Abstract
Purpose
This retrospective cohort study assessed semen and testicular tissue quality from adult and adolescent cancer patients who had samples cryopreserved in the Cryobank of Charité-Universitätsmedizin before and/or after cancer treatment.
Methods and Materials
Medical and cryopreservation data for all samples stored between 03/2004 and 05/2019 were collected retrospectively.
Results
We included information on 601 samples cryopreserved from 506 cancer patients for whom oncologic treatment data were available. The majority of the samples were cryopreserved prior to cancer treatment (460/600, 77%, median 5 days before treatment). Semen quality had a predisposed reduction in those collected from adolescents with testicular and/or hematological malignancies. Analyses of the 140 (23%) samples cryopreserved after treatment initiation (median of 84 days) revealed decreased median concentration and motility following high gonadotoxic-risk treatment. Rate of oligoasthenozoospermia was comparable in samples collected prior to treatment with those provided during follow-up spermiograms within 1 year after treatment initiation (45.5% vs 45.5%). However, an increase was seen in samples collected 1–2 (9.1% to 90.9%) and 2–3 (50.0% to 100.0%) years after treatment initiation.
Conclusion
Cancer diagnosis and treatment may impair spermatogenesis; therefore, patient counseling prior to cancer treatment by an oncologist and/or fertility specialist is crucial.
Introduction
The majority of male cancer patients express the wish to have (prospective) biological children.Citation1 However, cancer diagnosis and therapy can damage fertility, potentially making it impossible to fulfil this wish.Citation2–4 In males, direct damage of germinal cells and/or dysfunction of endocrine/paracrine control may result in impaired spermatogenesis, which may be reversible, but more likely persists permanently.Citation5 Disruption of spermatogenesis may present as reduced sperm count, loss of motility, and/or morphological abnormalities.Citation5,Citation6 The effects of gonadotoxic treatments depend on a variety of factors including patient age, type, cumulative dosage and/or combination of chemotherapeutic agents administered, site and dosage of irradiation, the potential synergic interaction of radio-/chemotherapy, site and type of surgery, as well as treatment duration.Citation7
Due to the sensitivity of rapidly dividing spermatogonia, they may be impaired or destroyed by chemotherapy, particularly by regimens containing alkylating agents, which may lead to azoospermia.Citation8,Citation9 A decrease in sperm concentration may occur within two months after treatment initiation.Citation9 Recovery of spermatogenesis (at earliest 12 weeks after end of therapy) depends on survival and differentiation of spermatogonial stem cells, varying with alkylating agent use and dosage.Citation10 Radiotherapy in the urogenital area may also disrupt spermiogenesis, potentially causing azoospermia after 18 weeks.Citation10 If spermatogonial stem cells are affected, it is questionable whether spermiogenesis will recover (repopulation of stem cells) after completion of therapy (after 9 months at earliest), or whether irreversible azoospermia (total dose of >2.5 Gray (Gy) or single dose >6 Gy in adults and total dose ≥4 Gy in younger patients) has to be expected.Citation10–12 Sperm quality may, however, already be impaired at diagnosis; azoospermia was found in 3–14% of patients with Hodgkin’s disease or testicular cancer at the time of diagnosis.Citation13,Citation14
To enable patients to fulfil their wish for prospective parenthood, despite being at risk for infertility, the use of fertility preservation (FP) prior to treatment can be crucial.Citation15,Citation16 Cryopreservation of sperm and/or testicular tissue is standard care for FP for adults and pubertal adolescents with cancer.Citation17 While long-term cryopreservation of up to 12 years does not seem to negatively influence sample quality,Citation18 only 50% of motile sperm were shown to survive the freeze–thaw process.Citation19 In the event of azoospermia, there remains a 44% chance of finding vital sperm in testicular tissue collected by biopsy for testicular sperm extraction (TESE).Citation20 When completed by intracytoplasmatic sperm injection (ICSI), live birth rates following TESE in cancer patients are 40% per couple per single ovarian hyperstimulation.Citation21 Therefore, in the event of azoospermia or if sperm banking is not possible, testicular tissue cryopreservation for TESE can be an effective FP option.Citation21
Objective
We examined the quality of semen and testicular tissue collected by biopsy for future TESE in a cohort of adolescent and adult cancer patients. Samples had been cryopreserved at the Charité-Universitätsmedizin Berlin, Germany from 03/2004 to 05/2019 prior to, and/or following the start of cancer treatment.
