Abstract
Purpose
Cetuximab (CET) combined with chemotherapy significantly improved the survival in RAS and RAF wild-type metastatic colorectal cancer (mCRC) patients, while clinical evidence was lacking on the use of maintenance therapy (MT). The study aimed to explore the role of maintenance therapy following Cetuximab + chemotherapy and the optimal Cetuximab-based maintenance therapy regimen.
Patients and Methods
We retrospectively reviewed data on the efficacy and safety of CET-based MT in patients with mCRC who achieved disease control after induction therapy.
Results
Eighty-one patients with mCRC who achieved disease control after CET + chemotherapy induction were enrolled. Overall median progression-free survival (PFS) was 10.5 (95% CI = 8.8–12.2) months and median maintenance/observation PFS (mnPFS) was 6.0 (95% CI = 5.0–7.0) months. Among these 81 patients, 61 patients were prescribed MT (CET alone for 21 patients and CET + chemotherapy for 40 patients). Median PFS and mnPFS in the MT group were significantly longer than those for the non-MT group. Different MT regimens did not affect PFS and mnPFS significantly. Univariate and multivariate analysis demonstrated MT, complete response/partial response during induction therapy, and absence of peritoneal metastasis to be positively associated with longer PFS and mnPFS. Treatment-related adverse events (AEs) were tolerable during MT, and AE-related deaths were not observed.
Conclusion
MT with CET or CET + chemotherapy was an appropriate option following initial induction chemotherapy for patients with RAS and RAF wild-type mCRC. This strategy endowed survival benefits and a tolerable safety profile.
Introduction
Colorectal cancer (CRC) is the third most common and second most lethal cancer type worldwide. CRC was estimated to cause 935,000 deaths worldwide in 2020.Citation1 Approximately 25% present with metastases at initial diagnosis and almost 50% of patients with colorectal cancer will develop metastases.Citation2,Citation3 Over the past two decades, the prognosis of patients with mCRC has been improved markedly due to multi-modal treatments, and a median overall survival (OS) of ~30 months has been documented.Citation4 5-Fluorouracil (5-FU)-based chemotherapy (combined with oxaliplatin or irinotecan) plus anti-epidermal growth factor receptor/vascular endothelial growth factor (anti-EGFR/VEGF) therapy is a first-line treatment for microsatellite-stable mCRC.Citation5–7
Cetuximab (CET) is a recombinant, human/mouse chimeric immunoglobulin monoclonal antibody that binds exclusively to the extracellular domain of the EGFR. CET interferes with the apoptosis and proliferation of cells, angiogenesis, and tumor metastasis. CET in combination with chemotherapy has been shown to improve the survival in RAS and RAF wild mCRC patients.Citation5 However, patients undergoing oxaliplatin- or irinotecan-based therapies tend to discontinue treatment prematurely due to severe neurotoxicity (oxaliplatin) or chronic diarrhea (irinotecan). Thus, switching to low-intensity or low-toxicity maintenance therapy (MT) can balance clinical efficacy and adverse effects (AEs) after disease control. Randomized trials have shown MT using bevacizumab plus fluoropyrimidine to be superior to intermittent treatment or continuous combined chemotherapy in patients with mCRC.Citation8,Citation9 However, the role of MT with CET is controversial.
The efficacy and safety of MT with different CET-based regimens in mCRC have been studied in several clinical trials. The MACRO-2 trial concluded that MT using CET alone was more tolerable than continued induction therapy.Citation10 Moreover, the MACBETH trial compared MT with CET alone or bevacizumab alone after chemotherapy plus induction therapy with CET. Greater clinical efficacy was achieved with MT using CET than with MT using bevacizumab, but the difference was not significant.Citation11 In the VALENTINO trial, MT with the anti-EGFR antibody panitumumab plus fluorouracil achieved longer progression-free survival (PFS) and OS than that by using panitumumab alone.Citation12 Clinical studies have explored CET combined with single-agent chemotherapy (eg, capecitabine or irinotecan) for MT and shown satisfactory efficacy and safety.Citation7 However, consensus regarding the best CET-based MT regimen (ie, single-agent CET or combined with chemotherapy) has not been reached.
