Abstract
Bevacizumab is a monoclonal antibody that binds and neutralizes vascular endothelial growth factor (VEGF)-A, a key player in the angiogenesis pathway. Despite benefits of bevacizumab in cancer therapy, it is clear that the VEGF pathway is complex, involving multiple isoforms, receptors, and alternative ligands such as VEGF-B, and placental growth factor, which could enable escape from VEGF-A-targeted angiogenesis inhibition. Recently developed therapies have targeted other ligands in the VEGF pathway (eg, aflibercept, known as ziv-aflibercept in the United States), VEGF receptors (eg, ramucirumab), and their tyrosine kinase signaling (ie, tyrosine kinase inhibitors). The goal of the current review was to identify comparative preclinical data for the currently available VEGF-targeted therapies. Sources were compiled using PubMed searches (2007 to 2012), using search terms including, but not limited to: “bevacizumab,” “aflibercept,” “ramucirumab,” and “IMC-18F1.” Two preclinical studies were identified that compared bevacizumab and the newer agent, aflibercept. These studies identified some important differences in binding and pharmacodynamic activity, although the potential clinical relevance of these findings is not known. Newer antiangiogenesis therapies should help further expand treatment options for colorectal and other cancers. Comparative preclinical data on these agents is currently lacking.
Introduction: why target angiogenesis?
Angiogenesis, the generation of new blood vessels, is an essential physiological process that can be dysregulated in various pathological conditions, including cancer.Citation1,Citation2 The vascular endothelial growth factor (VEGF) pathway is considered the most important and is a well-characterized contributor to angiogenesis.Citation3,Citation4 VEGF-A and other members of the VEGF family such as placental growth factor (PlGF) are upregulated in pathological conditions.Citation5–Citation8 VEGF-A, the first VEGF characterized, has served as a paradigm for the development of antiangiogenesis as a therapeutic strategy, including the clinical development of bevacizumab, a humanized monoclonal antibody targeting VEGF-A.Citation9 In a pivotal trial, the use of bevacizumab in combination with irinotecan, 5-fluorouracil (5-FU), and leucovorin (IFL) was shown to improve the survival of patients with metastatic colorectal cancer (mCRC),Citation10 resulting in its approval as the first antiangiogenic therapy.Citation9 Nonetheless, the overall impact of agents such as bevacizumab in prolonging survival has been limited.Citation2,Citation11 While 2-year survival has improved to the 24- to 28-month range, the overall prognosis of mCRC remains poor, with 5-year survival generally between 5% and 8%, despite the availability of such therapy.Citation11
Evidence is emerging that PlGF and other members of the VEGF family such as VEGF-B, although less well studied and understood, may also play a role in pathological angiogenesis.Citation8,Citation12,Citation13 For example, in genetically modified mice, expression of host and tumor PlGF was required for maximal tumor angiogenesis, whereas PlGF deficiency resulted in poorly vascularized tumors.Citation13 The upregulation of PlGF and other potentially angiogenic factors such as platelet-derived growth factors (PDGFs) and fibroblast growth factor (FGF) may also underlie disease progression in patients receiving bevacizumab.Citation14 There are multiple strategies for targeting angiogenesis, and in the present review, the currently available biological agents in Phase III or later development for mCRC that target the VEGF pathway are highlighted. Some of the biological anti-VEGF agents currently approved or in Phase III evaluation are shown in . In addition, in a systematic review of the more recent literature, comparative preclinical studies among these agents were identified.
Table 1 Biological anti-VEGF agents: currently approved and/or under phase III evaluation
Angiogenesis, the VEGF network: ligands and receptors
VEGF ligands
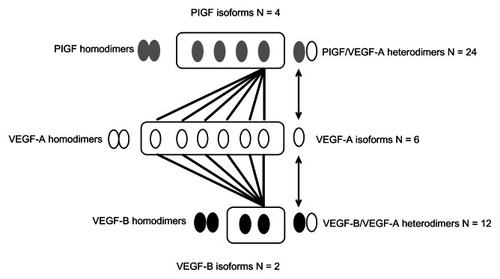
The VEGF family consists of five structurally related ligands, VEGF-A, -B, -C, and -D, and PlGF.Citation15 VEGF-A interacts with VEGF receptor-1 (VEGFR-1) and VEGFR-2 and has potent proangiogenic and vascular permeability increasing effects. VEGF-B, by comparison, interacts with VEGFR-1 only, and its function has not been well characterized.Citation16 While it does not appear to play a directly angiogenic role, there is evidence that VEGF-B may function as an angiogenesis survival factor.Citation12 VEGF-C and VEGF-D both interact with VEGFR-2 and VEGFR-3, and these factors are believed to play a role in both angiogenesis and lymphangiogenesis; PlGF, like VEGF-B, also interacts with VEGFR-1 only and its function is incompletely understood, although there is accumulating evidence for a role of PlGF in pathological angiogenesis, as described below.Citation17 The diversity in the VEGF ligand family is still further increased by the existence of multiple, alternatively spliced isoforms, each of which can have distinct and/or overlapping biological activities ().Citation5,Citation6,Citation18,Citation19 These distinct VEGF isoforms also have the potential to homo- and heterodimerize, which can result in a very wide array of homo- and heterodimeric signaling molecules, the impetus of which is just beginning to be explored (). For example, among ligands, which can interact with VEGFR-1, VEGF-A is known to exist in at least six isoforms, VEGF-B in at least two isoforms,Citation16,Citation20 and PlGF in at least four isoforms, resulting in at least some 36 potentially diverse signaling molecules arising from the heterodimeric combination of these isoforms ().Citation17 Although it has not been carefully studied, differences in properties such as heparin binding are observed among the different isoforms, and there could potentially be diverse biological functions of these molecules, and the implications of this for angiogenesis are not yet understood.Citation5,Citation6
Figure 1 Diversity of VEGF and PlGF isoforms: homo- and heterodimers.
Notes: The VEGF ligands, VEGF-A, VEGF-B, and PlGF can all interact with VEGFR-1, and the illustration provides an example of how diversity in isoforms can result in a wide array of signaling molecules that can interact with the receptor. The four PlGF isoforms can homo- and heterodimerize with any of six distinct isoforms of VEGF-A, as can the two isoforms of VEGF-B, resulting in many combinations that can potentially exhibit different biological activity.Citation5,Citation6,Citation17
Abbreviations: PlGF, placental growth factor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
In terms of amount and biological activity, VEGF-A 165 appears to be the dominant isoform;Citation4 however, it is also important to note that more recently, antiangiogenic isoforms of VEGF-A, of which there are at least five subtypes, have been identified.Citation18 These findings have brought into question some of the original thinking in terms of the design of antiangiogenesis therapy, in as much as it is no longer clear that selective targeting of a single factor and/or isoform will necessarily achieve the desired result of angiogenesis inhibition in the tumor microenvironment. It is clear that multiple alternative and/or overlapping angiogenesis pathways exist; the potential for these mechanisms to be exploited by tumors in the face of targeted inhibitory molecules must be recognized.