Materials and Methods
Study Population and Data Collection
From 01/2020 to 09/2021, we used hospital records to retrospectively collect medical and cryopreservation data associated with samples stored in the cryobank of the Charité-Universitätsmedizin Berlin (Clinic of Urology) from 03/2004 to 05/2019. We identified 603 cancer patients whose samples had been cryopreserved during this period. Oncologic treatment information was available for 506 (83.9%) of these patients. This study complies with the Declaration of Helsinki. All data was documented as pseudonymised. Our study was approved by the ethics committee of the Charité-Universitätsmedizin Berlin (EA4/158/19).
Cancer Diagnosis and Therapy Groups
Cancer diagnoses were stratified as hematological cancers (leukemia or lymphoma), brain tumors, testicular tumors, and solid tumors in other locations (non-testicular tumors). Depending on cryopreservation time points, samples were categorized as “collected before” or “after” initiation of oncologic treatment (chemotherapy and/or radiotherapy and/or surgery of either the brain, pelvis, or testes). According to previous guidelines,Citation8,Citation11 cancer treatment groups were defined as high, medium, low, or without increased gonadotoxic risk for adolescent cancer patients (<18 years old) and high/medium, low, and without increased gonadotoxic risk for adult cancer patients (≥18 years old).
Semen and Testicular Tissue Analyses and Cryopreservation
For the present analyses results of semen analyses, conducted prior to cryopreservation, were evaluated according to the World Health Organization (WHO) recommendations of 2010Citation19 assessing volume (mL), pH value, sperm concentration (106/mL), total sperm count (106 per ejaculate), progressive and non-progressive motility (%), immotility (%), and vitality (%). All parameters were routinely assessed using standard methodologies, except for the total sperm count per ejaculate, which was calculated for each semen sample by multiplying the sperm concentration by the ejaculate volume. Normozoospermia was defined as a sperm concentration ≥15 million sperm/mL or a total sperm count 39 million per ejaculate. Patients were advised to collect additional samples in the event of low sperm counts or sample volumes below WHO reference values.Citation19,Citation22 Despite cryptozoospermia or azoospermia, ejaculate sediments were cryopreserved to safeguard potentially viable sperm or upon patient demand. Patients with azoospermia were recommended to undergo testicular tissue cryopreservation for future TESE. Testicular tissue samples were analyzed within one hour after surgical removal according to WHO recommendations,Citation19,Citation22 including number of sperm per microscopic field and motility (%). If no sperm were found, or if the Johnsen Score (determined by a pathologist) was <7, samples were only cryopreserved upon patient’s request.Citation23
Statistical Analysis
Data analysis was conducted using R, version 3.6.1. If the day of cancer diagnosis was not specified, the first day of the corresponding month was used, and if either month or year was not specified, the data were analyzed as missing. If multiple samples were provided, the earliest collection date was analyzed. This included multiple samples cryopreserved on the same day, or, by the same modality, within 15 days. Follow-up time since the first cryopreservation was grouped as <1, 1–2, 2–3, >3 years. All continuous variables are presented as mean and standard deviations (SD) or median values and interquartile ranges (IQR). Comparisons according to treatment groups and for numerical variables were made using the one-way ANOVA or Kruskal–Wallis test (two groups) and t-test or Wilcoxon-Mann–Whitney test (three groups), depending on the data distribution. The Chi-square test was used to analyze categorical variables. We used Spearman’s rank correlation test to analyze the association between sperm concentration or progressive motility and year of collection. Multiple linear regressions were performed for sperm concentration and progressive motility, considering the covariates age at diagnosis and at cryopreservation and year of cryopreservation. Linear mixed models were used to analyze significant results for sperm concentration, progressive motility, or sperm diagnosis in our subgroup. While the WHO recommends the use of total sperm count over sperm concentration when defining normozoospermia, the use of the sperm concentration also allows a grading as mild, moderate, or severe oligoasthenozoospermia, which gives valuable information for fertility treatment outcomes. While we present both numbers in the characteristics, we therefore focused on the use of sperm concentration for the definition of normospermia. A p-value <0.05 was considered statistically significant.
Results
Patient Characteristics and Description of Cryopreserved Samples
Among the 506 patients who stored samples in the cryobank, the majority had been diagnosed with a testicular tumor (n=192, 37.9%), followed by those with hematological (n=191, 37.7%), non-testicular (n=105, 20.8%), and brain malignancies (n=18, 3.6%). Mean patient age at diagnosis was 29.4±9.1 years (range=9–71), including 53 (10.5%) patients aged <18 years. A total of 46 (9.1%) men requested their stored samples for assisted reproductive technology (ART) following cancer treatment. The cryobank stored 1–3 samples per patient (mean 1.2±0.4), of which 92.2% (554/601) were sperm samples. The majority of the samples were cryopreserved prior to oncologic treatment (460/600, 76.7%) with a median interval of 5 days (IQR=0–13). Samples that were stored following oncologic treatment initiation were collected after a median interval of 84 days (IQR=18-693.8). Generally, we observed stable sperm concentration and progressive motility values in adult cancer patient samples collected over the years. We observed an increase in both sperm concentration (R²=0.034; p=0.032) and progressive motility (R²=0.37; p=0.013) in adolescent’s samples collected after 2010; this may be due to technique precision improvement.
