Abstract
Background
Regorafenib, a novel multikinase inhibitor, has been approved by the US Food and Drug Administration as a standard treatment choice for metastatic colorectal cancer (mCRC). Nonetheless, its substantial cost places a significant burden on social health resources and patients. However, the cost-effectiveness (CE) of regorafenib compared to other third-line therapies is still undetermined.
Objective
This study aims to assess the CE of regorafenib compared to other third-line therapies for the treatment of mCRC.
Methods
We conducted a comprehensive literature search in PubMed, Medline, Scopus, Embase, Cochrane Library, as well as nine other databases to identify relevant studies published up to October 2023, focusing on patients with mCRC and examining the cost-effectiveness of regorafenib. Following the screening and extraction of pertinent data, the study quality was assessed using the Quality of Health Economic Studies (QHES) checklist.
Results
The literature search yielded 751 records, and after applying the inclusion criteria, 13 studies from 7 different countries were included. Of these, 7 studies evaluated the cost-effectiveness of regorafenib compared to trifluridine/tipiracil (TAS-102), 3 studies compared regorafenib with best supportive care (BSC), and 3 studies compared regorafenib with fruquintinib, serplulimab, and regorafenib dose optimization (ReDo).The quality of the included studies was high with an average QHES scores of 85.62. Regorafenib standard dose proves to be less cost-effective than alternative third-line therapies. Implementing a dose optimization strategy could potentially rectify this disparity and enhance the cost-effectiveness of regorafenib.
Conclusion
The use of the standard dose of regorafenib is generally regarded as not cost-effective when compared to other third-line therapies for patients with mCRC. However, implementing a dose-escalation strategy may enhance regorafenib’s cost-effectiveness. Consequently, significant price reductions or optimizing the dose of regorafenib are required to achieve cost-effectiveness.
Introduction
Colorectal cancer (CRC) is the third most common type of cancer and a prominent cause of cancer-related deaths worldwide.Citation1 In 2023, approximately 153,020 new cases of CRC and 52,550 deaths in the United States.Citation2 In the past decades, the incidence of CRC in individuals under the age of 50 years has increased rapidly, and this trend has been observed globally in both men and women.Citation3 Among all initial CRC diagnoses, approximately 25% of patients have metastatic CRC (mCRC) at the first diagnosis and at least 50% of patients eventually develop metastases.Citation4,Citation5 The prognosis of mCRC is poor, with a 5-year survival rate of less than 15%.Citation6 Although surgery with or without adjuvant chemotherapy can cure early-stage CRC, mCRC cannot be eradicated due to the substantial burden of disseminated cancer cells, which are composed of therapy-resistant metastasis-competent cells.
For decades, major therapeutic advances involving chemotherapy (fluoropyrimidine, oxaliplatin, and irinotecan) as a backbone combined with monoclonal antibodies targeting specific molecular subtypes have been achieved in mCRC, resulting in clinically relevant survival improvement.Citation4,Citation7 In recent years, drugs targeting elevated processes or pathways in tumor cells, such as angiogenesis and the epidermal growth factor receptor (EGFR)-mediated mitogen-activated protein kinase (MAPK) pathway, have been successfully employed in clinical practice.Citation8 In 2012, regorafenib was approved by the United States Food and Drug Administration (FDA) as a third-line therapy for mCRC refractory to fluoropyrimidine, oxaliplatin, and irinotecan-based chemotherapy, prior anti-VEGF therapy, and if KRAS wild-type, previous anti-EGFR therapy.Citation9
With the substantial price reduction of regorafenib and updates to relevant guidelines, several scholars have conducted comparative studies on the cost-effectiveness of regorafenib for patients with mCRC compared to other third-line or further treatment options. However, due to variations in the economic level, healthcare environment, and pricing of regorafenib in different countries, there may be differences in the methodologies or results of its economic evaluation. Therefore, this study aimed to evaluate the cost-effectiveness of regorafenib compared with other third-line or further treatments for mCRC to comprehensively assess its cost-effectiveness and provide guidance for clinical applications and healthcare decision-makers.
Materials and Methods
Sources and Search Strategy
This study conformed to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.Citation10 Eligible studies were searched using PubMed, Medline, Scopus, Embase, Cochrane Library, and nine other databases to identify relevant articles published in English from January 1, 2012, to October 31, 2023, with an abstract available for review. Titles and abstracts were initially searched using the following search algorithm: “Regorafenib OR Stivarga AND metastatic colorectal cancer OR mCRC AND cost-effectiveness OR cost-utility or pharmacoeconomics or economic evaluation or cost”.
