Abstract
Background
Isolated limb infusion (ILI) using cytotoxic agents has been demonstrated to be an effective and less invasive alternative modality than isolated limb perfusion for the treatment of melanoma localized to a limb. Percutaneous catheters were inserted into the axial artery and vein of the affected limb while using a pneumatic cuff to restrict limb vascular flow proximally to “isolate” the limb from the body and enable delivery of high-dose intra-arterial chemotherapy selectively to the limb. The ILI technique was developed at the Sydney Melanoma Unit (now renamed the Melanoma Institute Australia), and only a few other centers have reported separate results. We report our early results using the ILI technique for management of locally recurrent surgically nonresectable melanoma.
Methods and results
Twenty-eight ILI procedures were performed in 20 patients treated with one or more procedures between 1997 and 2007. Patient parameters and clinical responses were evaluated. The median follow-up duration was 15.9 months after the first ILI, with an overall response rate after one or more infusions of 70%, of which 35% were complete responders and 35% were partial responders, with a further 20% showing stable disease, giving a “clinically significant” response rate of 90%. After one ILI (n = 20), the overall response rate was 70%, with 20% complete responders and 50% partial responders, and 20% with stable disease. Low limb toxicities were generally observed, and no amputations were required.
Conclusion
ILI chemotherapy is a useful technique, which can be readily repeated for control of melanoma in the limb. It is generally well tolerated, and is capable of achieving a cure, delayed progression, or effective palliation in selected cases. The longest survivors in this series were 8 and 10 years from the last ILI.
Introduction
The incidence of melanoma in Australian men and women increased by 42% and 18%, respectively, from 1991 to 2009,Citation1 bringing with it a corresponding increase in the number of patients presenting with local recurrences following initial surgical excision. A study from the Sydney Melanoma Unit examining 4704 American Joint Committee on Cancer stage I and II patients reported a recurrence rate of 18.6% in 873 patients, of which 10.9% were local and 9.9% in-transit in type, over a median follow-up duration of 5.3 years after initial excision of primary melanoma.Citation2 Many of these are related to risk factors of the primary melanoma, such as high Breslow thickness or ulceration.
The modalities of treatment for patients with localized recurrence of limb melanoma are numerous, including local surgical excision, laser ablation, local injection, cryotherapy, and vaccine therapy. However, when metastases are numerous or bulky, these options are limited and other techniques should be considered, such as regional chemotherapy, radiotherapy, or systemic chemotherapy. Chemotherapy doses achievable using standard systemic chemotherapy are lowered due to risk of systemic side effects; however, higher doses of chemotherapy can be delivered to the limb if it is vascularly “isolated” from the systemic circulation. Isolated limb perfusion was developed in the 1950sCitation3 and used in many centers worldwide, but carries higher risks of morbidity and mortality because of its highly invasive nature. It requires use of vascular dissection and clamping as well as an extracorporeal bypass pump circuit, making it a complex and costly procedure. Overall response rates reported using isolated limb perfusion range from 71% to 88%, with complete response rates of 12%–73%.Citation4–Citation11 This wide range in complete response rates is likely related to differences in patient selection factors between studies. Known complications after isolated limb perfusion include compartment syndrome, neuropathy, infection, and arterial or venous thrombosis.Citation4–Citation18
Over the last two decades, isolated limb infusion (ILI) has been used at the Sydney Melanoma Unit (now known as the Melanoma Institute Australia) as an alternative to isolated limb perfusion. ILI has the advantage of being a much simpler procedure, circumventing the need for complex bypass, extensive surgical dissection, and long operating times, whilst being well tolerated and, if required, readily repeated, even in the elderly. High doses of chemotherapy, above systemically tolerable levels, can be delivered selectively to the affected limb, without leakage into the systemic circulation. Clinical response rates using ILI have been demonstrated to be comparable with those obtained using isolated limb perfusion.Citation15,Citation16
Patients and methods
Informed consent was obtained from all patients and the studies were approved by the Royal Adelaide Hospital human research ethics committee. Analysis was conducted on data from a total of 28 ILI procedures that were performed between July 1997 and May 2007 in 20 patients at the Royal Adelaide Hospital. The indication for treatment was recurrent lower or upper limb melanoma for which surgery was not feasible, presenting with either satellite or in-transit metastases and with or without previous surgically resected lymph node involvement. Patients with stage IV disease were treated for palliative purposes and were also included in this study to assess limb disease responses.