Herein, we retrospectively reviewed the data for efficacy and safety of CET-based MT in patients with mCRC or recurrent colorectal cancer in Qilu Hospital of Shandong University.
Materials and Methods
Inclusion Criteria
The inclusion criteria were: (1) Unresectable or recurrent mCRC with wild-type RAS and RAF status; (2) information on efficacy evaluation based on a complete response (CR), partial response (PR) or stable disease (SD) after first-line induction treatment with chemotherapy plus CET was available; (3) patients had adequate hematologic, hepatic, and renal functions; (4) at least one lesion could be measured in one dimension according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 ().
Study Population
We retrospectively collected the information of patients with RAS and RAF wild type (WT) mCRC who were treated with CET-based chemotherapy as first-line therapy and achieved disease control in Qilu Hospital of Shandong University (Jinan and Qingdao) from January 2016 to December 2020.
Treatment Methods
Eligible patients accepted observation or CET-based MT: CET, CET + irinotecan, CET + capecitabine, or CET + CF (5-FU and leucovorin (LV)). The dose of CET during induction and maintenance therapy is 500 mg/m2 on day 1 every 2 weeks. The dose of other agents followed international guidelines and the physician’s decision. The exact starting dose, dose modification, and dose interruption of CET and chemotherapy drugs were determined by the physician based on the results of clinical trials, general health status, and choice of individual patients.
Efficacy and Toxicities
Tumor responses were evaluated every 3–4 cycles of treatment according to the criteria in RECIST 1.1. Patients were evaluated at an early time point if significant signs of progressive disease presented rapidly. The “objective response” comprised the CR and PR. The “disease control rate” was defined as CR+PR and SD. Progression-free survival (PFS) was calculated from the beginning of induction therapy to the time of disease progression or death due to any cause. PFS during maintenance therapy/observation (mnPFS) was calculated from the beginning of MT/observation to the time of disease progression or death due to any cause. Toxicities were assessed based on Common Toxicity Criteria version 5.0 (CTC5.0) set by the US National Cancer Institute. The cutoff date for data use was 31 December 2021.
Statistical Analyses
The Pearson χ2 test was used to compare the difference in the characteristics at baseline and AEs between different groups of patients. PFS curves were constructed based on the Kaplan–Meier method. Median PFS and 95% confidence intervals (CIs) were estimated with the Log rank test. Cox regression analysis was employed to estimate significant factors in univariate and multivariate analysis. Statistical analyses were carried out using SPSS 22.0 (IBM, Armonk, NY, USA). p <0.05 (two-tailed) was considered significant.
Results
Patient Characteristics
A total of 81 patients were included in the study, which were treated with combination chemotherapy plus CET and achieved disease control after induction therapy, including 61 men (75.3%) and 20 women (24.7%). The median age of the study cohort was 64 (range, 22–83) years. The Eastern Cooperative Oncology Group performance score was 0/1 for 79 patients (97.5%) and ≥2 for two (2.5%) patients. Seventy-eight (96.3%) patients were diagnosed with left-sided primary CRC, and three (3.7%) patients were diagnosed with right-sided primary colon cancer. All patients were confirmed to be pMMR/MSS by immunohistochemical- or polymerase chain reaction-based assays. Fourteen (17.3%) patients had peritoneal metastasis. Sixty-one patients (75.3%) underwent resection of the primary tumor and twenty (24.7%) did not undergo surgical treatment. Among those patients, 39 (48.1%) received induction chemotherapy of CET with 5-FU/LV/oxaliplatin (mFOLFOX6), 32 (39.5%) received CET with 5-FU/LV/irinotecan (FOLFIRI), and 10 (12.3%) received CET with capecitabine/irinotecan (mXELIRI). Forty-nine (60.5%) patients experienced PR as the best response upon induction treatment, 32 (39.5%) patients had SD, and no patient achieved CR. The characteristics of patients at baseline are shown in .