VEGF receptors
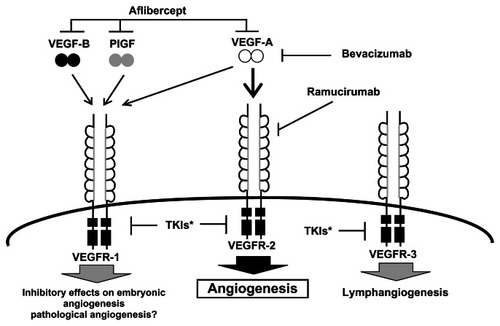
All three VEGFRs have intrinsic tyrosine kinase activity, and the neuropilins (NPs) also appear to serve as coreceptors and modulators of VEGFRs.Citation6,Citation15 shows the different VEGFRs and the ligands to which they bind.Citation4 The function of VEGFR-1 is less well characterized but it is believed that VEGFR-1 has both vascular and nonvascular functions, and it is expressed in tumor cells, as well as monocytes and macrophages,Citation4,Citation6,Citation17,Citation21 whereas VEGFR-2 is believed to be the primary mediator of VEGF-A action on angiogenesis and increased vascular permeability. This receptor was also expressed on tumor cells and has been implicated in the activation of autocrine oncogenic pathways.Citation6,Citation22 The expression of VEGFR-3 is largely limited to lymphatic epithelial cells and is believed to mediate a lymphangiogenic function.Citation4,Citation6
Figure 2 VEGF ligands, receptors, and inhibitors.Citation4,Citation5,Citation15,Citation19
Adapted with permission from Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull. 2011;34(12):1785–1788.Citation4
Abbreviations: PlGF, placental growth factor; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Role of VEGF-B and PlGF
As noted above, the role of VEGFR-1 and its ligands PlGF and VEGF-B is incompletely understood.Citation12,Citation17,Citation21 Gene targeting experiments have revealed no apparent impact of loss of PlGF on embryonic angiogenesis, even in combination with VEGF-B inactivation.Citation13 Loss of PlGF, however, did lead to reduced angiogenesis in pathological conditions, including ischemia, inflammation, and cancer,Citation13 and there is evidence for a benefit of anti-PlGF therapy in cancer cell models and inhibiting ocular neovascularization.Citation23 Some evidence suggests that anti-PlGF antibodies are effective in inhibiting pathological angiogenesis across multiple tumor models.Citation24 Notably, unlike anti-VEGFR-2 antibodies, anti-PlGF did not induce an angiogenic escape program.Citation24 Other data, however, are conflicting and have shown no effect of anti-PlGF antibodies on tumor angiogenesis in multiple cell lines, including those resistant to VEGF-A antibodies.Citation25 Significantly increased expression of PlGF isoforms has been observed in colorectal cancers, compared with normal tissue, and in those with poor outcomes.Citation7 There is also increasing evidence for a role of PlGF and other factors as possible escape mechanisms during antiangiogenesis therapy with bevacizumab.Citation14,Citation26 Increased expression of PlGF in particular was observed prior to progressive disease in patients receiving bevacizumab and chemotherapy.Citation14 Additional findings suggest that VEGFR-1, while apparently not promoting angiogenesis, may nonetheless participate in an autocrine/paracrine growth pathway, particularly in cells susceptible to anti-PlGF antibody.Citation27 In addition, while it is apparently dispensable for blood vessel growth, VEGF-B was necessary for blood vessel survival, and targeting of VEGF-B could inhibit pathological angiogenesis.Citation28 Taken together, these findings suggest that VEGF-B and PlGF, while perhaps not directly proangiogenic themselves, may nonetheless play an important role in activating VEGFR-1 under certain pathological conditions.
Bevacizumab: the first antiangiogenesis therapy in mCRC
Bevacizumab, the first antiangiogenesis therapy to be approved for use in mCRC, is a humanized monoclonal antibody that binds to all isoforms of VEGF-A.Citation9 Evidence for the clinical efficacy of bevacizumab in cancer, notably in the treatment of mCRC, has been reviewed elsewhere.Citation29 As noted earlier, the recent identification of alternatively spliced variants of VEGF-A, some of which may be antiangiogenic, complicates the initial rationale for inhibiting VEGF-A.Citation18 In the United States, bevacizumab is currently indicated in combination with intravenous 5-FU-based chemotherapy for the first- or second-line treatment of mCRC.Citation30
The mechanisms underlying the apparent benefit of bevacizumab in combination with chemotherapy are not well understood. It was previously thought that inhibition of VEGF would lead to vessel “normalization” and increase delivery of chemotherapies to the tumor.Citation31 Recent studies, however, have suggested that bevacizumab actually reduced tumor perfusion, with no evidence for improved drug delivery.Citation32 Notably, as a single agent, bevacizumab is also indicated for the treatment of glioblastoma in patients who have progressed on other therapies.Citation33 The efficacy of bevacizumab in glioblastoma, a highly vascularized tumor, could imply a greater dependence on angiogenesis in this tumor type and a greater susceptibility for inhibition.Citation34,Citation35 Indeed, recent data have shown that bevacizumab therapy reduces blood vessel number, tumor perfusion, and oxygenation in experimental glioblastoma models.Citation35
New biological agents targeting the VEGF pathway: mechanisms
Aflibercept (known in the United States as ziv-aflibercept)