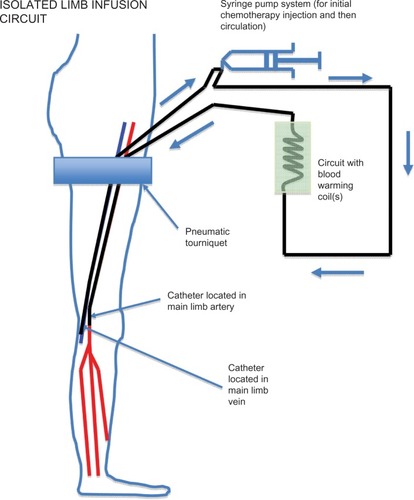
The technique used for ILI is shown in and have been detailed elsewhere.Citation15 In summary, a nonoxygenated low-flow extracorporeal circuit was created using percutaneously inserted arterial and venous catheters (6 French and 8 French, respectively). Under general anesthesia, the patient was systemically heparinized, followed by a rapid intra-arterial bolus injection of papaverine, and then isolation of the disease-affected limb using an infatable tourniquet, placed proximally, high on the limb. Rapid intra-arterial infusion of melphalan and actinomycin D followed. Using a high-flow, three-way stopcock valve, the infusate was manually circulated using a 60 mL syringe for up to 30 minutes. Blood warming coils were incorporated into this circuit to maintain limb temperature, and hot-air blankets were used to cover the patient’s limbs and body. Intramuscular and subcutaneous temperature probes were inserted into the limb for monitoring the temperatures throughout the procedure. Following circulation, the infusate was drained from the isolated circuit, discarded, and replaced by Hartmann’s solution to wash out the intravascular chemotherapeutic agent. Protamine was administered to reverse the heparinization and the catheters were removed. Pressure was applied to the puncture sites, followed by reversal of the general anesthetic.
Figure 1 Isolated limb infusion circuit in a lower limb showing positioning of the tourniquet, arterial and venous catheters inserted in the ipsilateral (or contralateral) groin to reach approximately the level of the popliteal artery and vein, respectively, and the extracorporeal warmed circuit using a hand-driven syringe pump.
Patients were restricted to bed rest with elevation of the treated limb for 48 hours following the procedure. Hourly limb observation, daily creatine kinase measurements, and limb toxicity assessments were made using the scale published by Wieberdink et alCitation19 (see ).
Table 1 Wieberdink gradesCitation19
Patients’ responses were clinically defined by World Health Organization criteria for reporting cancer treatment, where determinant measurements were taken not less than 4 weeks apart.Citation20 A complete response was defined by a 100% reduction in tumor mass; a partial response was a reduction in tumor mass by over 50%; stable disease was a reduction of less than 50% or an increase in mass of no more than 25%; whilst progressive disease was defined as any increase in mass greater than 25%. Overall response was defined as the combined complete response and partial response rates. Response duration was defined as the time elapsed between the ILI procedure and the development of new or progressive disease. Assessment of response was conducted by the same clinician on each occasion with measurement comparisons pre-ILI and on subsequent follow-up.
The results were analyzed as follows: assessment after the initial ILI procedure for all patients to assess initial outcome (n = 20); collectively for all patients, regardless of the number of ILI procedures performed, to assess overall outcome (n = 20); patients receiving a single ILI procedure alone (n = 15); assessment of all ILI treatment episodes for all patients; and assessment of the toxicity of each ILI procedure (n = 28).
Statistical analysis
An ordinal regression model was used to determine any association between patient characteristics and clinical response. A statistically significant difference was assumed at a probability value of <0.05. Statistical analyses were performed using Statistical Package for Social Sciences version 13 for MAC OSX (SPSS Inc, Chicago, IL, USA).
Results
Patient data
Between July 1997 and May 2007, 28 ILI procedures were performed in 20 patients at the Royal Adelaide Hospital. Fifteen patients underwent a single ILI procedure, two patients received two ILI procedures, and three had triple ILI procedures. The relevant patient and tumor data are shown in . Of the 20 patients who received ILI therapy, 19 involved the lower limb and one ILI was performed on the upper limb.
Table 2 Patient characteristics
The median follow-up duration after the initial ILI for all 20 patients was 15.9 (2.7–121.4) months. The patient population consisted of 14 women and six men of median age 72.6 (32.4–97.2) years. Computed tomography scans were performed at the time of ILI. All patients had either local recurrences or in-transit melanoma metastases in the limb, of whom 11 had no lymph node involvement, eight had previous regional lymph node involvement, and one had evidence of possible small pulmonary metastases.