Semen and Testicular Tissue Quality Related to Collection Time Point and Diagnosis
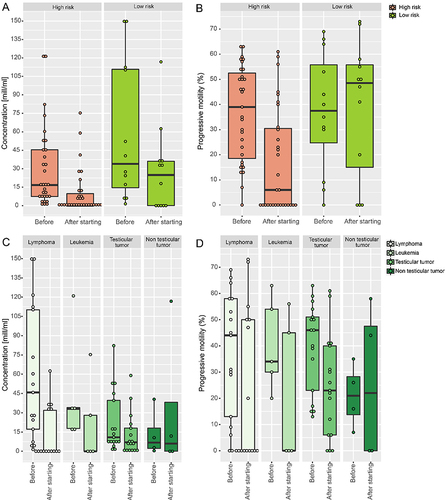
Prior to oncologic treatment initiation, we observed quality reduction in semen samples from adolescents with testicular or hematological malignancies (reduced median sperm concentration, mean vitality, and mean progressive motility) compared to WHO reference values (). Out of the 54 adolescent semen samples, 20 (37.0%) were oligoasthenozoospermic, and 3/5 (60.0%) testicular tissue samples (all collected from adolescents with a hematological malignancy) showed no sperm upon microscopic examination. In adult samples cryopreserved prior to oncologic treatment, sperm parameters were generally within WHO reference values, except for samples collected from testicular tumor patients (reduced mean sperm concentration). Yet, only 58.3% (221/379) of all adult samples were diagnosed with normozoospermia and 18.5% (70/379) with oligoasthenozoospermia. A quarter of adult testicular tissue samples (6/23, 26.1%) showed no sperm upon microscopic examination (). Type of cancer diagnosis most significantly influenced sperm concentration before treatment initiation in our regression model, whereas progressive motility depended on the collection year (R²=0.084).
Table 1 Sperm Sample Parameters Before and After Cancer Treatment Initiation, Relating to Diagnosis
Table 2 Testicular Tissue Sample Parameters Before and After Cancer Treatment Initiation, According to Diagnosis
Following cancer treatment initiation, the median sperm concentration was reduced–compared to WHO reference values in samples from adolescents and adults with testicular or hematological malignancies, and in adolescents with non-testicular tumors. Median progressive motility was below the normal range in adolescents with hematological malignancies, and in adults with non-testicular tumors (). Overall, 37.5% (9/24) of adolescent semen samples collected following treatment initiation and 43.0% (68/158) of adult samples were diagnosed as oligoasthenozoospermic or azoospermic. In adolescents, 2/3 (66.7%), and in adults, 7/15 (46.7%), of testicular tissue samples collected after oncologic treatment initiation showed no sperm upon microscopic examination ().
When comparing adolescent sample quality in relation to collection time point, no significant differences were revealed (), except for those from patients with testicular tumors (median sperm concentration, p=0.027). However, in adult samples, significant differences were seen depending on collection time point and diagnosis (testicular tumor: median concentration, p<0.001; mean volume p=0.004; hematological malignancies: p<0.001; non-testicular tumors: mean vitality, p=0.005; ). In adult samples, normozoospermia was less likely observed when collected following oncologic treatment initiation of hematological malignancies (p<0.001), testicular tumors (p<0.001), or non-testicular tumors (p=0.014).
Semen and Testicular Tissue Quality Related to Collection Time-Point and Treatment Gonadotoxicity
In samples collected following oncologic treatment initiation, semen quality parameters were reduced in all gonadotoxic-risk groups (low, medium, high) compared to samples collected following initiation of non-gonadotoxic treatment (). This included lower values in median sperm concentration in samples from adolescents (p=0.011) and adults (p=0.022), as well as reduced median progressive (p=0.001) and non-progressive motility (p=0.019) in adult samples. In multiple linear regressions, type of cancer diagnosis most significantly influenced median sperm concentration (R²=0.231), while no differences in any of the co-variables were found for progressive motility (R²=0.266).
