Abstract
Purpose
To evaluate Ki67 expression and prognostic value during neoadjuvant chemotherapy (NACT) in advanced epithelial ovarian cancer (EOC).
Patients and Methods
95 patients with advanced EOC receiving NACT followed by interval debulking surgery (IDS) were available for tissue samples from matched pre- and post-therapy specimens. The expression of Ki-67 was evaluated by immunohistochemistry and classified by percentage of stained cells. The optimal cutoff values of the Ki67 were assessed by receiver operating characteristic analysis. Kaplan-Meier analysis, the Log rank test, and Cox regression analysis were carried out to analyze survival.
Results
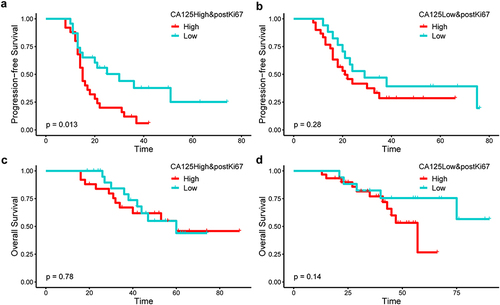
Post-NACT Ki67 was an independent prognostic factor for recurrence by univariate (HR: 1.8, 95% CI: 1.1–3.0, P-value: 0.023) and multivariate (HR: 1.88, 95% CI: 1.08–3.26, P-value: 0.025) analysis. Residual disease >1cm (HR: 2.69, 95% CI: 1.31–5.54, P-value: 0.0070) and pre-treatment CA125 ≥ 1432 U/mL (HR: 2.00, 95% CI: 1.13–3.55, P-value: 0.017) were also independent risk factors for progression-free survival (PFS) in multivariate analysis. Post-NACT Ki67 ≥ 20% was an independent risk factor for PFS, however, baseline Ki67 and Ki67 change did not suggest prognostic significance. In patients with high CA125, the median PFS for patients with high postKi67 (median PFS: 15.0 months, 95% CI: 13.4–16.6 months) was significantly (P-value: 0.013) poorer compared to patients with low postKi67 (median PFS: 30.0 months, 95% CI: 13.5–46.5 months).
Conclusion
Post-NACT Ki67 ≥ 20% was an independent factor associated with poorer PFS in patients with advanced-stage EOC undergoing NACT followed by IDS. The combination of post-NACT Ki67 and pretreatment CA125 could better identify patients with poorer PFS in NACT-administered patients.
Introduction
Epithelial ovarian cancer (EOC) is the fifth-leading cause of cancer-related deaths in women worldwide and the most lethal gynecologic malignancy.Citation1 Owing to the lack of specific symptoms and screening methods,Citation2 approximately 75% of patients are diagnosed at an advanced stage. The 5-year survival rate at the advanced stage is below 30%, contributing to the high death-to-incidence rate.Citation3 BRCA1/2 germline mutations are the strongest known genetic risk factors for EOCs and can be used for counseling regarding expected survival, as carriers usually respond better to platinum-based chemotherapies. However, only 6%-15% if EOC patients are diagnosed with BRCA mutations, Therefore, the search for an easily accessible, low-cost, reproducible biomarker represents an urgent need in clinical practice.Citation4
Primary debulking surgery (PDS) followed by chemotherapy has become the standard of care in advanced EOC since the 1980s.Citation5 For stage IIIC or IV EOC, PDS is preferred if there is a high likelihood of achieving cytoreduction to < 1 cm with acceptable morbidity. However, women with a high perioperative risk profile or a low likelihood of achieving cytoreduction to < 1 cm of residual disease should receive neoadjuvant chemotherapy (NACT).Citation6–8
Epithelial ovarian cancer lacks effective screening and monitoring biomarkers.Citation2,Citation3 CA125 and HE4 have been reliable biomarkers in the diagnosis and prediction of epithelial ovarian cancer.Citation9–11 The level of serum CA125 was found to be related to clinical stage and survival.Citation12,Citation13 However, the value of this marker in clinical practice has remained controversial.