The median volume infused into lower limb tissue was 6.75 (4.0–15.5) L, while the one case involving the upper limb had an infusion volume of 2.0 L. The median melphalan dose administered was 7.5 (6.0–13.3) mg per liter of tissue and the median actinomycin D dose was 50.0 (36.3–67.5) μg per liter of tissue. Median initial intramuscular and subcutaneous limb temperatures were 35.1°C (32.5°C–38.0°C) and 34.5°C (19.4°C–36.4°C), respectively, with median final temperatures of 34.9°C (28.7°C–39.0°C) and 34.9°C (21.8°C–38.2°C), respectively.
Initial single ILI outcomes
Toxicity
There was one case of grade I limb toxicity (5%), 14 cases of grade II limb toxicity (70%), three cases of grade III limb toxicity (15%), and two cases of grade IV limb toxicity (10%). No cases of grade V limb toxicity requiring limb amputation were observed. Of the two grade IV cases, one was considered to be a chemical cellulitis, which resolved without intervention. The other patient experienced severe local chemotherapy toxicity around the site of the catheter tips due to poor venous drainage subsequent to prior deep venous thrombosis years previously, but this was not declared until after the procedure, and required extensive debridement and grafting (longest bed stay). The median length of hospital stay for all patients was 8.5 (6–18) days.
Clinical responses
After clinical evaluation, there was an overall response rate of 70%, with a complete response rate of 20% and a partial response rate of 50%. Four patients were classified as having stable disease (20%), and if these were included, the overall “clinically significant” response rate was 90%. Another two patients were classed as having progressive disease (10%). In the four patients who achieved a complete response, only one has since developed further systemic disease; the median time from ILI until progression was 18.2 (4.1–45.7) months. Three of the four patients who achieved a complete response had regional lymph node involvement and nodal surgery prior to ILI and did not experience any recurrence. The other patient who had a complete response in the infused limb had stage IV disease, but did not experience limb recurrence. Of the ten patients who achieved a partial response, all but two have developed progressive or new disease, occurring after a median follow-up period of 7.3 (2.0–12.0) months after ILI. Of those ten patients with a partial response, eight were classified as having local or in-transit disease without regional nodal involvement, whilst the other two had regional nodal disease.
Overall final ILI outcomes
Toxicity
Limb toxicities after the final ILI for all patients (n = 20) were: grade 1, 5% (n = 1); grade 2, 75% (n = 15); grade 3, 10% (n = 2); and grade 4, 10% (n = 2).
Clinical responses
After the final ILI for all patients, there was an overall response rate of 70%, with a complete response rate of 35% (n = 7) and a partial response rate of 35% (n = 7). Stable disease was seen in 20% of cases (n = 4) and progressive disease in 10% (n = 2). If stable disease was included, the overall “clinically significant” response rate was 90%.
Outcomes in patients receiving only one ILI procedure
Toxicity
Limb toxicities for this patient subset (n = 15) were: grade 1, 6.7% (n = 1); grade 2, 73.3% (n = 11); grade 3, 6.7% (n = 1); and grade 4, 13.3% (n = 2).
Clinical responses
The overall response rate for patients who received one ILI alone was 66.6% (n = 15), with a complete response rate of 26.6% (n = 4), a partial response rate of 40% (n = 6), stable disease in 26.6% (n = 4), and progressive disease in one patient (6.8%). If stable disease was included, the overall “clinically significant” response rate was 93.2%.
Assessment of all ILI therapy episodes for overall toxicity
Limb toxicity following all ILI procedures (n = 28) was: grade 1, 3.6% (n = 1); grade 2, 71.4% (n = 20); grade 3, 17.8% (n = 5); and grade 4, 7.2% (n = 2). Of the 28 procedures, 19 (67.9%) showed raised creatine kinase levels postoperatively. The median duration until peak creatine kinase was four (1–6) days, while the median peak level was 680.5 (30–27,650) Units/liter. Ordinal regression showed that the peak creatine kinase level had a statistically significant association with Wieberdink toxicity grade (P = 0.012). The peak creatine kinase level did not show any association with clinical response or disease-free interval (P > 0.05).