Table 1 Clinicopathological and Disease Characteristics of 81 Metastatic Colorectal Cancer Patients with Disease Control After Induction Treatment (Chemotherapy Combined with CET) at Baseline
Maintenance Therapy
The methods of MT and treatment interruption/discontinuation are summarized in and , respectively. Most patients (61 of 81, 75.3%) were prescribed CET or CET + chemotherapy as MT. Among them, 21 (34.4%) accepted CET and 40 (65.6%) accepted CET plus chemotherapy (five patients (8.2%) had CET + CF, 15 patients (24.6%) had CET + irinotecan, and 20 patients (32.8%) had CET + capecitabine). Twelve patients (19.7%) experienced dose reduction. By the cutoff date, 37 (60.7%) patients had discontinued MT due to disease progression and 12 (19.7%) discontinued due to intolerable AEs.
Table 2 Treatment Modes After Induction Therapy
Clinical Efficacy
The median duration of follow-up was 16.5 months (range: 3.5–60.5 months). Overall median PFS for the 81 patients enrolled was 10.5 (95% CI, 8.8–12.2) months (). Overall median mnPFS was 6.0 (95% CI, 5.0–7.0) months (). The factors that influenced PFS and mnPFS according to COX univariate analyses are shown in . Then, we performed a multivariate analysis that depended on the outcomes of the univariate analyses. Multivariate analysis demonstrated MT, PR/CR during induction therapy, and an absence of peritoneal metastasis to be positively associated with PFS and mnPFS (). Among the 61 patients who had MT, a significant difference was not detected in PFS or mnPFS between the single-agent CET group or CET + chemotherapy group ().
Table 3 Univariate Analyses for PFS and PFS During Maintenance Therapy/Observation (mnPFS)
Table 4 Multivariate Analyses for PFS and PFS During Maintenance Therapy/Observation (mnPFS)
Table 5 Univariate Analyses for PFS and mnPFS with Maintenance Therapy
Figure 2 Kaplan-Meier plot of progression-free survival (PFS) and log-rank analysis of predictors of CET-based treatment in mCRC patients (n = 81 for a to d, n = 61 for e). (a) All patients (b) Maintenance/Observation (c) Best overall response during induction therapy (d) Peritoneal metastasis (e) Maintenance therapy regimen.
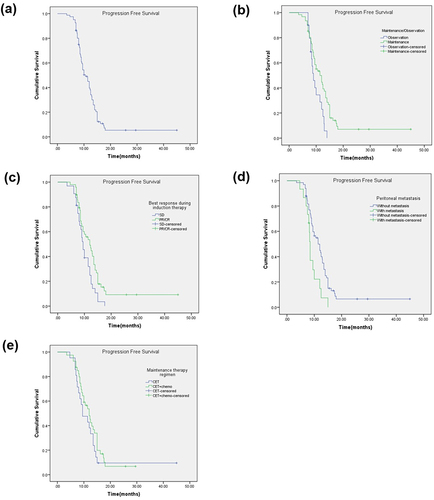
Figure 3 Kaplan-Meier plot of PFS during maintenance/observation (mnPFS) and log-rank analysis of predictors of CET-based treatment in mCRC patients (n = 81 for a to d, n = 61 for e). (a) All patients (b) Maintenance/Observation (c) Best overall response during induction therapy (d) Peritoneal metastasis (e) Maintenance therapy regimen.