Aflibercept is a recombinant fusion protein consisting of the second immunoglobulin (Ig) domain of VEGFR-1 and the third Ig domain of VEGFR-2, fused to human IgG1. It exhibits affinity for VEGF-A, VEGF-B, and PlGF.Citation36–Citation39 Aflibercept exhibits potent inhibition of human and mouse tumor xenografts in preclinical studies.Citation19 The biology of aflibercept and its antitumor effects in preclinical model systems has been reviewed in detail elsewhere.Citation39 In Phase I studies of patients with advanced solid tumors, aflibercept has displayed a manageable safety profile.Citation37 The recently reported Phase III VELOUR study investigated the efficacy of aflibercept in combination with irinotecan and 5-FU (FOLFIRI) in patients with mCRC who had progressed on a prior oxaliplatin-based regimen.Citation40 It is important to recognize that 30.4% of patients in this study had received prior bevacizumab treatment. Results of VELOUR showed significant improvements in the primary endpoint of overall survival (OS), as well as secondary endpoints of progression-free survival (PFS) and overall response rate (ORR) with aflibercept and FOLFIRI.Citation40 Median OS in the aflibercept arm was 13.50 months compared with 12.06 months with placebo (hazard ratio [HR] = 0.817; P = 0.0032); similarly, median PFS (6.90 months versus 4.67 months, respectively, HR = 0.758; P = 0.00007) and ORR (19.8% versus 11.1%; P = 0.0001) were also significantly improved with aflibercept relative to placebo.Citation40 Grade 3 or higher adverse events (AEs) with a 2% or higher incidence with aflibercept relative to placebo included proteinuria and hypertension, as well as diarrhea, asthenia/fatigue, and stomatitis/ulceration, infections, abdominal pain, and neutropenia.Citation40 The results of VELOUR suggest that targeting multiple ligands may be a viable option to inhibit angiogenesis in cancer, even in patients who have progressed after prior bevacizumab treatment. On the basis of VELOUR, aflibercept has now been approved by the US Food and Drug Administration (FDA) with the US generic name of ziv-aflibercept (ZALTRAP®) for use in combination with FOLFIRI in the treatment of mCRC that is resistant to or that has progressed following an oxaliplatin-containing regimen.Citation41
As in patients with mCRC, the use of aflibercept in patients with advanced ovarian cancer, advanced melanoma, metastatic pancreatic cancer,Citation42 and androgen independent prostate cancerCitation43 has been under investigation. It is worth noting that some of these studies have failed to reach their predetermined primary endpoint, in contrast to the results of VELOUR.Citation40 For example, in the VITAL study,Citation44 which examined the use of aflibercept in combination with docetaxel for second-line treatment of non-small-cell lung cancer (NSCLC), a significant improvement in OS was not observed (HR = 1.01); however, a benefit in PFS (HR = 0.82) and response rate (RR) (23.3% versus 8.9%) was seen with aflibercept.Citation45 AEs associated with the use of aflibercept are consistent with those typically seen with agents that inhibit VEGF and include hypertension, proteinuria, thrombosis, and hemorrhage.Citation46 As such, a pretreatment screening and management plan for hypertension and proteinuria should be in place, and patients receiving aflibercept should be educated about, and monitored for, the signs and symptoms of bleeding.Citation46
Ramucirumab
Ramucirumab (also known as IMC-1121C) is a fully human monoclonal antibody that binds to the extracellular domain of VEGFR-2.Citation47–Citation49 Anti-VEGFR-2 antibodies have shown antitumor activity in a range of tumor model systems.Citation50,Citation51 In a Phase I study of patients with advanced solid tumors, tumor perfusion and vascularity were decreased with ramucirumab therapy in 69% of the patients.Citation47 Ramucirumab is currently under investigation (in combination with chemotherapy) in a number of Phase III studies, including those of breast cancer, NSCLC, and as a second-line therapy for mCRC.Citation48,Citation49 Studies of ramucirumab currently underway in mCRC include a Phase III study of ramucirumab in combination with FOLFIRI chemotherapy in patients with progression following first-line combination therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine,Citation52 and a Phase II study of ramucirumab, cetuximab, and irinotecan versus cetuximab and irinotecan in patients with mCRC and progression following a bevacizumab-based regimen.Citation53 Toxicities associated with inhibition of the VEGF axis with ramucirumab (in Phase I studies) included hypertension, vascular thrombotic events, and proteinuria.Citation47 Ramucirumab is also under investigation in combination with mFOLFOX6 in patients with mCRC, in an open-label, randomized, Phase II study (estimated enrollment = 150), which is currently recruiting patients.Citation54 Patients in the trial had disease progression on an irinotecan-based, first-line chemotherapy regimen (FOLFIRI or CAPIRI), and the primary endpoint was PFS.Citation54 Also recently described at the American Society of Clinical Oncology (ASCO) 2012 Annual Meeting is the RAISE trial;Citation52 this is an ongoing, randomized, double-blinded, placebo-controlled Phase III trial of ramucirumab or placebo in combination with FOLFIRI in patients with mCRC who failed a first-line bevacizumab-, oxaliplatin-, or fluoropyrimidine-based regimen.Citation55 The primary endpoint of the trial will be OS, with secondary endpoints of PFS, RR, safety, and biomarker analysis.Citation55
IMC-18F1
Although it has received less attention than VEGFR-2, there is emerging evidence for a potential role of VEGFR-1 in human cancers, including mCRC, and the inhibition of VEGFR-1 signaling as a potential antiangiogenesis target is just beginning to be explored.Citation56 IMC-18F1 is a high-affinity human VEGFR-1 neutralizing antibody that specifically binds the extracellular domain of VEGFR-1 and prevents its interaction with all of its known ligands (VEGF-A, VEGF-B, and PlGF); as such, it effectively blocks its biological activity in multiple preclinical models, and exhibits antiangiogenic and antiproliferative activity.Citation57 There is also evidence from preclinical models that, similar to bevacizumab and aflibercept, this agent can potentiate the antitumor activity of cytotoxic chemotherapies.Citation57 In the same open-label, randomized, Phase II study as that described above for ramucirumab,Citation54 the combination of IMC 18F1 with mFOLFOX6 is under investigation in mCRC patients with disease progression on an irinotecan-based first-line chemotherapy regimen (FOLFIRI or CAPIRI), and results of this study should determine whether additional Phase III trials are warranted.