Eligibility Criteria
The inclusion criteria were as follows: (a) adopting cost-effectiveness analysis or cost-utility analysis (CUA); (b) patients diagnosed with mCRC and treatment with regorafenib; (c) incremental cost-effectiveness ratios (ICERs) adopted to compare treatments. The exclusion criteria were as follows: (a) not published in English; (b) not related to economic evaluation; and (c) non-specific drug studies (studies about screening strategies, intervention thresholds, medication adherence, etc.).
Data Extraction and Quality Assessment
Eligible studies were independently screened by two researchers to extract relevant data for inclusion in the study. Cross-validation was conducted to ensure accuracy, and discrepancies were discussed and reconciled. The extracted content included basic information on the research (first author, publication date, country, perspective, research type/model, and outcome index), study design (intervention, health outcomes, time horizon, and discounting), study outcomes, sensitivity analysis, and other necessary parameters. Importantly, ICERs were extracted as reported in the original article, and no adjustment for year or purchasing power parity was performed.
The Quality of Health Economic Studies (QHES) Checklist was used to evaluate the quality of the included studies.Citation11 The QHES is a validated grading system designed to assess the quality of economic health analyses. It evaluates each study across 16 criteria and assigns a score between 0 and 100, where 0 indicates the lowest quality and 100 represents the highest. The point values for each criterion were determined using regression analysis. After calculating the points for all 16 criteria, the studies were categorized into four categories: extremely poor quality (0–24), poor quality (25–49), fair quality (50–74), and high quality (75–100).Citation12
Results
Literature Search
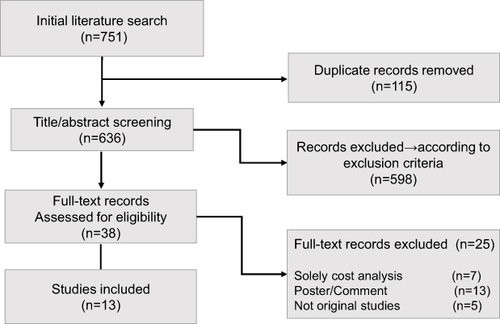
A total of 751 potentially relevant articles obtained from both manual and gray literature searches were identified in the databases. After screening, 738 articles were excluded as they were outside the scope of this study. Thirteen articles met our inclusion criteria. A flow diagram of the search and selection strategy is shown in .
Study Descriptions
As indicated in , these 13 studies were from seven different countries, four studies were conducted in the US,Citation13–16 three studies were from China,Citation17–19 two studies were from JapanCitation20,Citation21 and the rest were from Italy,Citation22 England and Wales,Citation23 Greece,Citation24 and the Czech.Citation25 The most commonly adopted perspective in the reviewed economic evaluation was the payer perspective; of the nine studies with the perspective of governmental healthcare, one used the third-party payer perspective. The majority of studies (11 of 13) incorporated cost-utility analysis (CUA), of which seven utilized the Markov model, whereas the remaining five adopted the partitioned survival model. Most studies selected life years (LYs), quality-adjusted life-years (QALYs), incremental cost-effectiveness ratio (ICER), and cost as the health output index. The results of the QHES assessment revealed that the overall quality of the included studies was relatively high, with an average QHES score of 85.62 ().
Table 1 General Characteristics of the Included Records
Table 2 Results Form the Quality of Health Economic Studies (QHES) Evaluation
Cost-Effectiveness Analysis
Clinical data included in the records were mainly selected from randomized controlled trials (RCT), meta-analyses, and clinical trials. Progression-free survival (PFS) and/or overall survival (OS) were used as the outcomes in these 11 studies with a model, two studies used no model. Eight studies applied a fixed time horizon, such as 2.5, 3, 5, or 10 years, whereas the others did not indicate the time horizon. Six studies applied a 5% discount rate for both costs and QALYs, two studies used 3.5%, and one study applied 3%, while the other three studies did not. All the 13 studies only considered the direct medical cost, including drug, pallicare per day, adverse drug reactions (ADRs) related management, outpatient chemotherapy, terminal care, and other non-medical-related expenses. Clinical data selection and cost analyses are presented in .