Ki67, encoded by the MKI67 gene, acts as a surfactant, dispersing chromosomes and enabling independent chromosome motility.Citation14 It is widely used as a proliferation marker in basic research and cancer prognosis.Citation15,Citation16 Posttreatment Ki67 levels provide prognostic information for patients with hormone-receptor-positive breast cancer and residual disease after neoadjuvant chemotherapy.Citation17 Nevertheless, the role of Ki67 in ovarian cancer prognosis is limited and controversial. Few studies have investigated the value of Ki67 measured after neoadjuvant chemotherapy.Citation18–20
This study aimed to examine the changes of Ki67 in ovarian cancer specimens during treatment with NACT to evaluate its possible clinical impact. We assumed Ki67 would be a useful biomarker to personalize the care for NACT-treated patients with advanced ovarian cancer.
Material and Methods
Patients and Study Design
This retrospective cohort study was conducted at the First Affiliated Hospital of Chongqing Medical University. Data were collected retrospectively through the electronic case system and followed up regularly through the medical record system or telephone.
The present study complied with the Declaration of Helsinki and was approved by the Chongqing Medical University Ethics Committee (Ethics approval number: 2020–674).
A list of patients with primary, previously untreated, histologically-confirmed epithelial ovarian, fallopian tube, or primary peritoneal cancers who underwent tumor biopsy at the first affiliated hospital of Chongqing Medical University between December 2014 and March 2022 was generated from our institutional registry (N=351). The following inclusion criteria were considered: 1) Advanced-stage (FIGO Stage III or IV) ovarian, fallopian tube, or primary peritoneal cancer which was diagnosed by exploratory surgery or paracentesis with pathological evidence; 2) received neoadjuvant chemotherapy and completed interval debulking surgery (IDS); 3) patients who completed standard treatment and were followed up regularly. Exclusion criteria were applied as follows: 1) patients without eligible surgical specimens were available for the evaluation of the Ki-67 immunostaining (N=204); 2) patients without standard surgical treatment or complete chemotherapy (N=33); 3) patients with other malignant tumors (N=2), patients who who were lost to follow-up or with missing medical records (N=17). Thus, a total of 95 patients were included as research subjects. All patients provided their written informed consent.
Outcomes include recurrence, progression-free survival (PFS), and overall survival (OS). Progression-free survival was defined as the time from diagnosis to the first tumor relapse or progression. Overall survival was defined as the interval from diagnosis to death from epithelial ovarian cancer or to the last observation for surviving patients. The diagnosis of recurrence was based on radiological imaging with/without elevation of tumor markers.
Immunohistochemistry
The postoperative specimens of all patients were immediately fixed with formalin tissue fixative and sent to the Pathology Laboratory Center of Chongqing Medical University within 20 minutes for embedding, sectioning, H&E staining, and immunohistochemical analysis by uniform standards. Immunohistochemical results of Ki67 were independently evaluated by two experienced pathologists and recorded as the percentage of positively stained tumor cells. Pathologists’ assessment for the proportion of positive tumor cells were considered to be consistent if the proportion differed ≤10%; If the initial assessment was considered to be inconsistent (the proportion differed >10%), then the results were re-evaluated (unblinded) and a consensus reached. Interpretation of Immunohistochemistry: five high-power fields were randomly observed in the most active tumor area (“hottest spot” of tumor), tumor cells with strong nuclear immunostaining were defined as positive cells, 100 tumor cells were evaluated in each field, and the average positive percentage (0–100%) was calculated in five fields. Ki67 was expressed as the percentage of positive tumor cells (0–100%).
Statistical Methods
Categorical variables are presented as frequencies and percentages. Continuous variables are presented as mean ± standard deviation (SD) if normally distributed and median and range if not normally distributed. Restricted cubic spline (RCS) curves were used to confirm whether the variable meets the application conditions of Cox proportional hazards: PH assumption and linearity. Univariate and multivariate analyses were realized by the Cox proportional-hazards model. The optimal threshold of the positive percentage of Ki67 was determined by the receiver operating characteristic curve (ROC). Progression-free survival was performed with the Kaplan-Meier method and a Log rank test was used to compare Kaplan-Meier curves. A P-value of <0.05 was considered to reject the null hypothesis. The R 4.3.2 project (https://www.r-project.org/) and SPSS 26.0 (IBM Statistics, Chicago, IL, USA) software were used for statistical analysis of the data.