Other strategies – tyrosine kinase inhibitors (TKIs)
Because all of the known VEGFRs share an intrinsic tyrosine kinase activity,Citation4,Citation6 another means of targeting the VEGF pathway is through the use of TKIs.Citation58 Many other cellular receptors utilize tyrosine kinases as a component in their signaling pathways; thus, many of these agents have unwanted “off-target” AEs associated with their inhibition of non-VEGFR kinases.Citation58,Citation59 The utility of TKIs as anti-VEGF agents can therefore be limited by their specificity for the various VEGFRs in relation to other receptor tyrosine kinases (ie, on-target and off-target effects).Citation59 In a published review of these small molecule TKIs, although no inter-agent comparisons were done, the on-target effects appeared to be related to VEGFR inhibition. The data were compiled from a review of studies involving approximately 3000 patients treated with various small-molecule, VEGF-targeted TKIs.Citation57 On-target AEs included VEGF inhibition-related events such as hypertension, proteinuria, and hemorrhage. Off-target AEs included events more likely related to the inhibition of other non-VEGF tyrosine kinases, such as fatigue, diarrhea, nausea, anorexia, and hand-foot reaction.Citation59 A number of kinase inhibitors are under investigation as single agents or in combination with chemotherapies for the treatment of mCRC, with many in Phase II or III development. A summary of these agents and the combinations under investigation (not meant to be exhaustive) is shown in .Citation60–Citation70 One of these agents, regorafenib, is discussed below; it was recently approved by the FDA for treatment in patients with mCRC in the third or fourth line, ie, for those who have been previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if KRAS wild type, an anti-EGFR therapy.Citation71
Table 2 Selected anti-VEGF kinase inhibitor agents currently under evaluation in mCRCTable Footnotea
Regorafenib (BAY 73-4506)
Regorafenib is an inhibitor of PDGF receptors, c-kit, FGF receptor, and all three VEGFRs.Citation58,Citation72 In preclinical studies, regorafenib inhibited tumor growth and microvascular density in glioblastoma xenograft models.Citation72 Regorafenib also inhibited tumor growth in breast and renal carcinoma xenograft models.Citation72 There are currently several Phase III trials of regorafenib underway or finished in patients with mCRC, including the completed CORRECT study.Citation54,Citation55,Citation72 Results of CORRECT, a randomized, Phase III study of regorafenib or placebo in patients with mCRC who have progressed after all approved drugsCitation67 have shown that the study met its primary objective of improvement in OS, with no new safety concerns.Citation73 The most common grade 3 or higher toxicities associated with regorafenib in this study included hand-foot skin reaction, fatigue, diarrhea, hyperbilirubinemia, and hypertension.Citation73 On the basis of this trial, regorafenib was approved by the FDA for third- or fourth-line treatment in patients with mCRC. A Phase III interventional, open-arm study of regorafenib in patients with mCRC who have progressed after all standard therapies (CONSIGN) is currently underway.Citation68
Comparing the Anti-VEGF agents: are there differences?
In our search, two studies that compared preclinical binding characteristics among the available anti-VEGF biological agents were identified.Citation38,Citation74 No direct comparative data comparing kinase inhibition or preclinical efficacy among the different TKIs under investigation in mCRC were identified.
Differences in VEGF-A–inhibitor complex formation
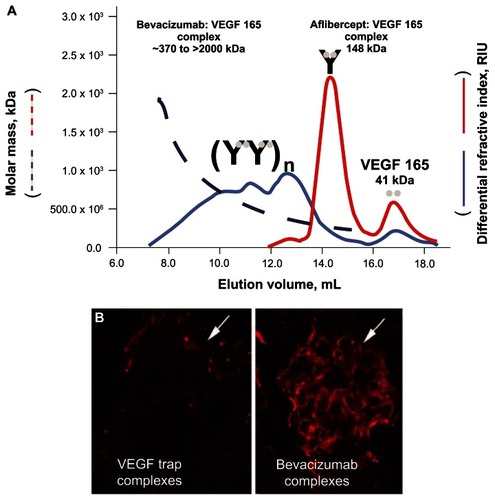
One study found in the search compared the VEGF-A binding characteristics of bevacizumab with those of aflibercept.Citation74 These investigators found that unlike bevacizumab, aflibercept formed stable complexes in the circulation that remained bound to VEGF-A. In addition, although aflibercept formed inert 1:1 complexes with VEGF-A, bevacizumab formed heterogeneous multimeric immune complexes that were rapidly cleared from the circulation ().Citation74 These differences in binding and complex formation between bevacizumab and aflibercept could have important implications in terms of the AE profile; for example, in terms of renal damage and proteinuria resulting from the deposition of VEGF-A– bevacizumab complexes in the kidney.Citation19,Citation74
Figure 3 Molecular masses of aflibercept-VEGF-A and bevacizumab-VEGF-A complexes. (A) Using a 1:2 molar ratio of aflibercept to VEGF 165, discrete peaks were observed at 17 mL and 14.5 mL. In contrast, a 1:2 molar ratio of bevacizumab to VEGF 165 revealed a heterogeneous multimeric complex that ranged in molar mass from 370 kDa to 2000 kDa.Citation74 (B) One milligram of a preformed complex of aflibercept (VEGF Trap) and VEGF 165 or bevacizumab and VEGF 165 were injected into the left ventricle of 2- to 3-month-old C57bl6 mice. After 10 minutes, the mice were killed, and their kidneys were processed for immunocytochemistry, using an antihuman Fc reporter antibody to the human Fc moiety present on both aflibercept and bevacizumab. Significant staining was observed in the glomeruli of bevacizumab/VEGF-treated mice but not in the glomeruli of aflibercept/VEGF-treated mice (white arrows).Citation74 Reprinted with permission from Rudge JS, Holash J, Hylton D, et al. VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc Natl Acad Sci U S A. 2007;104(47):18363–18370.Citation74 Copyright 2007 National Academy of Sciences, USA.
Abbreviations: RIU, refractive index units; VEGF, vascular endothelial growth factor.
Differences in VEGF binding
In another, more recent study, the binding characteristics of bevacizumab and aflibercept were compared using a variety of preclinical assessments.Citation38 In this study, aflibercept showed tight binding to VEGF-A 165, and the dissociation constant (KD) was significantly lower with aflibercept compared with dimerized VEGFR-1 or VEGFR-2. The KD of aflibercept (0.490 pM) was approximately 100-fold lower compared with bevacizumab (58 pM) ().Citation38 Notably, this suggests a 100-fold tighter binding to VEGF-A 165 by aflibercept. A lower KD for aflibercept in binding to VEGF-A 165 was predominantly attributable to its faster association rate (KA), which was 77-fold faster than that seen for bevacizumab ().Citation38 Consistent with its design, aflibercept was also shown to bind to VEGF-B, PlGF-2, and PlGF-1, whereas bevacizumab did not ().Citation38
Table 3 Bevacizumab and aflibercept: comparison of key biological activitiesCitation38Table Footnotea
Differences in biological activity
In terms of their biological activity as assessed via inhibition of VEGF-A or PlGF-2-induced activation of VEGFR-1, both bevacizumab and aflibercept inhibited VEGFR-1 activation induced by VEGF-A 165 or VEGF-A 121. Aflibercept, however, demonstrated 92-fold greater potency than bevacizumab when evaluated in this assay ().Citation38 In line with its binding profile, aflibercept also inhibited VEGFR-1 activation by PlGF-2, whereas bevacizumab did not show any inhibitory activity ().Citation38 When the ability to inhibit VEGF-A-induced activation of VEGFR-2 was examined in this study, aflibercept also inhibited activation of VEGFR-2 induced by VEGF-A 165 and was 51-fold more potent than bevacizumab ().Citation38 As expected, PlGF-2 did not activate VEGFR-2 in this assay system.
