was treated with 10 cycles of docetaxel and continuous dasatinib. After the first cycle, the dasatinib and docetaxel doses were reduced from 100 mg daily for dasatinib and 75 mg/m2 for docetaxel to 70 mg daily for dasatinib and 60 mg/m2 for docetaxel, due to stomatitis and mucositis. Docetaxel treatment was discontinued 190 days following the initiation of dasatinib and docetaxel treatment because maximal PSA response was achieved.
Figure 2B CT scans from patient 2 taken in (i) September 2008 and (ii) July 2012.
Note: Arrows in both panels indicate sites of metastases.
Abbreviation: CT, computed tomography.
Baseline study assessments prior to initiating dasatinib and docetaxel treatment (day 0), at docetaxel discontinuation (day 190), and at the last assessment on single-agent dasatinib (day 1373) are summarized in .
Discussion
Upon initial diagnosis with prostate cancer, androgen deprivation therapy is usually highly effective and successful in managing many patients’ disease.Citation2 Unfortunately, most patients develop resistance to androgen therapy and become castrate-resistant,Citation2 with AR-independent pathways becoming disease drivers. Docetaxel has become the standard of care over the last 10 yearsCitation13–Citation15 because it has demonstrated improvement in overall survival by treating the underlying prostate cancer and has ameliorated pain after progression. More than 10 different combinations with docetaxel have failed in previous phase III trials;Citation16–Citation19 however, we continue to strive for new treatment options to target AR-independent pathways and perhaps resensitize the AR to hormonal therapy. Here, we have explored the addition of an agent to docetaxel, which exploits multiple pathways within the prostate cancer tumor microenvironment.
Dasatinib is a potent oral tyrosine kinase inhibitor that has been approved for treatment of CML,Citation7 aiding in the transformation of this tumor type into a chronic disease by targeting the BCR-ABL kinase. Dasatinib combined with docetaxel in mCRPC provides synergistic efficacy, which has been demonstrated both preclinically and clinically.Citation8,Citation12 In addition, dasatinib has been shown preclinically to have antimetastatic activity that prevents the development of new metastasesCitation9,Citation10 and targets the underlying bone disease by potently inhibiting osteoclasts.Citation11
Dasatinib targets a number of different kinases, including ACK1, BCR-ABL, SRC family (SRC, LCK, YES, FYN), c-KIT, EPHA2, and PDGFRβ,Citation7 with SRC inhibition postulated to be the primary mechanism through which it works in mCRPC. Through directly targeting AR-independent pathways and with the potential mechanism of resensitizing AR, dasatinib is an important new treatment being explored in mCRPC.
Initial phase I–II study results, including a 60% RECIST response rate, prevention of metastases with the stabilization of bone lesions, and reduction in bone turnover markers, provide some evidence of efficacy for the combination of dasatinib with docetaxel in mCRPC.Citation12 However, long-term follow up of this study has allowed for the most intriguing part of this study to be reported: the ability of dasatinib, as a single agent, to hold the disease by preventing progression.
In the two presented cases, disease had progressed with bone or soft tissue metastases and appeared to have become resistant to androgen deprivation prior to study entry. Patient 1, who initially presented with bone metastasis, continued to have resolution of bone metastasis after discontinuation of docetaxel, followed by stabilization and prevention of new lesions while on single-agent dasatinib. He remains asymptomatic as of September 2012. Patient 2, who presented with lymph node metastasis, was able to achieve a maximal PSA response while on dasatinib therapy after discontinuing docetaxel treatment and remains asymptomatic as of September 2012 as well.
The two cases described in this report suggest that the addition of dasatinib to docetaxel can provide meaningful clinical benefit to patients, delaying disease progression, which will hopefully translate into prolonged survival.
Acknowledgments
We wish to thank Mark A Wildgust, PhD, for his insight in the development of this article, and Christopher Logothetis, MD, for his critical scientific review of earlier drafts of this case report. Professional medical writing and editorial assistance was provided by Ami P Modi, PhD, at StemScientific, and funded by Bristol-Myers Squibb (BMS).
Disclosure
GCT and PP are BMS employees and have stock ownership in BMS. The authors report no other conflicts of interest in this work.
References
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- ScherHISawyersCLBiology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axisJ Clin Oncol200523328253826116278481
- LamontKRTindallDJMinireview: Alternative activation pathways for the androgen receptor in prostate cancerMol Endocrinol201125689790721436259
- ChenYSawyersCLScherHITargeting the androgen receptor pathway in prostate cancerCurr Opin Pharmacol20088444044818674639
- GuoZDaiBJiangTRegulation of androgen receptor activity by tyrosine phosphorylationCancer Cell200610430931917045208
- AsimMSiddiquiIAHafeezBBBaniahmadAMukhtarHSrc kinase potentiates androgen receptor transactivation function and invasion of androgen-independent prostate cancer C4-2 cellsOncogene200827253596360418223692
- Bristol-Myers Squibb CompanySPRYCEL®(Dasatinib) [Package Insert]Princeton, NJBristol-Myers Squibb Company2012 Available from: http://packageinserts.bms.com/pi/pi_sprycel.pdfAccessed February 5, 2013
- KoreckijTNguyenHBrownYuEYVessellaRLCoreyEDasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysisBr J Cancer2009101226326819603032
- NamSKimDChengJQAction of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cellsCancer Res200565209185918916230377
- ParkSIZhangJPhillipsKATargeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse modelCancer Res20086893323333318451159
- de VriesTJMullenderMGvan DuinMAThe Src inhibitor AZD0530 reversibly inhibits the formation and activity of human osteoclastsMol Cancer Res20097447648819372577
- AraujoJCMathewPArmstrongAJDasatinib combined with docetaxel for castration-resistant prostate cancer: Results from a phase 1–2 studyCancer20121181637121976132
- NCCN [webpage on the Internet]Clinical Practice Guidelines in Oncology–Prostate Cancer V1.2012Fort WashingtonNational Comprehensive Cancer Network2012 Available from: http://www.nccn.orgAccessed March 27, 2012
- HorwichAParkerCBangmaCKatajaVESMO Guidelines Working GroupProstate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201021Suppl 5v129v13320555062
- SaadFHotteSJGuidelines for the management of castrate-resistant prostate cancerCan Urol Assoc J20104638038421191494
- AdamoVNotoLFranchinaTEmerging targeted therapies for castration-resistant prostate cancerFront Endocrinol (Lausanne)201237322666217
- NelsonJBFizaziKMillerKPhase 3, randomized, placebo- controlled study of zibotentan (ZD4054) in patients with castration- resistant prostate cancer metastatic to boneCancer2012118225709571822786751
- ScherHIJiaXChiKRandomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancerJ Clin Oncol201129162191219821483004
- Regeneron Pharmaceuticals IncSanofi and Regeneron Announce Regulatory and Clinical Update for ZALTRAP®(Aflibercept) [Press Release]TarrytownRegeneron Pharmaceuticals, Inc2012 Available from: http://files.shareholder.com/downloads/REGN/0x0x557779/94df2942-a7b5-4ea1-9c17-5ba3bd98c198/REGN_News_2012_4_5_General_Releases.pdfAccessed December 6, 2012