Results
Clinical and Pathological Characteristics
Ninety-five cases were included in the study (). The median patient age at diagnosis was 56 years (range: 36–79). The majority of tumors were high-grade serous histology (82, 86.3%), with rare cases of others. The majority of cases originated from either the ovary or fallopian tube (79, 83.2%), with 16.8% (16 cases) originating from the peritoneum. All cases were FIGO stage III (75, 78.9%) or IV (20, 21.1%). Residual disease was observed in 43 (45.3%) patients, measured ≤1 cm in 29 (30.5%) participants, and >1 cm in the other 14 participants (14.7%). Median pre-treatment serum CA-125 level (The CA125 in the following text refers to the pre-treatment serum CA125 level) at diagnosis was 1432 U/mL (range: 44.7–23,778.0). And median pre-treatment serum HE4 level at diagnosis was 475 pmol/L (range: 27–67,340). Recurrence occurred in 68 patients (71.6%), and death occurred in 36 patients (37.9%). For Ki67, the median primary Ki67 at biopsy (PrimaryKi67) was 60% (range: 0–90%). The median post-NACT Ki67 at IDS (PostKi67) was 20% (range: 0–80%).
Table 1 Baseline Clinical Characteristics
Univariate and Multivariate Analysis of Progression-Free and Overall Survival
We tested the proportionality assumption of the Cox PH model (Figure S1), and that all variables met the proportionality assumption. We used RCS to flexibly model and visualize the relation of Ki67 with recurrence, and that Ki67 met the linear assumption (P for non-linearity >0.05, Figure S2).
Univariate analysis showed that only higher postKi67 was associated with poorer PFS (P=0.023) (). None of the other variables were significant for PFS. The result demonstrated that the postKi67 is a risk factor for PFS (HR 1.8, 95% CI 1.1–3.0, P=0.023). The multivariate analysis revealed () that residual disease >1cm (HR 2.69, 95% CI 1.31–5.54, P=0.007), pre-treatment CA125 ≥1432 U/mL (HR 2.00, 95% CI 1.13–3.55, P=0.017), and postKi67 (HR 1.88, 95% CI 1.08–3.26, P=0.025) were all the independent risk factors for PFS. These Results were not translated in the death analysis (Table S1). None of the variables were significant for OS.
Table 2 Univariate and Multivariate Analysis of Factors Predicting Ovarian Cancer Recurrence
The Optimal Positive Threshold of Ki67 Associated with Recurrence
Univariate and multivariate analysis confirmed that postKi67 was an independent prognostic factor for recurrence in EOC. Furthermore, the ROC curve revealed that the optimal positive threshold of postKi67 for predicting recurrence was 17.5% (AUC = 0.7226; sensitivity = 83.3%; 1-specificity = 64.2%) (). According to the distribution characteristics of Ki67 and the convenience for clinical application, we selected 20% as the cut-off value. Patients with postKi67 index ≥20% and <20% were defined as High-postKi67 group and Low-postKi67 group, respectively. Comparison between the two groups showed that high-postKi67 expression was significantly associated with HGSOC (P=0.033) and Ki67 decrease < 30% (median) (P=<0.001). There is no difference between two groups in PrimaryKi67 ().
Table 3 Comparison of Clinic Pathological Parameters Between Low-postKi67 Group and High-postKi67 Group
Figure 1 The optimal cut-off value of postKi67 for predicting recurrence. (a)The ROC curve of postKi67 for predicting recurrence. The area under the curve at “black dot” is the largest, which suggests the optimal threshold of positive percentage of Ki67 is 17.5% (AUC = 0.7226; sensitivity = 83.3%; 1-specificity = 0.642%).
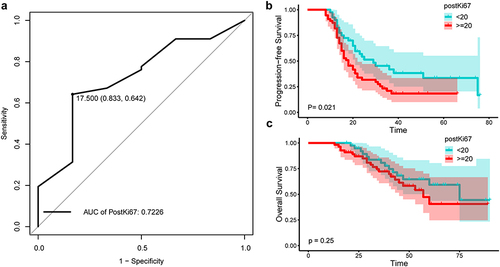
The median PFS for patients with high postKi67 (median PFS 18.0 months; 95% CI 14.4–21.6) was significantly (P=0.021) poor compared to patients with low postKi67 (median PFS 29.0 months; 95% CI 15.3–42.7) (). In OS analysis, the difference is not statistically significant between high postKi67 group (median OS 57.0 months; 95% CI 43.2–70.7) and low post Ki67 group (median OS 75.0 months; 95% CI 46.0–103.9) ().