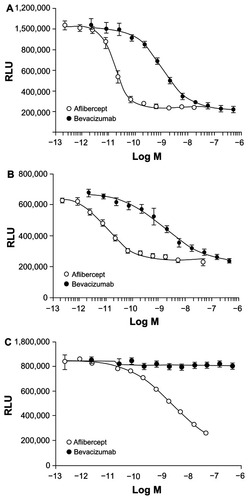
In cells expressing VEGFR-1, aflibercept was also more potent than bevacizumab in inhibiting luciferase activity as stimulated by either VEGF-A 121 or VEGF-A 165 ().Citation38 When the ability to inhibit calcium mobilization in endothelial cells was examined in this study, bevacizumab effectively blocked Ca2+ mobilization in endothelial cells (which express VEGFR-1 and VEGFR-2) when induced by VEGF-A 165 ().Citation38 The inhibitory concentration (IC) 50 for aflibercept was 27-fold lower than bevacizumab in this assay. Similarly, when the ability to inhibit human umbilical vein endothelial cell (HUVEC) migration induced by VEGF-A 165 or PlGF-2 was examined, aflibercept dose-dependently reduced HUVEC migration induced by VEGF-A 165 or PlGF-2.Citation38 Bevacizumab also inhibited HUVEC migration induced by VEGF-A 165, but required greater molar concentrations than aflibercept to produce the same level of inhibition. As expected, bevacizumab did not inhibit HUVEC migration induced by PlGF-2.Citation38
Figure 4 (A) Effect of aflibercept and bevacizumab on luciferase activity in cells expressing VEGFR-1 stimulated by VEGF-A 121.Citation38 (B) Effect of aflibercept and bevacizumab on luciferase activity in cells expressing VEGFR-1 stimulated by VEGF-A 165.Citation38 (C) Effect of aflibercept and bevacizumab on luciferase activity in cells expressing VEGFR-1 stimulated by PlGF.Citation38
Adapted from Papadopoulos N. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171–185. The article/figure is published under Creative Commons License 2.0 CC-BY.Citation38
Abbreviations: RLU, relative luciferase units; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
As a caveat to these findings, it must be noted that preclinical studies are not predictive of clinical efficacy in the treatment of cancer or other pathological conditions. It should also be mentioned that infusion and/or hypersensitivity reactions could distinguish agents such as bevacizumab and aflibercept from the TKIs, which are less likely to cause such AEs.Citation30
AEs
Safety considerations are of course a key aspect of antiangiogenesis therapy, but comparative data can only be gleaned in the context of randomized controlled trials, and the approved labeling of these agents when used in mCRC. Because AEs are also impacted by the concomitant regimen and patient population studied, these factors must also be considered, and as such no directly comparative data exist among the approved biologic agents, bevacizumab and aflibercept. Leukopenia, diarrhea, neutropenia, proteinuria, increased aspartate transaminase, stomatitis, fatigue, throm- bocytopenia, increased alanine transaminase, hypertension, decreased weight, decreased appetite, epistaxis, abdominal pain, dysphonia, increased serum creatinine, and headache were the most common AEs of 20% or greater incidence occurring at higher incidence (2% or more) with aflibercept when used in combination with FOLFIRI (VELOUR trial).Citation41 Neutropenia (37%), diarrhea (19%), hypertension (19%), leukopenia (16%), stomatitis (13%), fatigue (13%), proteinuria (8%), and asthenia (5%) were the most common Grade 3 to 4 adverse reactions of 5% or higher incidence reported at a higher incidence (2% or greater) in the aflibercept/FOLFIRI arm.Citation41 The prescribing information for bevacizumab lists the grade 3 and 4 toxicities (5% or higher incidence) of bevacizumab in combination with IFL as leukopenia (37%), diarrhea (34%), neutropenia (21%), hypertension (12%), asthenia (10%), deep vein thrombosis (9%), abdominal pain (8%), and pain (8%).Citation30 AEs associated with the use of bevacizumab in combination with FOLFIRI (ie, those most comparable to aflibercept) are not available from the prescribing information.
Although assessed in an open-label trial without a placebo control, when used as a first-line therapy in patients (n = 209) with mCRC, bevacizumab in combination with FOLFIRI was associated with grade 3 or 4 AEs (5% or more of patients) of neutropenia (29%), venous thromboembolic events ([VTE], 18%), diarrhea (12%), fatigue (10%), vomiting (7%), deep vein thrombosis (7%), pulmonary embolism (PE, 7%), nausea (6%), febrile neutropenia (6%), and hypertension (5%), with the VTE, PE, and febrile neutropenia events each including at least one event leading to death.Citation75 In addition, at least one grade 3 or 4 targeted AE (ie, those historically associated with the use of bevacizumab), including VTE, hypertension, bleeding, proteinuria, gastrointestinal perforation, and wound healing complications occurred in 35% of patients in this trial overall.Citation75 Taken together, although no directly comparative data are available, despite its broader binding specificity, aflibercept appears to have an AE profile related to inhibition of the VEGF axis, with no major distinguishing event from bevacizumab emerging as yet. Adverse reactions associated with the use of ramucirumab and IMC-18F1 will await results from randomized controlled clinical trials of these agents in mCRC.