Table 3 Sperm and Testicular Tissue Sample Quality Following Cancer Treatment Initiation, Related to Patient Age and Gonadotoxic-Risk of Treatment
In a subgroup analysis, semen quality from 44 patients was compared in samples that were cryopreserved prior to oncologic treatment; a follow-up spermiogram was available from each of these patients (Supplementary Table 1 and ). Following high gonadotoxic-risk treatment, a significant decrease in median sperm concentration (from 16.6 to 0.005 × 10⁶ mL⁻¹) and median motility (progressive sperm from 42.0% to 30.5%) was noted, while no significant changes were found following initiation of a low gonadotoxic-risk treatment. Simultaneously, azoospermia rates were significantly higher in follow-up samples collected after high gonadotoxic-risk treatment (p<0.05) (Supplementary Table 1 and ). The rate of oligiasthenozoospermic/azoospermic samples remained stable in those who provided follow-up spermiograms within 1 year after treatment initiation (before 45.5 vs after 45.5%), but increased in subgroups with follow-ups 1–2 (9.1 vs 90.9%), 2–3 (50.0 vs 100.0%), and more than 3 years (37.5 vs 50.0%) after treatment initiation. In the mixed model analysis, adjusted by time from treatment to follow-up spermiogram (range=0–132 months), type of cancer diagnosis influenced sperm concentration and progressive motility values, whereas collection time point (before or after treatment initiation) and time from treatment to follow-up did not. While cryopreservation time point influenced sperm diagnosis, no differences were seen regarding time since treatment to follow-up spermiogram and cancer diagnosis.
Figure 1 Quality of sperm samples before and after initiation of cancer treatment: (A) concentration according to gonadotoxic-risk of treatment (B) progressive motility according to gonadotoxic-risk of treatment (C) concentration according to cancer diagnosis (D) progressive motility according to cancer diagnosis.
Discussion
This study enriches the current knowledge on standard practice for sperm and testicular tissue cryopreservation in both adolescent and adult cancer patients. We describe the impact of cancer diagnosis and treatment on sperm and testicular tissue quality, specifically addressing sample collection time point (before vs after treatment initiation).
Current guidelines recommend cryopreservation before the start of potentially gonadotoxic treatment.Citation11,Citation15,Citation16 This is especially important as fertility may already be impaired at diagnosis, and would likely further decrease following cancer treatment.Citation24 In our study, only about half of the samples collected from adolescents (47%) and adults (58%) were normozoospermic prior to treatment. This rate is slightly higher if total sperm count is considered instead of sperm concentration (67.9 vs 70.0% in adolescents and 32.1 vs 30.0% in adults, with a total of 15 (2 adolescents and 13 adults) additional normozoospermic samples identified). Samples from patients with hematological or testicular malignancies showed a predisposed reduction in semen quality before cancer treatment. Reduced sperm quality at diagnosis has also been previously reported,Citation2,Citation24–27 and may be caused by impaired spermatogenesis, parenchymal damage due to tumor replacement in testicular tumors, hypothalamic-pituitary-gonadal disorders, or mitochondrial dysfunction (oxidative stress, DNA fragmentation).Citation28–30 Yet, studies on the rate of cryopreservation have shown that only a few use this chance of fertility preservation, with only 8% of adult and 19–28% of adolescent cancer patients cryopreserving samples.Citation31–33 In our cohort, samples from adolescents showed higher rates of oligoasthenozoospermia compared to those from adults (38% vs 19%). Adolescents were also more likely than adults to have testicular tissue cryopreserved prior to oncologic treatment, despite azoospermia (60% vs 26%). This rate may reflect an actual reduction in spermatogenesis in younger patients, but more likely highlights a conscious decision for cryopreservation despite impaired sample quality. Having already completed a collection process, patients (and their families) may have opted for, rather than against, cryopreservation. Newly diagnosed cancer patients, faced with the threat of potential therapy-induced infertility, may strive for FP, regardless of sample quality.