Combination of Pre-Treatment CA125 and Post-NACT Ki67 Stratified Prognosis
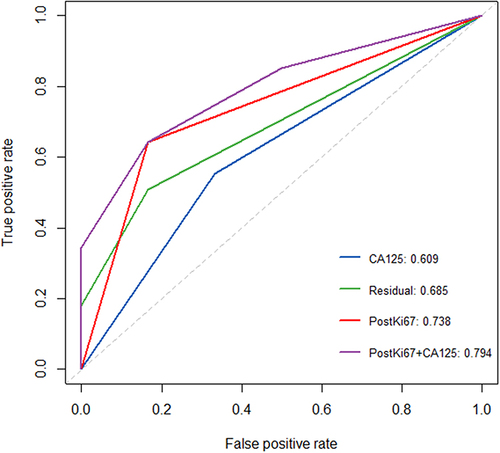
No residual tumor (R0) after PDS is the most important prognostic factor for survival.Citation21 CA125 is the most commonly used serum biomarker in epithelial ovarian cancer.Citation10 Our results also showed that residual disease (R2), pre-treatment serum CA125 (≥median), and postKi67 (≥20%) were all risk factors for PFS of EOC patients. To further illustrate the clinical value of postKi67 for predicting recurrence in EOC, the ROC curve and area under the curve (AUC) were used to compare the predictive performance of various predictive markers and their combinations.
From , the following key results can be drawn: 1. The AUC of Ki67 (AUC=0.738) was greater than other single predictors, including pathological parameters residual (AUC=0.685) and CA125 (AUC=0.609). 2. Ki67 combined with classical molecular indicators (pre-treatment serum CA125) can improve predictive performance (AUC=0.794).
Figure 2 The ROC curve of various predictive markers and their combinations for predicting recurrence.
As shown in , higher postKi67 (postKi67 index ≥20%) was significantly associated with poorer PFS in patients with advanced-stage EOC undergoing NACT followed by IDS. CA125, as an extensively studied tumor marker, can be easily detected from serum before surgery. We further calculated the association of the combination of pre-treatment CA125 level and post-NACT Ki67 with OS and PFS. As shown in , we stratified the population according to initial CA125. In patients with high pre-treatment CA125 (≥median), the median PFS for patients with high postKi67 (median PFS 15.0 months; 95% CI 13.4–16.6) was significantly (P=0.013) poor compared to patients with low postKi67 (median PFS 30.0 months; 95% CI 13.5–46.5) (). In patients with low CA125, the difference is not statistically significant between the high postKi67 group (median PFS 21.0 months; 95% CI 13.8–28.2) and low postKi67 group (median PFS 29.0 months; 95% CI 10.8–47.1) ().There was no difference in OS analysis ( and ). These findings implied that the combination of pre-treatment CA125 and post-NACT Ki67 can stratify PFS well.
Discussion
In EOC patients with advanced stage, high risks of complications, or who have been evaluated for potentially unsatisfactory PDS, neoadjuvant chemotherapy followed by IDS is not only effective but also safer.Citation3,Citation6,Citation8 Though EOC initially responds to treatment, the recurrence rate is pretty high. Emerging techniques such as proteomics have advanced the dissection of underlying molecular signaling events and uncovered new predictive and therapeutic markers to improve the prognosis of ovarian cancer. However, only a select few biomarkers have been FDA approved, especially in EOC, due to the paucity of validation tools from their discovery in the lab to implementation in the clinical setting.Citation22,Citation23 Therefore, it is important to fully investigate the clinical risk factors for recurrence to better predict the prognosis of EOC undergoing NACT followed by IDS, and then provide individualized guidance for patients.