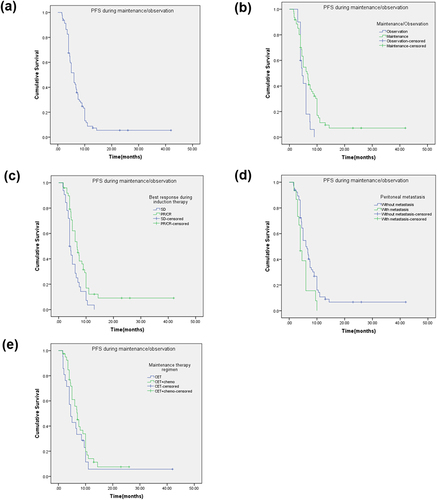
Subgroup analysis showed that PFS and mnPFS were 11.4 (95% CI = 9.0–13.8) months and 6.4 (95% CI = 4.8–8.0) months in the MT group, respectively, which were significantly better than those in the observation group (9.0 (95% CI = 7.7–10.3) months and 4.6 (95% CI = 3.3–5.9) months, respectively; p = 0.014 and 0.019 by Log rank test) ( and ). Median PFS of patients who achieved a PR/CR was 12.0 (95% CI = 9.4–14.6) months compared with 9.5 (95% CI = 8.4–10.6) months for patients with SD during induction therapy (p = 0.001 by Log rank test) (). PFS for patients who did not have peritoneal metastasis was significantly longer than that of patients who had peritoneal metastasis (11.4 months (95% CI = 9.3–13.5) vs 8.5 months (95% CI = 8.2–8.8), p = 0.009) (). mnPFS was significantly longer (p = 0.005) in patients with a PR/CR during induction therapy (6.7 months, 95% CI = 5.0–8.4) compared with that of patients with SD (4 months, 95% CI = 3.4–4.6) (). Patients with peritoneal metastasis had much shorter mnPFS (4.0 months, 95% CI = 3.0–5.0) compared with patients who did not have peritoneal metastasis (6.0 months, 95% CI = 4.6–7.4; p = 0.001 by Log rank test) (). There was no significant difference among other subgroups. In the MT group, median PFS with CET alone or CET + chemotherapy was 9.5 months (95% CI = 5.9–13.1) and 12.0 months (95% CI = 9.7–14.3), respectively (p = 0.296) (). Median mnPFS with CET alone or CET + chemotherapy was 4.5 months (95% CI = 3.0–6.0) and 7.0 months (95% CI = 5.5–8.5), respectively (p = 0.227) ().
Safety
Toxicity was assessed for all 81 patients. The prevalence of AEs during MT was lower than that during induction chemotherapy (). The prevalence of toxicity of any grade during induction therapy was 100% (81/81). Common treatment-related AEs of any grade were diarrhea (22/81, 27.2%), hand–foot syndrome (18/81, 22.2%), rash acneiform (57/81, 70.3%), anemia (26/81, 32.1%), nausea and vomiting (55/81, 67.9%), fatigue (69/81, 85.2%), and neutropenia (65/81, 80.2%). The prevalence of grade-3 toxicity was 39.5% (32/81), including diarrhea (n = 4), hand–foot syndrome (n = 3), rash acneiform (n = 9), anemia (n = 2), nausea and vomiting (n = 4), fatigue (n = 9), and neutropenia (n = 7). Toxicity of grade ≥4 was not observed. During MT, the most common AEs were of grade 1–2, and the prevalence of any AE of grade 3 was low. Toxicities such as diarrhea, anemia, fatigue, nausea, and neutropenia were alleviated during MT compared with that during induction therapy. Treatment-related toxicity and AEs were well tolerated during MT, with no AE-related deaths during the entire treatment. AEs of grade 3–4 were observed in 14.8% (nine of 61) of patients during MT, including rash acneiform, hand–foot syndrome, anemia, fatigue, oral mucositis, and neutropenia. Patients who had MT using CET + chemotherapy had more AEs than those who had MT with CET alone, including nausea and vomiting (27.5% vs 4.8%, p = 0.034), fatigue (55.0% vs 23.8%, p = 0.020), and neutropenia (42.5% vs 9.5%, p = 0.008). However, the prevalence of AEs of grade 3–4 AEs between MT regimens was not significantly different.