In some cases, FP may not be possible prior to treatment. A quarter of the samples in our cohort (23%) were cryopreserved after cancer treatment initiation, which may be attributed to treatment demands, lack of information, or other reasons.Citation34,Citation35 Both diagnosis and therapy gonadotoxicity seemed to affect sample quality following treatment initiation. An increased risk of azoospermia following cancer treatment reduces the chances of natural conception, and of viable sperm in ejaculate and/or testicular tissue for ART. In adults, the quality of semen and testicular tissue samples collected following treatment initiation was significantly reduced compared to those collected at diagnosis. Semen samples were cryopreserved when motile sperm was found microscopically in the native sample or in the sediment after centrifugation (such as in cryptozoospermia); or at the patient’s special request. Therefore, the number of patients with poor sample quality before the start of treatment might be underrepresented. Unfortunately, we have no data on the number of patients whose attempted collection was not cryopreserved due to azoospermia. We can state, however, that the rate of azoospermic samples cryopreserved from cancer patients following treatment initiation was higher than the rates of samples cryopreserved prior to initiation. Following treatment initiation, most of adult samples were cryopreserved following a high gonadotoxic-risk treatment, whereas samples collected from adolescents were at most collected following low gonadotoxic-risk treatment (45.8%, 11/24). According to the guideline definitions that we used to classify treatment gonadotoxicity, we observed the highest rate of azoospermic samples following high gonadotoxic-risk treatment in both adults and adolescents. However, it needs to be acknowledged that semen parameters were also reduced in the moderate and low gonadotoxic-risk groups compared to those who received non-gonadotoxic treatment. This emphasizes the necessity of fertility surveillance for all patients, not only following high gonadotoxic-risk treatment but also after a moderate/low gonadotoxic-risk treatment—especially as these patients may be less aware of the potential gonadotoxic effect of their treatment. Patient counselling and fertility monitoring after cancer treatment are therefore essential for all childhood, adolescent, and adult cancer patients, and should include annual documentation of puberty and fertility development, including hormone analyses and semen analyses, as part of clinical routine.Citation36
The course of spermatogenesis following cancer treatment is not fully predictable. Fertility impairment may be detected as early as at diagnosis, or it can occur during or shortly following the end of cancer treatment. Patients who underwent high gonadotoxic-risk treatment such as is required before allogeneic hematopoietic stem cell transplantation are especially at risk, with infertility occurring at a median of 2.6 years.Citation37 However, for some patients, fertility impairment can manifest years after cancer treatment.Citation37 Sperm counts often remain stable within the first 2 months following the start of cancer treatment.Citation8 In our subgroup analyses, we saw no change in the percentage of oligoasthenozoospermic/azoospermic samples collected within 1 year after treatment initiation. However, the rate of azoospermic samples increased following this time (highest in samples from patients who followed up after 1 to 2 years). Semen quality parameters showed no differences regarding time from treatment to sperm analysis. The potential of gonadal recovery is not fully understoodCitation38 and remains unpredictable,Citation9 as only a few studies have examined semen quality prior to oncologic treatment.Citation2 While recovery of impaired spermatogenesis is possible, it is more likely persistent. Post-treatment recovery of spermatogenesis was previously not only documented in moderately impaired patients who had normozoospermia at diagnosis (within 66 months) but also in those more significantly impaired (at a peak of >66 months).Citation2 After certain oncologic treatments, a recovery of spermatogenesis occurred within 24–31 months post-treatment. This included treatment with 3–4 cycles of bleomycin, etoposide, and cisplatin (BEP regimen) or radiotherapy, as well as following treatment with daily oral cyclophosphamide for periods of 5–34 months.Citation24,Citation39 Further research should look into the dynamics of fertility development in men following a cancer treatment on a more individual level in order to improve prediction strategies.
The use of sperm banking in cancer survivors is a standard fertility preservation treatment.Citation40 Samples may be used for in-vitro fertilization (IVF) or – with better clinical outcomes than IVF or intrauterine insemination (IUI) – for intracytoplasmic sperm infection (ICSI).Citation41 The success rates of assisted reproductive techniques (ART) with fresh oocytes are comparable for couples where the male partner has a history of cancer treatment, regardless of whether fresh or cryopreserved semen is utilized with fresh oocytes.Citation42 The outcome of embryo transfers is significantly higher when cryopreserved semen is used, but live birth rates remain similar to those using fresh sperm.Citation42,Citation43 In ART, particularly more in ICSI, sperm with DNA breakage are naturally excluded from selection due to concerns regarding potential alterations in the sperm epigenome. Consequently, this exclusion may lead to improved fertilization rates, embryo quality, and implantation rates.Citation44,Citation45 Patients should therefore be advised to collect sperm before cytotoxic treatments, since genetic alterations may persist after cancer treatment.Citation46 DNA fragmentation tests are increasingly used as part of sperm testing within the recent years’ increasing knowledge about the chance of success in ART, especially in samples of very limited quality (use for ICSI treatment).Citation47 These results could help to decide whether a fresh sperm sample collected after therapy, or a sample cryopreserved before treatment initiation, should be used for ART. Nonetheless, it should be noted that large studies have shown no increased risk of congenital malformation or cancer development in offspring born to cancer survivors following spontaneous conception or ART use.Citation48–50 Providing this information to patients could help to reduce fears.