Ki67 has been widely used as a tumor marker, while its use in ovarian cancer remains controversial and ambiguous. Early reports indicated the prognostic significance of baseline Ki-67 of primary tumors and observed poorer survival rates in highly proliferative ovarian cancers. As reported by Anttila et al,Citation24 high Ki67 (≥ 20%) was associated with poor OS. And Khouja et alCitation25 reported that high Ki67 (≥ 10%) was associated with poor PFS in stage III ovarian cancer. Yet, an 11-year cohort study of Chinese patients reported that low Ki67 expression (< 40%) in HGSOC is significantly associated with platinum resistance and decreased PFS.Citation26 EOC appears to be a heterogeneous disease with different clinical outcomes, which can be divided into two main categories: relatively indolent type I and biologically aggressive type II.Citation27 Studies on the value of Ki67 in neoadjuvant chemotherapy for EOC are limited. A comparison of 40 individual paired results from pretreatment and posttreatment samples revealed that rather than the baseline level, an increased Ki67 after chemotherapy was associated with poorer PFS.Citation18 A study with 13 cases confirmed that the Ki67-decrease and the lower post-NACT Ki67 were independent factors associated with favorable PFS.Citation20 In addition, Heayn et alCitation19 reported that post-NACT ki67>20% was a risk factor for OS, with 123 post-neoadjuvant chemotherapy samples.
Our study had a larger sample size of 95 fully paired pretreatment and posttreatment samples. Our results showed that post-NACT Ki67 ≥ 20% was an independent risk factor for PFS. However, baseline Ki67 and Ki67-change did not suggest prognostic significance. Ki67 had no prognostic difference in OS in our results. Our results highlighted the importance of Ki67 after neoadjuvant chemotherapy. In addition, the combination of post-NACT Ki67 and pretreatment CA125 can better stratify and identify patients with poor PFS, thus providing individualized treatment strategies.
This study has certain limitations. The retrospective design in a single institution was one of the shortcomings of the present study. Then, the relatively short follow-up period made it difficult to assess whether there was a difference in overall survival. Moreover, there is no unified standard procedure for the interpretation of parameter Ki67. The “hot spot” method of assessment was used in this study, which was also applied in most similar studies.Citation28 Also, the immunohistochemical scoring of KI67 is subjective to a certain extent. Despite the joint scoring by two experienced pathologists in this study, there will still inevitably be a certain degree of inter-observer bias. Finally, although the sample size was among the largest compared to other similar studies, it still needs to be verified by a multi-center prospective experiment.
Conclusion
In summary, post-NACT Ki67 ≥ 20% was an independent factor associated with poorer PFS in patients with advanced stage EOC undergoing NACT followed by IDS. Baseline Ki67 of primary tumor did not suggest prognostic significance. And the combination of post-NACT Ki67 and pretreatment CA125 can better identify patients with poorer PFS.
Abbreviations
EOC, epithelial ovarian cancer; PDS, primary debulking surgery; IDS, interval debulking surgery; NACT, neoadjuvant chemotherapy; PFS, progression-free survival; OS, overall survival; ROC, receiver operating characteristic curve; AUC, area under the curve.
Disclosure
The authors report no conflicts of interest in this work.
References
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
- Menon U, Gentry-Maharaj A, Burnell M, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK collaborative trial of ovarian cancer screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397(10290):2182–2193. doi:10.1016/S0140-6736(21)00731-5
- Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–1253. doi:10.1016/S0140-6736(18)32552-2
- Shah S, Cheung A, Kutka M, Sheriff M, Boussios S. Epithelial ovarian cancer: providing evidence of predisposition genes. Int J Environ Res Public Health. 2022;19(13):8113. doi:10.3390/ijerph19138113
- Griffiths CT, Fuller AF. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg Clin North Am. 1978;58(1):131–142. doi:10.1016/S0039-6109(16)41440-4
- Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of gynecologic oncology and American society of clinical oncology clinical practice guideline. Gynecol Oncol. 2016;143(1):3–15. doi:10.1016/j.ygyno.2016.05.022