Table 6 Adverse Events Related to Treatment
Discussion
Targeted therapy combined with chemotherapy is first-line treatment for mCRC or recurrent CRC. After stable disease or better response is achieved, switching to low-intensity or low-toxicity MT can balance the need for quality of life (QoL) while maintaining treatment efficacy for a longer period. Most clinical trials have shown MT with bevacizumab to result in survival benefits without seriously compromising QoL.Citation8,Citation9,Citation13 For patients with RAS and RAF wild-type mCRC, clinical evidence is lacking on the use of MT after 5-FU-based chemotherapy combined with anti-EGFR therapy. The role of MT following chemotherapy plus use of anti-EGFR antibody and the optimal regimen to be adopted has not been established.
We showed that standard first-line CET-based treatment plus CET-based MT could significantly improve the PFS and mnPFS of patients with RAS/RAF wild-type mCRC. This regimen had an acceptable toxicity profile. MT achieved the expected anti-tumor activity with tolerable AEs, which is similar to results reported from CET-related clinical trials.Citation6,Citation7,Citation10,Citation11 PFS and mnPFS were 11.4 months and 6.4 months, respectively, in the MT group, which were significantly better than those in the non-MT group (9.0 months and 4.6 months, respectively). In the 61 patients of the MT group, MT with CET alone or combined with chemotherapy did not affect PFS or mnPFS. Multivariate analysis revealed peritoneal metastasis and non-achievement of a PR/CR during induction therapy to be independent predictors of significantly shorter PFS and mnPFS. AE prevalence during MT was lower than that during induction chemotherapy. However, MT with CET + chemotherapy led to a higher prevalence of AEs compared with the use of CET alone, including nausea, fatigue, and neutropenia.
Bevacizumab was the first evidence-based option because of results from clinical trials (Stop and Go, MACRO, and CAIRO3).Citation8,Citation13,Citation14 MT with bevacizumab has become a standard strategy against advanced CRC. Findings from a phase-III randomized controlled trial demonstrated significantly improved median PFS (11.7 months vs 8.5 months in the observation group, p < 0.0001) with MT of CET plus bevacizumab after induction therapy in patients with mCRC,Citation8 which agreed with observations from another randomized controlled trial.Citation13
For patients with RAS and RAF wild-type left-sided advanced CRC, chemotherapy with an anti-EGFR agent is standard first-line treatment. Previous studies have shown that biweekly cetuximab in combination with chemotherapy is tolerable and effective, supporting the use of biweekly cetuximab.Citation15,Citation16 Although not studied extensively, MT with CET against advanced CRC has been suggested.Citation10,Citation17 Coin-B is a phase-II trial comparing differences between an observation group and CET MT group in patients with mCRC after 12 weeks of chemotherapy combined with CET. Results revealed failure-free survival (FFS) in the MT group (14.3 months) to be longer than that in the observation group (12.2 months).Citation18 In the MACROII study, after eight cycles of CET plus mFOLFOX6, patients were assigned to CET + chemotherapy or to MT with CET. The effects of MT with CET followed by chemotherapy were not inferior to those of CET + chemotherapy.Citation10 The MACBETH study explored the role of MT with cetuximab or bevacizumab in patients with RAS and RAF wild-type CRC. Median PFS was 13.3 (95% CI = 11.2–17.3) months in the CET group and 10.8 (95% CI = 9.3–13.9) months in the bevacizumab group (hazard ratio (HR) = 0.73, 95% CI = 0.46–1.17).Citation11 Similarly, in the NORDIC-VII trial, MT with CET alone, with median PFS of 7.5 months and median OS of 21.4 months, was more clinically efficacious than FLOX (5-FU/LV and oxaliplatin) combined with CET.Citation17 Another phase-II clinical trial evaluated the biological activity and safety of capecitabine + CET as novel MT for patients with RAS and RAF wild-type CRC. Median PFS using MT was 7.2 (95% CI = 5.8–8.6) months, median PFS was 12.7 (95% CI = 11.8–15.4) months, and median OS was 27.4 (95% CI = 21.4–35.5) months.Citation7 A prospective study investigated patients with treatment response after induction therapy either entered observation (stop treatment) or maintenance treatment 1 (cetuximab plus irinotecan) groups. After 6–12 cycles of MT-1, patients entered MT-2 (CET only). Median failure-free survival (mFFS) was significantly longer in the MT-1 group compared with that in the observation group (12.7 vs 3.0 months; HR = 0.202, 95% CI = 0.111–0.369, p < 0.001). Overall, mFFS was 19.0 months and 9.3 months in the MT group and observation group, respectively (HR = 0.211, 95% CI = 0.117–0.380, p < 0.001).Citation19 Similar to our study, those studies implied that an MT regimen with CET offered greater efficacy without high toxicities.