Discussion
Recurrence of melanoma in a limb represents a spectrum of disease, but is predominantly either cutaneous or subcutaneous, or a combination of both. Involvement of limb bones or muscle is rare, but can occur, sometimes associated with deep and large subcutaneous tumor deposits, and/or enlargement of lymph node metastases in the deep calf, popliteal, or epitrochlear regions. Bulky disease may occur if superficial deposits enlarge and/or become confluent. Essentially all cutaneous and subcutaneous recurrent melanoma arises from lymphatic metastatic spread that is in-transit to the regional lymph nodal basin. This fact was recognized in the 2001 and 2009 American Joint Committee on Cancer staging classification, where intransit lesions were classified as stage III melanoma.Citation21 Despite this, local surgical resection of superficial limb deposits, in small numbers or appearing sequentially, is often surprisingly effective for control of recurrent disease of the limb, even in the longer term. However, there are difficult situations where melanoma metastases develop inexorably in a limb and are not amenable to standard local therapies.Citation22 Systemic chemotherapy is usually ineffective for treatment of advanced melanoma recurrence in a limb, and radiotherapy may have an effect, but this is often unpredictable.Citation23 Recent therapies, such as B-raf inhibitory antibodies, can have some effect, although this is often temporary or short-lived. Over half of all melanoma patients are B-raf mutant-negative and do not show responsiveness. About half of B-raf-positive patients show responses, but most of these eventually fail. Also, dose-limiting adverse events occur in about 40% of patients treated with B-raf. Citation24,Citation25
Regional chemotherapy for locally recurrent or in-transit metastatic melanoma distal to the axilla or groin has been shown to be an effective treatment option in many situations where surgical resection is not possible or has failed.Citation13,Citation18 Isolated limb perfusion has been used extensively internationally, whereas ILI has become widely used by specialist melanoma centers in Australia, with gradual interest internationally.Citation26,Citation27 The advantages of ILI are its technical simplicity and practicality, good tolerability in patients (especially the elderly), and the relative ease of repeating the procedure(s). ILI also has a relatively low complication rate compared with isolated limb perfusion, and does not require specialized extracorporeal bypass facilities, invasive surgery, or blood transfusion.Citation18 Further, the reduced operative time and cost required per procedure makes it economically more attractive for health budgets than isolated limb perfusion. Lower rates of limb toxicity and morbidity, including compartment syndrome, peripheral neuropathy, infection, and venous thrombosis, are also significant additional economic advantages.Citation17
The current analysis shows that the patients treated at the Adelaide Melanoma Unit experience very low levels of limb toxicity, with only one significant long-term complication recorded so far. However, this occurred in a patient with a specific venous stasis problem that was only reported after ILI. Our overall toxicity rates assessing each ILI episode as an independent event (n = 28) demonstrated relatively low morbidity (75% Wieberdink grade 1 or 2). For overall outcomes (n = 20) after the final ILI, the toxicity profile (grade 1, 5%; grade 2, 75%; grade 3, 10%; grade 4, 10%) was not significantly different from either independently analyzed ILI procedures or first ILI procedures. This indicates that the post-ILI toxicity profile remained similar regardless of the number of procedures that were performed per patient, which is in accordance with previous reports on repeat ILI.Citation28 The median follow-up period after initial ILI for all 20 patients was 15.9 (2.7–121.4) months, providing a good indication that successful longer-term results can be achieved.
After the final ILI, there was an overall clinical response rate of 70%, with a complete response rate of 35% and a partial response rate of 35%. Stable disease was seen in 20% and progressive disease in 10% of patients. If stable disease was included, the overall “clinically effective” response rate was 90%, where any clinically significant or meaningful measurable response was observed.
Our response rates compare favorably with previously published ILI studies. At the time of publication, the largest ILI study, performed by the Sydney Melanoma Unit, consisted of 185 patients. Citation17 That study showed an overall response rate of 84% (complete response 38%, partial response 46%) using a technique similar to that used in this analysis after one ILI. After repeated ILI procedures (n = 48), the overall response was 83% (complete response 23% and partial response 60%).Citation28 The first and to date only multicenter study investigating the ILI procedure showed a slightly lower but still satisfactory response rate in comparison with the Sydney Melanoma Unit study with an overall response rate of 64% (complete response 31%, partial response 33%), after one or more ILI procedures.Citation26
Measurement of visible melanoma nodules or deposits was performed with good reproducibility by measuring in two directions perpendicular to each other. However, for deeper deposits, imaging was required and this was done by either Doppler ultrasound or computed tomography scanning. An alternative would be to use contrast-enhanced ultrasound or dynamic contrast-enhanced ultrasonography for evaluations using microbubble contrast.Citation29 However, the most ideal lesions for dynamic contrast-enhanced ultrasonography are those that are over 2 cm in size, with sufficient necrosis and more vasculariza-tion. Smaller deposits can be more difficult to evaluate, and were the main type of in-transit deposit encountered in our study.