Beyond colorectal cancer
Although antiangiogenesis therapy has been most successful in colorectal cancer, and in combination with chemotherapy, increasing diversity of biological agents may allow for a broader use across certain cancers, including glioblastomaCitation76,Citation77 and prostate cancer.Citation78,Citation79 The AVAglio trial is designed to assess the efficacy and safety of bevacizumab in combination with temozolomide in newly diagnosed glioblastoma patients,Citation77 and aflibercept is also currently under investigation in a Phase II study of patients with recurrent malignant glioma that did not respond to temozolomide.Citation80 Bevacizumab has been used in combination with docetaxel and prednisone (DP) in men with metastatic castration-resistant prostate cancer in the CALGB 90401 trial.Citation78 Recently reported results showed no significant improvement in OS with DP plus bevacizumab compared with placebo (HR = 0.91; P = 0.181), although PFS (9.9 versus 7.5 months; P < 0.001) and objective response (49.4% versus 35.5%; P = 0.0013) were significantly improved.Citation78 Aflibercept was also being studied (in combination with DP) in the Phase III VENICE trial of patients with metastatic androgen-independent prostate cancer. It was announced in early 2012, however, that the trial did not meet the prespecified endpoint of improvement in OS.Citation79 Ramucirumab is also currently under investigation in patients with recurrent glioblastoma multiforme,Citation81 and in patients with advanced androgen-independent prostate cancer.Citation82
Conclusion
Angiogenesis continues to be a viable therapeutic target for pathological conditions, including cancer. The development and investigation of bevacizumab as a therapeutic agent has provided a basis for understanding the clinical potential for biological therapies that target angiogenesis. Nonetheless, it is clear that VEGF family members other than VEGF-A (eg, VEGF-B, PlGF) may have roles as angiogenic mediators in these conditions that are currently less clearly defined. There is additional evidence that these and other factors could also contribute to disease progression in patients treated with mono-targeted therapies for VEGF-A such as bevacizumab.Citation14 The development of alternative biological therapies targeting angiogenesis, including those that target multiple ligands (eg, aflibercept), those targeting the VEGFRs (eg, ramucirumab), and the TKIs, are certainly expanding the potential for antiangiogenesis therapy. As recently pointed out by Zoppoli et alCitation83 however, important unanswered questions remain, including how to determine which patients and which tumors are the best candidates for these therapies; clearly, identifying antiangiogenesis biomarkers will be an essential component of the safer, more efficient, and cost-effective application of these therapies in mCRC and other cancers. The availability of such markers would favor a more personalized approach to the treatment of mCRC, which considers both patient- and tumor-specific factors; a good example of this is the negative predictive value of the KRAS gene with the use of epidermal growth factor receptor (EGFR) inhibitors, cetuximab and panitumumab, in mCRC.Citation84 The availability of this marker prevents the unnecessary use of these costly therapies in patients who are unlikely to respond.Citation84 In addition, with an increasing number of targeted antiangiogenesis therapies available and used in combination, it may be necessary to move beyond a solely biomarker-centered approach to a more comprehensive view of angiogenesis as a complex biologic system; a recent review considers this topic in detail in the context of the EGFR inhibitors.Citation84
In a review of the available preclinical literature of the antiangiogenic agents,Citation59 it was found that inter-agent and preclinical comparisons between the anti-VEGF TKIs are currently lacking. It will be of interest to determine whether such differences, if observed, can be related to efficacy.Citation59 It also remains to be seen whether differences in kinase inhibitory activity among these agents (ie, on-target versus off-target effects) will be of predictive value in determining which patients and/or tumors will benefit from which TKIs. There is, however, some evidence for notable preclinical differences in binding, as well as assessable biological activity, among the available antiangiogenic biological therapies.Citation38 Whether these differences will translate into improved efficacy and/or expanded indications for these agents remains to be further explored. Another important unresolved issue is how these agents can best be integrated into a sequential treatment plan for mCRC patients. The recent results from the CORRECT trial have, for example, established a role for the more broadly-targeted regorafenib in the third-line setting.Citation73 In addition, results of the VELOUR trial have established the efficacy of aflibercept in a population of mCRC patients, approximately one third of whom had progressed on a regimen containing prior bevacizumab.Citation40 There is a need to better understand whether this relates to the broader specificity of aflibercept as compared to single-targeted bevacizumab; this could form the basis for the logical use of these agents in sequence.
Acknowledgments
Medical editorial assistance was provided by Susan DePetris, PhD, of Phase Five Communications Inc, and supported by Sanofi-aventis US LLC, in collaboration with Regeneron Pharmaceuticals.
Disclosure
The author declares no conflicts of interest in this work.
References
- FolkmanJKlagsbrunMAngiogenic factorsScience198723547874424472432664
- SamantRSShevdeLARecent advances in anti-angiogenic therapy of cancerOncotarget20112312213421399234
- FerraraNVascular endothelial growth factor as a target for anticancer therapyOncologist20049 Suppl 121015178810
- TakahashiSVascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapyBiol Pharm Bull201134121785178822130231
- CrawfordYFerraraNVEGF inhibition: insights from preclinical and clinical studiesCell Tissue Res2009335126126918766380
- CaoYPositive and negative modulation of angiogenesis by VEGFR1 ligandsSci Signal2009259re119244214
- Escudero-EsparzaAMartinTADaviesMLJiangWGPGF isoforms, PlGF-1 and PlGF-2, in colorectal cancer and the prognostic significanceCancer Genomics Proteomics20096423924619657001
- CarmelietPJainRKMolecular mechanisms and clinical applications of angiogenesisNature2011473734729830721593862
- FerraraNHillanKJNovotnyWBevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapyBiochem Biophys Res Commun2005333232833515961063
- HurwitzHFehrenbacherLNovotnyWBevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancerN Engl J Med2004350232335234215175435
- ChuEAn update on the current and emerging targeted agents in metastatic colorectal cancerClin Colorectal Cancer201211111321752724
- LiXLeeCTangZVEGF-B: a survival, or an angiogenic factor?Cell Adh Migr20093432232719684473
- CarmelietPMoonsLLuttunASynergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditionsNat Med20017557558311329059
- KopetzSHoffPMMorrisJSPhase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistanceJ Clin Oncol201028345345920008624
- KorpantyGSullivanLASmythECarneyDNBrekkenRAMolecular and clinical aspects of targeting the VEGF pathway in tumorsJ Oncol2010201065232020628530
- OlofssonBKorpelainenEPepperMSVascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cellsProc Natl Acad Sci U S A1998952011709117149751730
- RibattiDThe discovery of the placental growth factor and its role in angiogenesis: a historical reviewAngiogenesis200811321522118568405