Studies have explored the efficacy and safety of MT using CET compared with observation or continuous combined chemotherapy. However, a comparison between use of CET alone or combined with chemotherapy has not been made. We compared the efficacy of MT using CET monotherapy or combination chemotherapy (irinotecan, 5-FU, capecitabine). We found that combination therapy tended to increase PFS, but not significantly so. Hence, use of cetuximab alone could achieve a similar effect to that of combined chemotherapy + CET.
We found that the best overall response during induction therapy was associated significantly with PFS and MT duration. Early shrinkage of tumors is associated with longer survival in patients receiving CET-based systemic chemotherapy for liver metastases from CRC.Citation20,Citation21 Early tumor response is predicted for R0–R1 liver resection and overall survival hazard ratio in multivariate analyses.Citation22 Achieving tumor shrinkage is an important goal of chemotherapy and targeted therapy for CRC because it can translate to resection.Citation23,Citation24 Thus, early shrinkage of a tumor could be an important clinical objective for rapid relief from symptoms, identification of the best candidates for conversion-to-resectionCitation25,Citation26 and ultimately lengthening of OS. We showed that tumor shrinkage was associated with longer PFS and MT efficacy, which is consistent with previous research results. Our results suggest that patients who achieve a PR/CR after induction therapy are good candidates for MT.
Peritoneal metastasis (PM) is associated with a worse prognosis than that for other sites of metastasis.Citation27 PM occurs in approximately one in four CRC patients and is a factor of CRC staging and an important prognostic factor of CRC.Citation28 Studies have found peritoneal metastasis to be an independent prognostic factor for advanced CRC.Citation29 As a tumor-specific location, no definite trial on PM for testing systemic chemotherapy for CRC has been reported. A recent publication reported a mean survival for patients having PM of 16.3 (13.5–18.8) months compared with 19.1 (18.3–19.8) months for patients having liver isolated metastasis.Citation30 On the basis of those findings, we explored the prognostic value of peritoneal metastasis, which indicated that PFS and mnPFS were shorter for patients with CRC who had peritoneal metastasis.
Maintaining treatment at the initial dose is linked to cumulative AEs (eg, sensory neuropathy after using oxaliplatin) and a reduction in QoL. However, ceasing first-line therapy completely can lead to disease progression. One of the most effective strategies to solve these problems is an MT approach with a less aggressive agent, lowering toxicity using different drugs (usually irinotecan or oxaliplatin), initiating a dose-reduction schedule, or using a different drug. This strategy avoids cumulative AEs while retaining more aggressive drugs to combat future disease progression. Consequently, another major goal of MT is to reduce the toxicity of continuous chemotherapy and improve the QoL of patients suffering from mCRC. We found that the prevalence of AEs during MT was lower than that during induction chemotherapy. During MT, the most common AEs were of grade 1–2, and the prevalence of any grade-3 AE was low. For treatment using capecitabine + cetuximab, diarrhea, rash acneiform, and hand–foot syndrome may be the first AEs to appear.
Our retrospective study had two main limitations. First, the sample size was small, especially the group without MT, which hampered subgroup analyses. Second, the duration of follow-up was short, and the OS value for the whole cohort could not be obtained. Thus, the findings of our study must be confirmed in large prospective studies.
Conclusion
In patients with RAS and RAF wild-type mCRC, cetuximab-based MT after induction therapy improves PFS and mnPFS with tolerable AEs. There was no significant advantage for combined MT compared with use of CET alone. However, diarrhea, neutropenia, and nausea were observed more often in patients who had combined MT.