Table 3 Cost Analysis of the Included Publications
Regorafenib versus BSC
As shown in , three studies evaluated the CE of regorafenib in a third-line setting in patients with mCRC. Goldstein et alCitation14 assessed the cost-effectiveness of regorafenib for mCRC from the US payer perspective and found that regorafenib provides minimal incremental benefit at a high incremental cost per QALY, with a 50% chance that regorafenib is cost-effective at a willingness-to-pay (WTP) value of approximately $900,000 per QALY. Zhang S et alCitation19 compared the cost-effectiveness of regorafenib with placebo plus best supportive care (BSC) in Chinese patients with mCRC, and indicated that regorafenib is not cost-effective at the WTP threshold of $27,576 when compared with the placebo group. Mlcoch et alCitation25 examined the cost-effectiveness of regorafenib using a propensity score-weighted cohort from the Czech Registry. They concluded that, based on data from the registry and RCTs in the Czech Republic, regorafenib represents a cost-effective therapeutic option for patients with mCRC at a WTP threshold of €47,000 per QALY.
Table 4 Health Output Assessment of Included Publications
Regorafenib versus Trifluridine/Tipiracil
As indicated in , seven studies evaluated the cost-effectiveness of regorafenib compared to trifluridine/tipiracil (TAS-102) for the treatment of patients with mCRC. Two studies were from the USACitation13,Citation15 two studies were from Japan 21,26Citation20,Citation21 and the rest were from Italy,Citation22 England and Wales,Citation23 and Greece.Citation24 Five studies evaluated the cost-effectiveness of regorafenib standard dose with TAS-102 and concluded that TAS-102 is more clinically and cost-effective than regorafenib.Citation13,Citation20,Citation21,Citation23,Citation24 Two additional studies examined the cost-effectiveness of regorafenib dose optimization (ReDo) in comparison to TAS-102. They concluded that the optimal dosing strategy for regorafenib has enhanced its benefit-to-toxicity ratio and relative cost-effectiveness when compared to TAS-102.Citation15,Citation22 Cho SK et alCitation15 conducted a cost-effectiveness analysis comparing ReDo to TAS-102 in combination with bevacizumab (TAS-BEV). Their findings indicated that ReDo was more cost-effective than TAS-BEV, as it offered a higher QALY at a lower cost.
Regorafenib versus Other Molecular Target Drugs
As shown in , Two studies conducted in China investigated the cost-effectiveness of regorafenib compared to fruquintinib or serplulimab as a third-line treatment for patients with mCRC. Guan et alCitation17 developed a three-state Markov model to evaluate the cost-effectiveness of regorafenib versus fruquintinib in the context of China. Their study findings indicated that fruquintinib is the more cost-effective option, as it is associated with an increase of approximately 0.05 quality-adjusted life years (QALYs) and results in a cost saving of about $11,454. Ma Y et alCitation18 developed a three-state Markov model to estimate the costs and health outcomes of serplulimab and regorafenib in China. They concluded that serplulimab is more cost-effective than regorafenib for patients with previously treated metastatic colorectal cancer (mCRC) in China.
Sensitivity Analysis Results and Main Limitations
As shown in , most studies (11 out of 13) performed a sensitivity analysis to evaluate the uncertainty and robustness of the model, of which three studies adopted one-way and probabilistic sensitivity analysesCitation15,Citation17,Citation24 Three studies conducted univariate and probabilistic sensitivity analysesCitation14,Citation16,Citation19 Two studies used scenario analysis based on one-way and probabilistic sensitivity analyses;Citation18,Citation20 one study used scenario analysis based on univariate and probabilistic sensitivity analyses,Citation13 the rest employed one-way sensitivity analyses and probabilistic sensitivity analyses, respectively.Citation23,Citation25 The sensitivity analysis results revealed that drug prices, baseline utility value, and exposure to regorafenib exerted the most significant influence on the ICER. Moreover, nine studies identified the primary limitations of the study, with the lack of local data and direct clinical evidence being the most frequently cited constraints in the included studies.
Table 5 Sensitivity Analyses, Conclusions and Limitations of Included Studies
Discussion
This study systematically reviewed and assessed the quality of selected economic evaluation studies on the cost-effectiveness of regorafenib versus other third-line or further molecular-targeted drugs for mCRC. The study findings indicate that administering regorafenib treatment yields marginal gains at considerably high incremental costs per QALY for patients with mCRC. Regorafenib is projected to have lower cost-effectiveness compared to other established treatment choices like TAS-102, fruquintinib, and serplulimab. Nevertheless, the implementation of a dose optimization strategy has the potential to alter this scenario, rendering regorafenib more cost-effective than TAS-102. Therefore, we performed a cost-effectiveness analysis of regorafenib versus other third-line and molecular-targeted drugs for the treatment of mCRC to facilitate treatment strategies for patients and clinicians.