- Meyer LA, Cronin AM, Sun CC, et al. Use and effectiveness of neoadjuvant chemotherapy for treatment of ovarian cancer. J Clin Oncol. 2016;34(32):3854–3863. doi:10.1200/JCO.2016.68.1239
- Vergote I, Coens C, Nankivell M, et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018;19(12):1680–1687. doi:10.1016/S1470-2045(18)30566-7
- Felder M, Kapur A, Gonzalez-Bosquet J, et al. MUC16 (CA125): Tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014;13:129. doi:10.1186/1476-4598-13-129
- Zhang M, Cheng S, Jin Y, Zhao Y, Wang Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim Biophys Acta Rev Cancer. 2021;1875(2):188503. doi:10.1016/j.bbcan.2021.188503
- Ghose A, McCann L, Makker S, et al. Diagnostic biomarkers in ovarian cancer: advances beyond CA125 and HE4. Ther Adv Med Oncol. 2024;16:17588359241233225. doi:10.1177/17588359241233225
- Cooper BC, Sood AK, Davis CS, et al. Preoperative CA 125 levels: an independent prognostic factor for epithelial ovarian cancer. Obstet Gynecol. 2002;100(1):59–64. doi:10.1016/s0029-7844(02)02057-4
- Salminen L, Nadeem N, Jain S, et al. A longitudinal analysis of CA125 glycoforms in the monitoring and follow up of high grade serous ovarian cancer. Gynecol Oncol. 2020;156(3):689–694. doi:10.1016/j.ygyno.2019.12.025
- Cuylen S, Blaukopf C, Politi AZ, et al. Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature. 2016;535(7611):308–312. doi:10.1038/nature18610
- Whitfield ML, George LK, Grant GD, Perou CM. Common markers of proliferation. Nat Rev Cancer. 2006;6(2):99–106. doi:10.1038/nrc1802
- von Minckwitz G, Schmitt WD, Loibl S, et al. Ki67 measured after neoadjuvant chemotherapy for primary breast cancer. Clin Cancer Res. 2013;19(16):4521–4531. doi:10.1158/1078-0432.CCR-12-3628
- Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174–183. doi:10.1016/S1470-2045(09)70262-1
- Polcher M, Friedrichs N, Rudlowski C, et al. Changes in Ki-67 labeling indices during neoadjuvant chemotherapy for advanced ovarian cancer are associated with survival. Int J Gynecol Canc. 2010;20(4):555–560. doi:10.1111/IGC.0b013e3181c104c0
- Heayn M, Skvarca LB, Zhu L, et al. Impact of Ki-67 labeling index on prognostic significance of the chemotherapy response score in women with tubo-ovarian cancer treated with neoadjuvant chemotherapy. Int J Gynecol Pathol. 2021;40(3):278–285. doi:10.1097/PGP.0000000000000706
- Kaya R, Takanashi H, Nakajima A, et al. Prognostic significance of Ki67 during neoadjuvant chemotherapy in primary unresectable ovarian cancer. J Obstet Gynaecol Res. 2021;47(11):3979–3989. doi:10.1111/jog.14981
- du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized Phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009;115(6):1234–1244. doi:10.1002/cncr.24149
- Ghose A, Gullapalli SVN, Chohan N, et al. Applications of Proteomics in Ovarian Cancer: dawn of a New Era. Proteomes. 2022;10(2):16. doi:10.3390/proteomes10020016
- Hollis RL, Meynert AM, Michie CO, et al. Multiomic characterization of high-grade serous ovarian carcinoma enables high-resolution patient stratification. Clin Cancer Res. 2022;28(16):3546–3556. doi:10.1158/1078-0432.CCR-22-0368
- Anttila M, Kosma VM, Ji H, et al. Clinical significance of alpha-catenin, collagen IV, and Ki-67 expression in epithelial ovarian cancer. J Clin Oncol. 1998;16(8):2591–2600. doi:10.1200/JCO.1998.16.8.2591
- Khouja MH, Baekelandt M, Nesland JM, Holm R. The clinical importance of Ki-67, p16, p14, and p57 expression in patients with advanced ovarian carcinoma. Int J Gynecol Pathol. 2007;26(4):418–425. doi:10.1097/pgp.0b013e31804216a0
- Chen M, Yao S, Cao Q, Xia M, Liu J, He M. The prognostic value of Ki67 in ovarian high-grade serous carcinoma: an 11-year cohort study of Chinese patients. Oncotarget. 2017;8(64):107877–107885. doi:10.18632/oncotarget.14112
- Pavlidis N, Rassy E, Vermorken JB, et al. The outcome of patients with serous papillary peritoneal cancer, fallopian tube cancer, and epithelial ovarian cancer by treatment eras: 27 years data from the SEER registry. Cancer Epidemiol. 2021;75:102045. doi:10.1016/j.canep.2021.102045
- Honma N, Horii R, Iwase T, et al. Ki-67 evaluation at the hottest spot predicts clinical outcome of patients with hormone receptor-positive/HER2-negative breast cancer treated with adjuvant tamoxifen monotherapy. Breast Cancer. 2015;22(1):71–78. doi:10.1007/s12282-013-0455-5