Abstract
Background
Thromboembolic events are important causes of morbidity and mortality in cancer patients. Clinical presentation in a community-based setting has not been fully clarified. The purpose of this study was to evaluate the incidence, risk factors, role of thrombophilia, and subsequent survival following thrombosis in cancer patients.
Methods
A retrospective review was undertaken of clinical data for all consecutive patients with histologically confirmed cancer seen by the author at a regional cancer center, with emphasis on cancer-related thrombosis.
Results
Between 2005 and 2012, of 1874 cancer patients, 307 (16.4%) developed thrombosis during their lifetime. Of these patients, 37 (2%) had a history of thrombosis, while the remaining 270 (14.4%) patients developed thrombosis 3 months before or any time after diagnosis of cancer, which was considered to be cancer-related. These patients included 230 (12.3%) with venous thrombosis, 28 (1.5%) cases with arterial occlusion, and 12 (0.6%) with combined venous and arterial thrombosis. Patients of Caucasian ancestry were more prone to develop thrombosis, with a higher frequency of multiple genetic thrombophilia compared with other ethnic groups. In regression analysis, only advanced stages of cancer and the presence of atherosclerosis were predictive of thrombosis. There were no significant differences between venous and arterial thrombosis. The worst survival was noted in patients who developed thrombosis 3 months prior to or shortly after their diagnosis of cancer. There has been a recent improved survival outcome following therapy.
Conclusion
In addition to venous thrombosis, arterial occlusion with stroke and anginal symptoms is relatively common among cancer patients, especially those of Caucasian ancestry, and is possibly related to genetic predisposition.
Introduction
The association between cancer and vascular thrombosis has been known since 1865 when Trousseau reported an association between migratory thrombophlebitis in patients with malignancy.Citation1 During the last two decades, there has been a great interest in investigating this relationship. The increased risk of thrombosis in cancer patients tends to correlate well with advanced stage of disease, increased tumor volume, and prolonged hospitalization.Citation2 The syndrome is associated with a poorer prognosis than in matched cancer patients without thrombosis.Citation3,Citation4
The overall prevalence and clinical outcome of such disorders in cancer patients have been largely based on a cohort of studies in patient populations at risk, hospital-based statistics, and retrospective studies in selected groups of patients. Such coagulation disorders have rarely been assessed in a large cohort of patients seen and followed at a regional cancer center. Further, the role of ethnic background and genetic factors in the prevalence of these disorders has been evaluated infrequently. In the present study, the frequency of various types of thrombosis in cancer patients was investigated, factors that predispose them to clot formation were determined, the potential relationship with inherited thrombophilia was examined, and these factors were correlated with subsequent survival.
Materials and methods
Data collection
The medical records of cancer patients previously seen and closely followed by the author were retrospectively reviewed, with special emphasis on the incidence and consequences of thromboembolic events, risk factors for thrombus formation, and subsequent survival. The study included all consecutive adult patients with histologically confirmed malignancy, with the exception of nonmelanoma skin cancer and carcinoma in situ of the uterine cervix. All data collected were de-identified of any patient identification to comply with the Health Information Protection Act. The study was approved by the institutional review board at St Rita’s Medical Center.
Data grouping
Patient age was estimated based on the initial clinic visit. Elderly patients were defined as being aged ≥65 years. Ethnic background was categorized as Caucasian, Native American Indian (from both parents), African American, and others. Smoking status was categorized as nonsmoking, mild smoking (≤29 pack-years), and heavy smoking (>30 pack-years). Body mass index (BMI, kg/m2) was categorized as underweight (<18.4), normal (18.5–24.9), and overweight (>25). Primary tumor site was classified as head and neck (including brain and thyroid), breast, lung (and pleura), gastrointestinal, hepatobiliary (including pancreas), gynecologic and genitourinary, lymphoma, hematologic, dermatologic, and others. Stage of disease was defined according to the American Joint Committee of Cancer Staging Classification (Seventh Edition, 2010). Patients with acute leukemia were considered to be stage IV. Other cancer types for which there is no defined staging system were categorized as stage I if localized, stage II if there was regional lymph node involvement, stage III if locally or regionally advanced, and stage IV if there was evidence of distant metastases.
For patients with multiple malignancies, the date of the first primary diagnosis was considered to be the date of initial diagnosis, and the most advanced stage of any primary site was recorded as the stage of disease. Patients were questioned about any previous history of thromboembolic events, and closely followed for such a possibility. Only patients with clinical manifestation and/or radiologic evidence of deep venous thrombosis, pulmonary embolism, a combination of deep venous thrombosis and pulmonary embolism, or other thrombotic manifestations were considered to have had thromboembolic events. For patients with recurrent thrombosis, the date of initial vascular thrombosis was considered to be the date of thrombosis.