ILI chemotherapy could be combined with approved or experimental systemic therapies, such as interleukin-2, standard chemotherapy, radiotherapy, or serine–threonine protein kinase B-Raf inhibitory antibodies (B-Raf), Cytotoxic T lymphocyte associated antigen-4 (CTLA-4), mitogen-activated protein kinase kinase (MEK) Programmed cell Death Receptor-1 (PD-1), Programmed cell Death Ligand-1 (PD-L1) inhibitor therapy, or local intra-lesional therapies such as coxsackie virus or rose bengal, and be practice-changing. ILI using non-cytotoxic agents, perhaps including some of those above, might be explored.
Although ILI is essentially a regional local treatment confined to the melanoma-affected limb, long-term survival is not uncommon. This raises the distinct possibility of systemic immune modulation from “autovaccination” events as a result of tumor necrosis induced by ILI chemotherapy, which has been proposed previously.Citation30 Similar reports have been made for other local therapies associated with direct lesional injection of melanoma metastases, and topical desensitising therapies such as diphencyprone (DPCP), where “bystander”-type regressions have been noted in noninjected metastases.Citation31–Citation34
Conclusion
The current analysis of our procedures indicates that ILI is a particularly useful method for controlling metastatic limb melanoma, with high efficacy and results comparable with those reported using isolated limb perfusion chemotherapy regimens, avoiding a more invasive and costly procedure. ILI offers the advantage of being a relatively simple method with lower morbidity. Our findings validate previous studies using ILI. Larger multicenter studies are required to investigate the true efficacy of ILI for treating locally advanced melanoma confined to the limb. Further work on the ILI method is currently required to improve and refine this promising technique for treatment of limb melanoma. Long-term survivors are reported after ILI. Combination therapy using ILI chemotherapy and other systemic therapies may be a useful future direction.
Acknowledgments
The authors wish to thank Professor John F Thompson, Sydney Melanoma Unit, Royal Prince Alfred Hospital, Sydney, for his guidance and advice in the early stages of establishment of ILI therapy, and Dr R Waugh, Radiology Department, Royal Prince Alfred Hospital, for his advice regarding catheter selection and technique. Thanks are also extended to Dr G Freer for his anesthetic expertise in pioneering the ILI procedure at our institution. Additional thanks for statistical support go to Nancy Briggs and Tom Sullivan of the Department of Public Health, The University of Adelaide, and to our radiological colleagues at the Royal Adelaide Hospital for their catheter expertise. We thank Hidde Kroon (The Netherlands) for reviewing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- Australian Institute of Health and Welfare and Australasian Association of Cancer Registries 2012Cancer in Australia: an overview, 2012Australian Cancer Incidence and Mortality Workbooks Melanoma Cancer series no 74 Catalog number CAN 70Canberra AustraliaAustralian Institute of Health and Welfare2012
- FranckenABAccorttNAShawHMPrognosis and determinants of outcome following locoregional or distant recurrence in patients with cutaneous melanomaAnn Surg Oncol2008151476148418196345
- CreechOJrKrementzETRyanRFChemotherapy of melanoma of the extremities by isolated perfusionCancer Chemother Rep19621657958113882088
- HayesAJNeuhausSJClarkMAThomasJMIsolated limb perfusion with melphalan and tumor necrosis factor alpha for advanced melanoma and soft-tissue sarcomaAnn Surg Oncol20071423023817066234
- KnorrCMeyerTJanssenTHyperthermic isolated limb perfusion (HILP) in malignant melanoma. Experience with 101 patientsEur J Surg Oncol20063222422716289716
- NoordaEMVrouenraetsBCNiewegOEKroonBBIsolated limb perfusion in regional melanomaSurg Oncol Clin N Am20061537338416632221
- TakkenbergRBVrouenraetsBCvan GeelANPalliative isolated limb perfusion for advanced limb disease in stage IV melanoma patientsJ Surg Oncol20059110711116028280
- AloiaTAGrubbsEOnaitisMPredictors of outcome after hyperthermic isolated limb perfusion: role of tumor responseArch Surg20051401115112016301451