As a broad-spectrum, antiangiogenic, multikinase inhibitor, regorafenib was used as third-line therapy for patients with mCRC who progressed after standard therapy.Citation26 In CORRECT trial, regorafenib demonstrated a median OS improvement of 1.4 months compared with placebo.Citation27 Three studies conducted in different countries compared the cost-effectiveness of regorafenib with that of a placebo in the third-line treatment of mCRC and presented varying outcomes. The research from the United States and the Czech Republic concluded that regorafenib is a cost-effective therapy,Citation14,Citation25 and another study from China revealed that regorafenib is not cost-effective when compared with placebo group.Citation19 The inconsistencies in research findings may stem from variations in research perspectives, models, and data sources. Furthermore, differences in the selection of price points before and after the reduction in regorafenib price could also contribute to these inconsistencies.
TAS-102, a fluoropyrimidine-derivative drug, was approved by the FDA in 2015 as third-line therapy or beyond for unselected patients with mCRC.Citation7 A previous study revealed that treatment with TAS-102 significantly improved the OS of patients with mCRC compared with placebo.Citation28 Seven studies from five different countries compared the cost-effectiveness of TAS-102 with regorafenib in third-line or further treatment of patients with mCRC from 5 different countries. Of which, five studies shown that TAS-102 is more cost-effective than regorafenib with a standard-dose strategy. However, two studies showed that the adoption of a dose optimization strategy could reverse the situation and make regorafenib more cost-effective than TAS-102, with a low cost per month of OS-gain (510.41€) and an ICER of $37,966 relative to TAS-102.Citation15,Citation22 The phase 2 trial known as C-TASKFORCE has provided data supporting the utilization of TAS-BEV as a viable treatment alternative for patients grappling with chemotherapy-refractory mCRC.Citation29 Cho SK et alCitation15 founded that ReDO was both less costly and more effective than TAS-BEV. Furthermore, two studies demonstrated that regorafenib is less cost-effective than either fruquintinib or serplulimab.
This study has several limitations. First, databases were searched in English, and studies published in other languages were not included. In addition, the 13 articles included in this study came from seven different countries, which are different in medical services, drug costs, and willingness-to-pay threshold, so it is impossible to compare the results directly. Finally, publication bias could have influenced the available evidence. This problem may be particularly important here, as most studies lack direct clinical evidence for a comparison between regorafenib and other third-line or further molecular-target drugs.
In conclusion, regorafenib has a cost-effectiveness advantage over BSC for patients with mCRC and is less cost-effective than other third-line or further molecular target drugs, while a dose optimization strategy could reverse the situation and make regorafenib more cost-effective.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Data Sharing Statement
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233–254. doi:10.3322/caac.21772
- Spaander MCW, Zauber AG, Syngal S, et al. Young-onset colorectal cancer. Nat Rev Dis Primers. 2023;9(1):21. doi:10.1038/s41572-023-00432-7
- Ciardiello F, Ciardiello D, Martini G, Napolitano S, Tabernero J, Cervantes A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J Clin. 2022;72(4):372–401. doi:10.3322/caac.21728
- Morris VK, Kennedy EB, Baxter NN, et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J Clin Oncol. 2023;41(3):678–700. doi:10.1200/JCO.22.01690
- Alice E, Shin F, Giancotti G, Anil K. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci. 2023;44.
- Shin AE, Giancotti FG, Rustgi AK. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci. 2023;44(4):222–236. doi:10.1016/j.tips.2023.01.003
- Raghav K, Ou FS, Venook AP, et al. Acquired Genomic Alterations on First-Line Chemotherapy With Cetuximab in Advanced Colorectal Cancer: circulating Tumor DNA Analysis of the CALGB/SWOG-80405 Trial (Alliance). J Clin Oncol. 2023;41(3):472–478. doi:10.1200/JCO.22.00365
- Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20(8):1070–1082. doi:10.1016/S1470-2045(19)30272-4
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi:10.1136/bmj.n160
- Ofman JJ, Sullivan SD, Neumann PJ, et al. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manag Care Pharm. 2003;9(1):53–61. doi:10.18553/jmcp.2003.9.1.53
- Spiegel BM, Targownik LE, Kanwal F, et al. The quality of published health economic analyses in digestive diseases: a systematic review and quantitative appraisal. Gastroenterology. 2004;127(2):403–411. doi:10.1053/j.gastro.2004.04.020
- Cho SK, Hay JW, Barzi A. Cost-effectiveness Analysis of Regorafenib and Tas-102 in Refractory Metastatic Colorectal Cancer in the United States. Clin Colorectal Cancer. 2018;17(4):e751–e761. doi:10.1016/j.clcc.2018.08.003
- Goldstein DA, Ahmad BB, Chen Q, et al. Cost-Effectiveness Analysis of Regorafenib for Metastatic Colorectal Cancer. J Clin Oncol. 2015;33(32):3727–3732. doi:10.1200/JCO.2015.61.9569
- Cho SK, Bekaii-Saab T, Kavati A, Babajanyan S, Hocum B, Barzi A. Value-Based Analysis of Therapies in Refractory Metastatic Colorectal Cancer in US. Clin Colorectal Cancer. 2022;21(4):277–284. doi:10.1016/j.clcc.2022.09.003
- Barzi A, Cho SK, Hay JW, et al. Cost-effectiveness (CE) of regorafenib dose optimization schedule in metastatic colorectal cancer. J clin oncol. 2019;37.