Study Limitations
Due to the retrospective study design, we were unable to collect medical data from all cancer patients who had samples stored in the Charité cryobank, especially those who came from external oncological clinics. Unsuccessful cryopreservation attempts were not regularly documented, especially prior to treatment. Exclusion of these patients due to missing data resulted in selection bias. Very few patients had follow-up spermiograms, which led to a limited subgroup analysis to identify semen quality development over time. Although we have contacted all patients and treating fertility centers, there has been a lack of feedback regarding the use of the samples for ART. Therefore, essential sources of information on the type of use, and the results regarding successful pregnancies by the use of cryopreserved samples, are missing. Another issue is that clinical practices have changed over the years in both cancer treatment and FP. In our study, the techniques for cryopreservation refer to the specifications of the stricter recommendations of the “WHO laboratory manuals for the examination and processing of human ejaculate” from 2010, which may not reflect the results according to the former standards when samples were analyzed.
Conclusions
Sperm and testicular tissue banking are effective options for FP in adolescents and adults. Nevertheless, these methods are insufficiently implemented in cancer patients. Oncologic treatment, especially high, as well as moderate and low gonadotoxic-risk treatment, can impair spermatogenesis, resulting in infertility. However, a relevant proportion of adolescents and adults with cancer are predisposed to azoospermia at the time of diagnosis. Therefore, fertility counselling and assessment should be conducted at initial diagnosis in order to enable patients— if desired—to undergo fertility preservation measures.
Ethical Approval
Our study was approved by the ethics committee of the Charité-Universitätsmedizin Berlin (EA4/158/19).
Disclosure
Ina Wilkemeyer and Magdalena Balcerek share equal last authorship. The authors declare no conflicts of interest in this work.
Additional information
Funding
References
- Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S. Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol. 2002;20(7):1880–1889. doi:10.1200/JCO.2002.07.175
- Bahadur G, Ozturk O, Muneer A, et al. Semen quality before and after gonadotoxic treatment. Hum Reprod. 2005;20(3):774–781. doi:10.1093/HUMREP/DEH671
- Tomlinson M, Meadows J, Kohut T, et al. Review and follow-up of patients using a regional sperm cryopreservation service: ensuring that resources are targeted to those patients most in need. Andrology. 2015;3(4):709–716. doi:10.1111/ANDR.12045
- Kawai K, Nishiyama H. Preservation of fertility of adult male cancer patients treated with chemotherapy. Int J Clin Oncol. 2018;24(1):34–40. doi:10.1007/S10147-018-1333-0
- Meistrich ML. Male gonadal toxicity. Pediatr Blood Cancer. 2009;53(2):261–266. doi:10.1002/pbc.22004
- Stukenborg JB, Jahnukainen K, Hutka M, Mitchell RT. Cancer treatment in childhood and testicular function: the importance of the somatic environment. Endocr Connect. 2018;7(2):R69–R87. doi:10.1530/EC-17-0382
- Agarwal A, Ranganathan P, Kattal N, et al. Fertility after cancer: a prospective review of assisted reproductive outcome with banked semen specimens. Fertil Sterility. 2004;81(2):342–348. doi:10.1016/j.fertnstert.2003
- Trottmann M, Becker AJ, Stadler T, et al. Semen quality in men with malignant diseases before and after therapy and the role of cryopreservation. Eur Urol. 2007;52(2):355–367. doi:10.1016/J.EURURO.2007.03.085
- Okada K, Fujisawa M. Recovery of spermatogenesis following cancer treatment with cytotoxic chemotherapy and radiotherapy. World J Mens Health. 2018;37(2):166–174. doi:10.5534/WJMH.180043
- Ulrich U, Buchweitz O, Greb R, et al. National German Guideline (S2k): guideline for the diagnosis and treatment of endometriosis. Geburtshilfe Frauenheilkd. 2014;74(12):1104–1118. doi:10.1055/S-0034-1383187/ID/R852-17/BIB
- Dittrich R, Kliesch S, Schüring A, et al. Fertility preservation for patients with malignant disease. Guideline of the DGGG, DGU and DGRM (S2k-Level, AWMF Registry No. 015/082, November 2017) - recommendations and statements for girls and women. Geburtshilfe Frauenheilkd. 2018;78(6):567–584. doi:10.1055/A-0611-5549
- Wasilewski-Masker K, Seidel D, Mertens AC, et al. Male infertility in long-term survivors of pediatric cancer: a report from the Childhood Cancer Survivor Study. J Cancer Surviv. 2014;8(3):437–447. doi:10.1007/s11764-014-0354-6
- Van Der Kaaij MAE, Heutte N, Van Echten-Arends J, et al. Sperm quality before treatment in patients with early stage Hodgkin’s lymphoma enrolled in EORTC-GELA Lymphoma Group trials. Haematologica. 2009;94(12):1691. doi:10.3324/HAEMATOL.2009.009696
- Van Casteren NJ, Boellaard WPA, Romijn JC, Dohle GR. Gonadal dysfunction in male cancer patients before cytotoxic treatment. Int J Androl. 2010;33(1):73–79. doi:10.1111/J.1365-2605.2009.00956.X
- Lambertini M, Peccatori FA, Demeestere I, et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines y on behalf of the ESMO Guidelines Committee. Ann Oncol. 2020. doi:10.1016/j.annonc.2020.09.006
- Oktay K, Harvey BE, Partridge AH, et al. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2018;36:1994–2001. doi:10.1200/JCO.2018.78.1914
- Pacey AA, Eiser C. Banking sperm is only the first of many decisions for men: what healthcare professionals and men need to know. Human Fertility. 2011;14(4):208–217. doi:10.3109/14647273.2011.634480
- Pariz JR, Monteiro RAC, Hallak J. Long-term sperm cryopreservation does not affect post-thaw survival rates. J Bras Reprod Assist. 2020;24(1):3–8. doi:10.5935/1518-0557.20190066
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. Published on behalf of the World Health Organization [by] Cambridge University Press; 2010.