Abbreviations
CET, Cetuximab; mCRC, metastatic colorectal cancer; MT, maintenance therapy; PFS, progression-free survival; mnPFS, maintenance/observation progression-free survival; FFS, failure-free survival; OS, overall survival; AE, adverse event; CR, complete response; PR, partial response; SD, stable disease; QoL, quality of life.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Qilu Hospital (Qingdao) of Shandong University (protocol code KYLL-KS-2023131). Individual consent was waived because of the retrospective nature of our study. Patients’ data were anonymized and maintained with confidentiality.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel R, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- O’Connell J, Maggard M, Ko C. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96(19):1420–1425. doi:10.1093/jnci/djh275
- Van Cutsem E, Oliveira J. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;61–63. doi:10.1093/annonc/mdp130
- Sonbol M, Mountjoy L, Firwana B, et al. The role of maintenance strategies in metastatic colorectal cancer: a systematic review and network meta-analysis of randomized clinical trials. JAMA Oncol. 2020;6(3):e194489. doi:10.1001/jamaoncol.2019.4489
- Lenz H, Ou F, Venook A, et al. Impact of consensus molecular subtype on survival in patients with metastatic colorectal cancer: results from CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2019;37(22):1876–1885. doi:10.1200/jco.18.02258
- Heinemann V, von Weikersthal L, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, Phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075. doi:10.1016/s1470-2045(14)70330-4
- Wang L, Liu Y, Yin X, et al. Effect of reduced-dose capecitabine plus cetuximab as maintenance therapy for RAS wild-type metastatic colorectal cancer: a Phase 2 clinical trial. JAMA Network Open. 2020;3(7):e2011036. doi:10.1001/jamanetworkopen.2020.11036
- Simkens L, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385(9980):1843–1852. doi:10.1016/s0140-6736(14)62004-3
- Hegewisch-Becker S, Graeven U, Lerchenmüller C, et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2015;16(13):1355–1369. doi:10.1016/s1470-2045(15)00042-x
- Aranda E, García-Alfonso P, Benavides M, et al. First-line mFOLFOX plus cetuximab followed by mFOLFOX plus cetuximab or single-agent cetuximab as maintenance therapy in patients with metastatic colorectal cancer: Phase II randomised MACRO2 TTD study. Eur J Cancer. 2018;101:263–272. doi:10.1016/j.ejca.2018.06.024
- Cremolini C, Antoniotti C, Lonardi S, et al. Activity and safety of cetuximab plus modified FOLFOXIRI followed by maintenance with cetuximab or bevacizumab for RAS and BRAF wild-type metastatic colorectal cancer: a randomized Phase 2 clinical trial. JAMA Oncol. 2018;4(4):529–536. doi:10.1001/jamaoncol.2017.5314
- Pietrantonio F, Morano F, Corallo S, et al. Maintenance therapy with panitumumab alone vs panitumumab plus fluorouracil-leucovorin in patients with RAS wild-type metastatic colorectal cancer: a Phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1268–1275. doi:10.1001/jamaoncol.2019.1467
- Yalcin S, Uslu R, Dane F, et al. Bevacizumab + capecitabine as maintenance therapy after initial bevacizumab + XELOX treatment in previously untreated patients with metastatic colorectal cancer: Phase III “Stop and Go” study results--a Turkish Oncology Group Trial. Oncology. 2013;85(6):328–335. doi:10.1159/000355914
- Díaz-Rubio E, Gómez-España A, Massutí B, et al. First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD study. Oncologist. 2012;17(1):15–25. doi:10.1634/theoncologist.2011-0249