- HilmiCGuyotMPagèsGVEGF spliced variants: possible role of anti-angiogenesis therapyJ Nucleic Acids2012201216269222013509
- ChuQSAflibercept (AVE0005): an alternative strategy for inhibiting tumour angiogenesis by vascular endothelial growth factorsExpert Opin Biol Ther20099226327119236257
- OlofssonBPajusolaKvon EulerGChilovDAlitaloKErikssonUGenomic organization of the mouse and human genes for vascular endothelial growth factor B (VEGF-B) and characterization of a second splice isoformJ Biol Chem19962713219310193178702615
- FischerCMazzoneMJonckxBCarmelietPFLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy?Nat Rev Cancer200881294295619029957
- HamerlikPLathiaJDRasmussenRAutocrine VEGF-VEGFR2- Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growthJ Exp Med2012209350752022393126
- Van de VeireSStalmansIHeindryckxFFurther pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye diseaseCell2010141117819020371353
- FischerCJonckxBMazzoneMAnti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vesselsCell2007131346347517981115
- BaisCWuXYaoJPlGF blockade does not inhibit angiogenesis during primary tumor growthCell2010141116617720371352
- LogesSSchmidtTCarmelietPAntimyeloangiogenic therapy for cancer by inhibiting PlGFClin Cancer Res200915113648365319470735
- YaoJWuXZhuangGExpression of a functional VEGFR-1 in tumor cells is a major determinant of anti-PlGF antibodies efficacyProc Natl Acad Sci U S A201110828115901159521709213
- ZhangFTangZHouXVEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesisProc Natl Acad Sci U S A2009106156152615719369214
- TolJPunkCJMonoclonal antibodies in the treatment of metastatic colorectal cancer: a reviewClin Ther201032343745320399983
- Avastin®(bevacizumab) [prescribing information]South San Francisco, CAGenentech, Inc2013
- JainRKNormalization of tumor vasculature: an emerging concept in antiangiogenic therapyScience20053075706586215637262
- Van der VeldtAALubberinkMBahceIRapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugsCancer Cell2012211829122264790
- MaitlandMLLouXJRamirezJVascular endothelial growth factor pathwayPharmacogenet Genomics201020534634920124951
- JainRKdi TomasoEDudaDGLoefflerJSSorensenAGBatchelorTTAngiogenesis in brain tumoursNat Rev Neurosci20078861062217643088
- KeunenOJohanssonMOudinAAnti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastomaProc Natl Acad Sci U S A201110893749375421321221
- HolashJDavisSPapadopoulosNVEGF-Trap: a VEGF blocker with potent antitumor effectsProc Natl Acad Sci U S A20029917113931139812177445
- TewWPGordonMMurrenJPhase 1 study of aflibercept administered subcutaneously to patients with advanced solid tumorsClin Cancer Res201016135836620028764
- PapadopoulosNMartinJRuanQBinding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumabAngiogenesis201215217118522302382
- TengLSJinKTHeKFZhangJWangHHCaoJClinical applications of VEGF-trap (aflibercept) in cancer treatmentJ Chin Med Assoc201073944945620875616
- Van CutsemETaberneroJLakomyRAddition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimenJ Clin Oncol201230283499350622949147
- ZALTRAP®(ziv-aflibercept) [prescribing information]Bridgewater, NJRegeneron Pharmaceuticals, Inc/sanofi-aventis US LLC2012
- SanofiA Multinational, randomized, double-blind study, comparing the efficacy of aflibercept once every 2 weeks versus placebo in patients treated with gemcitabine for metastatic pancreatic cancer (VANILLA)ClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2012 Available from: http://www.clinicaltrials.gov/ct2/show/NCT00574275. NLM identifier: NCT00574275Accessed April 26, 2013
- SanofiA multicenter, randomized, double blind study comparing the efficacy and safety of aflibercept versus placebo administered every 3 weeks in patients treated with docetaxel/prednisone for metastatic androgen-independent prostate cancer (VENICE)ClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2012 Available from: http://clinicaltrials.gov/show/NCT00519285. NLM identifier: NCT00519285Accessed April 26, 2013
- SanofiA multinational, randomized, double-blind study comparing aflibercept versus placebo in patients treated with second-line docetaxel after failure of one platinum based therapy for locally advanced or metastatic non-small-cell lung cancer (VITAL)ClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2012 Available from: http://clinicaltrials.gov/show/NCT00532155. NLM identifier: NCT00532155Accessed April 26, 2013
- Sanofi-aventis and Regeneron Report Top-line Results from Phase III Study with aflibercept (VEGF Trap) in Second-Line Non-Small Cell Lung Cancer [webpage on the Internet]PR Newswire3102011 Available from: http://www.prnewswire.com/news-releases/sanofi-aventis-and-regeneron-report-top-line-results-from-phase-iii-study-with-aflibercept-vegf-trap-in-second-line-non-small-cell-lung-cancer-117757228.htmlAccessed November 30, 2012
- JinKShenYHeKXuZLiGTengLAflibercept (VEGF Trap): one more double edged sword of anti-VEGF therapy for cancer?Clin Transl Oncol201012852653220709650
- SpratlinJRamucirumab (IMC-1121B): monoclonal antibody inhibition of vascular endothelial growth factor receptor-2Curr Oncol Rep20111329710221222245
- KrupitskayaYWakeleeHARamucirumab, a fully human mAb to the transmembrane signaling tyrosine kinase VEGFR-2 for the potential treatment of cancerCurr Opin Investig Drugs2009106597605
- GrotheyAGalanisETargeting angiogenesis: progress with anti-VEGF treatment with large moleculesNat Rev Clin Oncol20096950751819636328
- BrunsCJLiuWDavisDWVascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastasesCancer200089348849910931447
- PrewettMHuberJLiYAntivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumorsCancer Res199959205209521810537299
- Eli Lilly and Company/ImClone LLCA randomized, double-blind, multicenter phase 3 study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progressive during or following first-line combination therapy with bevacizumab, oxaliplatin, and a fluoropyrimidineClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2012 Available from: http://clinicaltrials.gov/show/NCT01183780. NLM identifier: NCT01183780Accessed April 26, 2013
- National Cancer Institute (NCI)A randomized phase II study of irinotecan and cetuximab with or without the anti-angiogenic antibody, ramucirumab (IMC-1121B), in advanced, K-ras wild-type colorectal cancer following progression on bevacizumab-containing chemotherapy )ClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2012 Available from: http://clinicaltrials.gov/show/NCT01079780. NLM identifier: NCT01079780Accessed April 26, 2013
- ImClone LLCAn open-label, multicenter, randomized phase 2 study evaluating the safety and efficacy of 5 FU/FA and oxaliplatin (modified FOLFOX 6) in combination with IMC-1121B or IMC-18F1 or without investigational therapy as second line therapy in patients with metastatic colorectal cancer following disease progression on first line irinotecan-based therapyClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2012 Available from: http://clinicaltrials.gov/show/NCT01111604. NLM identifier: NCT01111604Accessed April 26, 2013
- GrotheyATaberneroJRougierPA randomized, double-blind, phase (Ph) III study of the irinotecan-based chemotherapy FOLFIRI plus ramucirumab (RAM) or placebo (PL) in patients (pts) with metastatic colorectal carcinoma (mCRC) progressive during or following first-line therapy with bevacizumab (BEV), oxaliplatin (OXALI), and a fluoropyrimidine (FP) (RAISE) (NCT01183780)J Clin Oncol201230Suppl Abstract TPS3634
- SchwartzJDRowinskyEKYoussoufianHPytowskiBWuYVascular endothelial growth factor receptor-1 in human cancer: concise review and rationale for development of IMC-18F1 (human antibody targeting vascular endothelial growth factor receptor-1)Cancer2010116Suppl 41027103220127948