- Picton HM, Wyns C, Anderson RA, et al.; Diseaseson behalf of the ETFOFPIS. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod. 2015;30(11):2463–2475. doi:10.1093/HUMREP/DEV190
- Levi-Setti PE, Negri L, Baggiani A, et al. Testicular sperm extraction and intracytoplasmic sperm injection outcome in cancer survivors with no available cryopreserved sperm. J Assist Reprod Genet. 2020;37(4):875–882. doi:10.1007/S10815-020-01697-7
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. Published on behalf of the World Health Organization [by] Cambridge University Press; 1999.
- Johnsen SG. Testicular biopsy score count – a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Horm Res Paediatr. 1970;1(1):2–25. doi:10.1159/000178170
- Vomstein K, Reiser E, Pinggera GM, et al. Sperm banking before gonadotoxic treatment: is it worth the effort? Asian J Androl. 2021;23(5):490. doi:10.4103/AJA.AJA_16_21
- Appaneravanda LC, Gerstl B, Nagaraju A, Kumar A, Balamukund IS, Gunasheela D. A descriptive study exploring semen quality among Indian cancer patients. J Adolesc You Adult Oncol. 2021;10(6):690–696. doi:10.1089/JAYAO.2021.0005
- Williams DH, Karpman E, Sander JC, Spiess PE, Pisters LL, Lipshultz LI. Pretreatment Semen parameters in men with cancer abbreviations and acronyms GI gastrointestinal NCRMN national cooperative reproductive medicine network NHL nonHodgkin’s lymphoma. JURO. 2009;181:736–740. doi:10.1016/j.juro.2008.10.023
- Depalo R, Falagario D, Masciandaro P, et al. Fertility preservation in males with cancer: 16-year monocentric experience of sperm banking and post-thaw reproductive outcomes. Ther Adv Med Oncol. 2016;8(6):412–420. doi:10.1177/1758834016665078
- Van Der Kooi ALLF, Van Den Heuvel-Eibrink MM, Van Den Berg SAA, Van Dorp W, Pluijm SMF, Laven JSE. Changes in anti-müllerian hormone and inhibin b in children treated for cancer. J Adolesc You Adult Oncol. 2019;8(3):281–290. doi:10.1089/JAYAO.2018.0130
- Selvam MKP, Agarwal A, Pushparaj PN. Altered molecular pathways in the proteome of cryopreserved sperm in testicular cancer patients before treatment. Int J Mol Sci. 2019;20(3):677. doi:10.3390/IJMS20030677
- Parekh NV, Lundy SD, Vij SC. Fertility considerations in men with testicular cancer. Transl Androl Urol. 2020;9(Suppl 1):S14. doi:10.21037/TAU.2019.08.08
- Ferrari S, Paffoni A, Filippi F, Busnelli A, Vegetti W, Somigliana E. Sperm cryopreservation and reproductive outcome in male cancer patients: a systematic review. Reprod BioMed Online. 2016;33:29–38. doi:10.1016/j.rbmo.2016.04.002
- Klosky JL, Wang F, Russell KM, et al. Prevalence and predictors of sperm banking in adolescents newly diagnosed with cancer: Examination of adolescent, parent, and provider factors influencing fertility preservation outcomes. J Clin Oncol. 2017;35(34):3830. doi:10.1200/JCO.2016.70.4767
- Balcerek M, Schilling R, Byrne J, et al. Determinants of utilization of cryopreservation of germ cells in adolescent cancer patients in four European countries. Eur J Pediatr. 2020;179(1):51–60. doi:10.1007/S00431-019-03459-9
- Overbeek A, van den Berg M, Louweé L, et al. Practice, attitude and knowledge of Dutch paediatric oncologists regarding female fertility. 2014. https://pubmed.ncbi.nlm.nih.gov/24930460/. Accessed June 22, 2022.