- Guan X, Li H, Xiong X, et al. Cost-effectiveness analysis of fruquintinib versus regorafenib as the third-line therapy for metastatic colorectal cancer in China. J Med Econ. 2021;24(1):339–344. doi:10.1080/13696998.2021.1888743
- Ma Y, Zhou J, Ye Y, Wang X, Ma A, Li H. The cost-effectiveness analysis of serplulimab versus regorafenib for treating previously treated unresectable or metastatic microsatellite instability-high or deficient mismatch repair colorectal cancer in China. Front Oncol. 2023;13:1113346. doi:10.3389/fonc.2023.1113346
- Zhang S, Ma AX, Li H, Rascati K. Pcn117 Cost-Effectiveness Analysis of Regorafenib Used in Chinese Patients with Metastatic Colorectal Cancer. Value Health. 2020;23:S43. doi:10.1016/j.jval.2020.04.1608
- Kashiwa M, Matsushita R. Comparative Cost-utility Analysis of Regorafenib and Trifluridine/Tipiracil in The Treatment of Metastatic Colorectal Cancer in Japan. Clin. Ther. 2020;42(7):1376–1387. doi:10.1016/j.clinthera.2020.05.014
- Kimura M, Usami E, Iwai M, Go M, Teramachi H, Yoshimura T. Comparison of cost-effectiveness of regorafenib and trifluridine/tipiracil combination tablet for treating advanced and recurrent colorectal cancer. Mol Clin Oncol. 2016;5(5):635–640. doi:10.3892/mco.2016.1020
- Giuliani J, Fiorica F, Ponturo G, Azzurro M, Ruzzenente A, Bonetti A. Dose-escalation strategy in refractory metastatic colorectal cancer: a change in terms of cost-effectiveness. J Oncol Pharm Pract. 2021;27(4):974–977. doi:10.1177/1078155221992546
- Bullement A, Underhill S, Fougeray R, Hatswell AJ. Cost-effectiveness of Trifluridine/tipiracil for Previously Treated Metastatic Colorectal Cancer in England and Wales. Clin Colorectal Cancer. 2018;17(1):e143–e151. doi:10.1016/j.clcc.2017.09.001
- Gourzoulidis G, Maniadakis N, Petrakis D, Souglakos J, Pentheroudakis G, Kourlaba G. Economic evaluation of trifluridine and tipiracil hydrochloride in the treatment of metastatic colorectal cancer in Greece. J Comp Eff Res. 2019;8(3):133–142. doi:10.2217/cer-2018-0076
- Mlcoch T, Tereza H, Zadak J, Vesela S, Marian M, Dolezal T. Regorafenib in Metastatic Colorectal Cancer: cost-Effectiveness Analysis Based on Propensity Score Weighted Cohort of Czech Registry. Value Health. 2018;21.
- Van Cutsem E, Martinelli E, Cascinu S, et al. Regorafenib for Patients with Metastatic Colorectal Cancer Who Progressed After Standard Therapy: results of the Large, Single-Arm, Open-Label Phase IIIb CONSIGN Study. Oncologist. 2019;24(2):185–192. doi:10.1634/theoncologist.2018-0072
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, Phase 3 trial. Lancet. 2013;381(9863):303–312. doi:10.1016/S0140-6736(12)61900-X
- Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of Tas-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. doi:10.1056/NEJMoa1414325
- Per P, Mette Y, Sören M, et al. Tas-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21:412–420. doi:10.1016/S1470-2045(19)30827-7