- Matsuda A, Yamada T, Jamjittrong S, et al. Comparison between biweekly and weekly cetuximab in patients with metastatic colorectal cancer: a meta-analysis. Anticancer Res. 2020;40(6):3469–3476. doi:10.21873/anticanres.14333
- Zekri J, Abbas Baghdadi M, Belal Ibrahim R, Meliti A, Sobahy TM. Biweekly cetuximab in combination with capecitabine and oxaliplatin (XELOX) or irinotecan (XELIRI) in the first-line and second-line treatment of patients with RAS wild-type metastatic colorectal cancer. ecancermedicalscience. 2022;16. doi:10.3332/ecancer.2022.1490
- Pfeiffer P, Sorbye H, Qvortrup C, et al. Maintenance therapy with cetuximab every second week in the first-line treatment of metastatic colorectal cancer: the NORDIC-7.5 study by the Nordic Colorectal Cancer Biomodulation Group. Clin Colorectal Cancer. 2015;14(3):170–176. doi:10.1016/j.clcc.2015.03.002
- Wasan H, Meade A, Adams R, et al. Intermittent chemotherapy plus either intermittent or continuous cetuximab for first-line treatment of patients with KRAS wild-type advanced colorectal cancer (COIN-B): a randomised phase 2 trial. Lancet Oncol. 2014;15(6):631–639. doi:10.1016/s1470-2045(14)70106-8
- Jiang T, Chen H, Zheng J, et al. Cetuximab maintenance therapy in patients with unresectable wild-type RAS and BRAF metastatic colorectal cancer: a Single-Institute Prospective Study. Adv Ther. 2020;37(6):2829–2840. doi:10.1007/s12325-020-01360-8
- Piessevaux H, Buyse M, Schlichting M, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2013;31(30):3764–3775. doi:10.1200/jco.2012.42.8532
- Cremolini C, Loupakis F, Antoniotti C, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26(6):1188–1194. doi:10.1093/annonc/mdv112
- Bouchahda M, Boige V, Smith D, et al. Early tumour response as a survival predictor in previously- treated patients receiving triplet hepatic artery infusion and intravenous cetuximab for unresectable liver metastases from wild-type KRAS colorectal cancer. Eur J Cancer. 2016;68:163–172. doi:10.1016/j.ejca.2016.09.011
- Chiappa A, Makuuchi M, Lygidakis N, et al. The management of colorectal liver metastases: expanding the role of hepatic resection in the age of multimodal therapy. Crit Rev Oncol Hematol. 2009;72(1):65–75. doi:10.1016/j.critrevonc.2008.11.003
- Ksienski D, Woods R, Speers C, Kennecke H. Patterns of referral and resection among patients with liver-only metastatic colorectal cancer (MCRC). Ann Surg Oncol. 2010;17(12):3085–3093. doi:10.1245/s10434-010-1304-9
- Ye L, Wei Y, Zhu D, Chen T, Xu J. Impact of early tumor shrinkage on clinical outcome in wild-type-KRAS colorectal liver metastases treated with cetuximab. J Gastroenterol Hepatol. 2015;30(4):674–679. doi:10.1111/jgh.12847
- Douillard J, Siena S, Peeters M, Koukakis R, Terwey J, Tabernero J. Impact of early tumour shrinkage and resection on outcomes in patients with wild-type RAS metastatic colorectal cancer. Eur J Cancer. 2015;51(10):1231–1242. doi:10.1016/j.ejca.2015.03.026
- Roth L, Russo L, Ulugoel S, et al. Peritoneal metastasis: current status and treatment options. Cancers. 2021;14(1):60. doi:10.3390/cancers14010060
- Ceelen W, Ramsay R, Narasimhan V, Heriot A, De Wever O. Targeting the tumor microenvironment in colorectal peritoneal metastases. Trends Cancer. 2020;6(3):236–246. doi:10.1016/j.trecan.2019.12.008
- Mendoza-Moreno F, Diez-Alonso M, Matías-García B, et al. Prognostic factors of survival in patients with peritoneal metastasis from colorectal cancer. J Clin Med. 2022;11(16):4922. doi:10.3390/jcm11164922
- Franko J, Shi Q, Meyers J, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709–1719. doi:10.1016/s1470-2045(16)30500-9