- Vindrola-Padros C, Dyer KE, Cyrus J, Lubker IM. Healthcare professionals’ views on discussing fertility preservation with young cancer patients: a mixed method systematic review of the literature. Psychooncology. 2017;26(1):4–14. doi:10.1002/PON.4092
- Balcerek M, Wolff von M, Borgmann-Staudt A. Paediatric Cancer. Fertil Preserv Oncol Non-Oncological Dis. 2020;93–103. doi:10.1007/978-3-030-47568-0_12
- Pfitzer C, Orawa H, Balcerek M, et al. Dynamics of fertility impairment and recovery after allogeneic haematopoietic stem cell transplantation in childhood and adolescence: results from a longitudinal study. J Cancer Res Clin Oncol. 2014;141(1):135–142. doi:10.1007/S00432-014-1781-5
- Nangia AK, Krieg SA, Kim SS. Clinical guidelines for sperm cryopreservation in cancer patients. Fertil Steril. 2013;100(5):1203–1209. doi:10.1016/J.FERTNSTERT.2013.08.054
- Bujan L, Walschaerts M, Moinard N, et al. Impact of chemotherapy and radiotherapy for testicular germ cell tumors on spermatogenesis and sperm DNA: a multicenter prospective study from the CECOS network. Fertil Steril. 2013;100(3):673–680.e2. doi:10.1016/J.FERTNSTERT.2013.05.018
- Bitan R, Magnezi R, Kedem A, et al. Autologous sperm usage after cryopreservation—the crucial impact of patients’ characteristics. Andrology. 2024;12(3):527–537. doi:10.1111/ANDR.13502
- Li Q, Lan QY, Zhu WB, Fan LQ, Huang C. Fertility preservation in adult male patients with cancer: a systematic review and meta-analysis. Hum Reprod Open. 2024;2024(1). doi:10.1093/HROPEN/HOAE006
- Papler TB, Vrtacnik-Bokal E, Drobnic S, Stimpfel M. The outcome of IVF/ICSI cycles in male cancer patients: retrospective analysis of procedures from 2004 to 2018. Radiol Oncol. 2021;55(2):221–228. doi:10.2478/RAON-2021-0011
- Melli B, Morini D, Daolio J, et al. Semen cryopreservation in men undergoing cancer treatment: a ten-year study. Minerva Obstet Gynecol. 2022;75(3):227–235. doi:10.23736/S2724-606X.22.04994-6
- Zini A, Jamal W, Cowan L, Al-Hathal N. Is sperm DNA damage associated with IVF embryo quality? A systematic review. J Assist Reprod Genet. 2011;28(5):391. doi:10.1007/S10815-011-9544-6
- Sadeghi MR, Hodjat M, Lakpour N, et al. Effects of sperm chromatin integrity on fertilization rate and embryo quality following intracytoplasmic sperm injection. Avicenna J Med Biotechnol. 2009;1(3):173.
- Beaud H, Tremblay AR, Chan PTK, Delbes G. Sperm DNA damage in cancer patients. Adv Exp Med Biol. 2019;1166:189–203. doi:10.1007/978-3-030-21664-1_11
- World Health Organization. 2021 WHO laboratory manual for the examination and processing of human semen. Geneva World Heal Organ. 2021;6:1–276.
- Sommerhäuser G, Borgmann-Staudt A, Astrahantseff K, et al. Health outcomes in offspring born to survivors of childhood cancers following assisted reproductive technologies. J Cancer Surviv. 2021;15(2):259–272. doi:10.1007/S11764-020-00929-0/TABLES/4
- Spaan M, Van Den Belt-Dusebout AW, Van Den Heuvel-Eibrink MM, et al. Risk of cancer in children and young adults conceived by assisted reproductive technology. Hum Reprod. 2019;34(4):740–750. doi:10.1093/HUMREP/DEY394
- Van Dorp W, Haupt R, Anderson RA, et al. Reproductive function and outcomes in female survivors of childhood, adolescent, and young adult cancer: a review. J Clin Oncol. 2018;36(21):2169–2180. doi:10.1200/JCO.2017.76.3441