- WuYZhongZHuberJAnti-vascular endothelial growth factor receptor-1 antagonist antibody as a therapeutic agent for cancerClin Cancer Res200612216573658417085673
- BhargavaPRobinsonMODevelopment of second-generation VEGFR tyrosine kinase inhibitors: current statusCurr Oncol Rep201113210311121318618
- IvySPWickJYKaufmanBMAn overview of small-molecule inhibitors of VEGFR signalingNat Rev Clin Oncol200961056957919736552
- PfizerA randomized, phase 2 study of FOLFOX Or FOLFIRI with AG-013736 or bevacizumab (Avastin) in patients with metastatic colorectal cancer after failure of an irinotecan or oxaliplatin-containing first-line regimenClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltrials.gov/ct2/show/NCT00615056. NLM identifier: NCT00615056Accessed April 26, 2013
- PfizerA phase II trial of single agent axitinib as maintenance therapy for patients with first line metastatic colorectal cancer (mCRC)ClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://www.clinicaltrials.gov/ct2/show/NCT01490866. NLM identifier: NCT01490866Accessed April 26, 2013
- Boehringer Ingelheim PharmaceuticalsA phase I-II study of BIBF 1120 and FOLFOX compared to bevacizumab and FOLFOX in first line metastatic colorectal cancer patientsClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltrials.gov/show/NCT00904839. NLM identifier: NCT00904839Accessed April 26, 2013
- NCIC Clinical Trials GroupA phase III randomized study of Brivanib Alaninate (BMS-582664) in combination with cetuximab (Erbitux®) versus placebo in combination with cetuximab (Erbitux®) in patients with K-RAS wild type tumors previously treated with combination chemotherapy for metastatic colorectal carcinomaClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2013 Available from: http://clinicaltrials.gov/show/NCT00640471. NLM identifier: NCT00640471Accessed April 26, 2013
- AstraZenecaA randomised, double-blind, multicentre phase II/III study to compare the efficacy of Cediranib (RECENTIN™, AZD2171) in combination with 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX), to the efficacy of bevacizumab in combination with FOLFOX in patients with previously untreated metastatic colorectal cancer (HORIZON III)ClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2013 Available from: http://clinicaltrials.gov/show/NCT00384176. NLM identifier: NCT00384176Accessed April 26, 2013
- PfizerA multicenter, randomised, double-blind, phase 3 study of sunitinib in metastatic colorectal cancer patients receiving irinotecan, 5-fluorouracil and leucovorin (FOLFIRI) as first line treatmentClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2013 Available from: http://clinicaltrials.gov/show/NCT00457691. NLM identifier: NCT00457691Accessed April 26, 2013
- AIO-Studien-gGmbHA controlled randomized double-blind multicenter phase II study of FOLFOX6 or FOLFIRI combined with sorafenib versus placebo in second-line metastatic colorectal carcinomaClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2013 Available from: http://www.clinicaltrials.gov/ct2/show/NCT00889343. NLM identifier: NCT00889343Accessed April 26, 2013
- BayerA randomized, double-blind, placebo-controlled phase III study of regorafenib plus BSC versus placebo plus BSC in patients with metastatic colorectal cancer (CRC) who have progressed after standard therapyClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2013 Available from: http://clinicaltrials.gov/show/NCT01103323. NLM identifier: NCT01103323Accessed April 26, 2013
- BayerAn open-label phase IIIb study of regorafenib in patients with metastatic colorectal cancer (CRC) who have progressed after standard therapy (CONSIGN)ClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2013 Available from: http://clinicaltrials.gov/show/NCT01538680. NLM identifier: NCT01538680Accessed April 26, 2013
- AstraZenecaA phase II, double-blind, placebo controlled, randomized study to assess the efficacy and safety of 2 doses of ZD6474 (Vandetanib) in combination with FOLFOX vs FOLFOX alone for the treatment of colorectal cancer in patients who have failed therapy with an irinotecan and fluoropyrimidine regimenClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2013 Available from: http://clinicaltrials.gov/show/NCT00500292. NLM identifier: NCT00500292Accessed April 26, 2013
- AstraZenecaA phase II, double blind, placebo controlled, randomised study to assess the efficacy and safety of 2 doses of ZACTIMA™(ZD6474) in combination with FOLFIRI vs FOLFIRI alone for the treatment of colorectal cancer in patients who have failed therapy with anoxaliplatin and fluoropyrimidine containing regimenClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine2011 Available from: http://clinicaltrials.gov/show/NCT00454116. NLM identifier: NCT00454116Accessed April 26, 2013
- Stivarga®(regorafenib) [prescribing information]Wayne, NJBayer HealthCare Pharmaceuticals Inc2013
- WilhelmSMDumasJAdnaneLRegorafenib (BAY73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activityInt J Cancer2011129124525521170960
- GrotheyAVan CutsemESobreroACORRECT Study GroupRegorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trialLancet2013381986330331223177514
- RudgeJSHolashJHyltonDVEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockadeProc Natl Acad Sci U S A200710447183631837018000042
- SobreroAAcklandSClarkeSAVIRI Trial investigatorsPhase IV study of bevacizumab in combination with infusional fluorouracil, leucovorin and irinotecan (FOLFIRI) in first-line metastatic colorectal cancerOncology200977211311919628950
- NarayanaAGruberDKunnakkatSA clinical trial of bevacizumab, temozolomide, and radiation for newly diagnosed glioblastomaJ Neurosurg2012116234134522035272
- ChinotOLde La Motte RougeTMooreNAVAglio: phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiformeAdv Ther201128433434021432029
- KellyWKHalabiSCarducciMRandomized, double-blind, placebo- controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401J Clin Oncol201230131534154022454414
- Sanofi/RegeneronSanofi and Regeneron Announce Regulatory and Clinical Update for Zaltrap® (aflibercept) [press release]Paris: Sanofi/Tarrytown, NYRegeneron452012 Available from: http://en.sanofi.com/Images/30138_20120405_ZALTRAP_BLA_VENICE_en.pdfAccessed November 30, 2012
- National Cancer Institute (NCI)Phase II single arm trial of VEGF trap in patients with recurrent temozolomide-resistant malignant gliomasClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine Available from: http://clinicaltrials.gov/show/NCT00369590. NLM identifier: NCT00369590Accessed April 26, 2013
- National Cancer Institute (NCI)/ImClone LLCAn open label, phase 2 study evaluating the safety and efficacy of IMC-3G3 or IMC-1121B in patients with recurrent glioblastoma multiformeClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine Available from: http://clinicaltrials.gov/show/NCT00895180. NLM identifier: NCT00895180Accessed April 26, 2013
- ImClone LLCA phase 2, multicenter, randomized study of IMC-A12 or IMC-1121B plus mitoxantrone and prednisone in metastatic androgen-independent prostate cancer (AIPC) following disease progression on docetaxel-based chemotherapyClinicalTrials.gov [website on the internet]Bethesda, MDUS National Library of Medicine Available from: http://clinicaltrials.gov/show/NCT00683475. NLM identifier: NCT00683475Accessed April 26, 2013
- ZoppoliGFerrandoVScabiniSOn biomarkers and pathways in rectal cancer: What’s the target?World J Gastrointest Surg201241227527723493582
- BallestreroAGarutiACirmenaGPatient-tailored treatments with anti-EGFR monoclonal antibodies in advanced colorectal cancer: KRAS and beyondCurr Cancer Drug Targets201212431632822385512