- ThompsonJFHuntJAShannonKFKamPCFrequency and duration of remission after isolated limb perfusion for melanomaArch Surg19971329039079267277
- ReintgenMReintgenCNoboCGiuliano ShiversRSReintgenDRegional therapy for recurrent metastatic melanoma confined to the extremity: hyperthermic isolated limb perfusion vs isolated limb infusionCancers201224350
- DerooseJPEggermontAMvan GeelANVerhoefCIsolated limb perfusion for melanoma in-transit metastases: developments in recent years and the role of tumor necrosis factor alphaCurr Opin Oncol20112318318821150602
- TestoriAVerhoefCKroonHMTreatment of melanoma metastases in a limb by isolated limb perfusion and isolated limb infusionJ Surg Oncol201110439740421858835
- VrouenraetsBCKroonBBNiewegOEThompsonJFIsolated limb perfusion for melanoma: results and complicationThompsonJFMortonDLKroonBBTextbook of MelanomaLondon, UKMartin Dunitz2004
- KoopsHSVagliniMSuciuSProphylactic isolated limb perfusion for localized, high-risk limb melanoma: results of a multicenter randomized phase III trial. European Organization for Research and Treatment of Cancer Malignant Melanoma Cooperative Group Protocol 18832, the World Health Organiation Melanoma Program Trial 15, and the North American Perfusion Group Southwest Oncology Group-8593J Clin Oncol199816290629129738557
- ThompsonJFKamPCWaughRCHarmanCRIsolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusionSemin Surg Oncol1998142382479548607
- LindnerPDoubrovskyAKamPCThompsonJFPrognostic factors after isolated limb infusion with cytotoxic agents for melanomaAnn Surg Oncol2002912713611888868
- KroonHMMoncrieffMKamPCThompsonJFOutcomes following isolated limb infusion for melanoma. A 14-year experienceAnn Surg Oncol2008153003301318509706
- KroonHMThompsonJFIsolated limb infusion: a reviewJ Surg Oncol200910016917719365813
- WieberdinkJBenckhuysenCBraatRPDosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactionsEur J Cancer Clin Oncol1982189059106891640
- World Health OrganizationWHO Handbook for Reporting Results of Cancer TreatmentGeneva SwitzerlandWorld Health Organization1979
- BalchCMBuzaidACSoongSJFinal version of the American Joint Committee on Cancer staging system for cutaneous melanomaJ Clin Oncol2001193635364811504745
- TestoriAFariesMBThompsonJFLocal and intralesional therapy of in-transit melanoma metastasesJ Surg Oncol201110439139621858834
- HongAFogartyGRole of radiation therapy in cutaneous melanomaCancer J20121820320722453022
- ChapmanPBHauschildARobertCImproved survival with vemurafenib in melanoma with BRAF V600E mutationN Engl J Med20113642507251621639808
- SosmanJAKimKBSchuchterLSurvival in BRAF V600-mutant advanced melanoma treated with vemurafenibN Engl J Med201236670771422356324
- BeasleyGMCaudleAPetersenRPA multi-institutional experience of isolated limb infusion: defining response and toxicity in the USJ Am Coll Surg200920870671519476821
- DupratNeto JPMauroACMolinaASIsolated limb infusion with hyperthermia and chemotherapy for advanced limb malignancy: factors influencing toxicityANZ J Surg9242012 [Epub ahead of print.]
- KroonHMLinDYKamPCThompsonJFEfficacy of repeat isolated limb infusion with melphalan and actinomycin D for recurrent melanomaCancer20091151932194019288571
- LassauNChamiLChebilMDynamic contrast-enhanced ultrasonography (DCE-US) and anti-angiogenic treatmentsDiscov Med201111182421276407
- CoventryBJAshdownMLComplete clinical responses to cancer therapy caused by multiple divergent approaches: a repeating theme lost in translationCancer Manag Res2012413714922740774
- ThompsonJFHerseyPWachterEChemoablation of metastatic melanoma using intralesional rose bengalMelanoma Res20081840541118830132
- FooteMCBurmeisterBHThomasJMark SmithersBA novel treatment for metastatic melanoma with intralesional rose bengal and radiotherapy: a case seriesMelanoma Res201020485120009957
- ShafrenDRAuGGNguyenTSystemic therapy of malignant human melanoma tumors by a common cold-producing virus, coxsackievirus A21Clin Cancer Res200410536014734451
- DamianDLShannonKFSawRPThompsonJFTopical diphencyprone immunotherapy for cutaneous metastatic melanomaAustralasian Journal of Dermatology20095026627119916970