Patients were considered to have cancer-related thromboembolic events if they developed vascular thrombosis 3 months before or at any time after initial diagnosis of cancer, and were categorized into venous or arterial thrombosis. Time to vascular thrombosis was measured from initial histologic diagnosis of cancer to the date of initial vascular thrombosis. The final status of the patient, whether alive or dead, was recorded. Survival following thrombosis was calculated from the date of initial thrombus formation, and overall survival from the date of initial histologic diagnosis of cancer until the day of death, or if the patient was confirmed to be alive by the end of December 2012.
Only patients who agreed to genetic testing for thrombophilic susceptibility had blood drawn for factor V Leiden mutation, prothrombin-20210G>A, methylenetetrahydrofolate reductase (MTHFR)-A1298c, and MTHFR-c677t gene mutation studies. Patients with one or more abnormal genes were considered to have defective gene(s), and the cumulative number of abnormal genes in all studies was calculated (cumulative gene mutations), with consideration of homozygous gene mutation as two defective genes.
Management of thrombosis
All patients with thrombosis were treated according to standard guidelines. For deep venous thrombosis and pulmonary embolism, they received heparin products (unfractionated heparin or low molecular weight heparin) for 3–5 days followed by warfarin for 3 months in the case of deep venous thrombosis, 6 months for pulmonary embolism, and indefinitely if thrombosis was recurrent or if the patient had inherited thrombophilia. Long-term anticoagulant therapy with low molecular weight heparin was not generally practiced because of financial constraints for most patients. Vena caval filters were inserted in a few patients (n = 64, 25%) when anticoagulant therapy was not tolerated or contraindicated. Patients with other thrombotic episodes were treated less aggressively with aspirin, clopidogrel, or supportive care. For catheter-related thrombophlebitis, the catheter was removed and anticoagulant therapy was administered if there was evidence of concomitant deep venous thrombosis.
Statistical analysis
Differences between categorical groups were tested using the Pearson Chi-square test. Analysis of variance was used to analyze the differences between means of continuous variables, such as age in years, and expressed as the mean ± standard deviation. The Chi-square test was used to examine the relationship between qualitative variables. Odds ratios and their 95% confidence intervals were used for risk estimation. Multivariate analysis was done using the logistic regression method for significant factors affecting outcome variables on univariate analysis. The full cohort of patients was used in the multivariate method. Factors influencing survival were estimated by Kaplan-Meier survival analysis, and the log-rank test was used to compare the distribution of survival between groups. In all analyses, a two-sided P-value less than 0.05 was considered to be statistically significant. Missing data were deleted from the analysis. Statistical analysis was conducted using Statistica version 10 software (Stat Soft Inc, Tulsa, OK, USA). Multivariate regression analysis was analyzed using the Statistical Package for the Social Sciences advanced statistics version 20 software (IBM, Armonk, NY, USA).
Results
Incidence of thrombosis
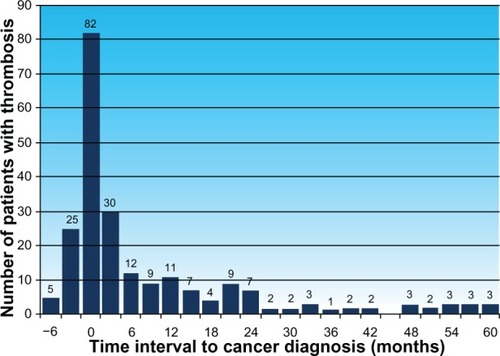
Between January 2005 and December 2012, of 1874 consecutive cancer patients with histologically confirmed malignancies, 307 (16.4%) had vascular thrombosis at some time during their lifetime. Thirty-seven (2%) patients developed thrombosis 6.6 months to 47.8 years prior to development of cancer, with no recurrent thrombosis afterwards, regardless of cancer therapy or disease status, and were considered to be unrelated to development of cancer. The remaining 270 (14.4%) patients developed thrombosis 3 months before or at any time after histologic diagnosis of cancer. They were considered to have cancer-related thrombosis, and included four patients who developed recurrent thrombosis as the first clinical manifestation of development of a second cancer 3.4–5 months prior to histologic diagnosis of cancer. Patients with cancer-related thrombosis included 230 (12.3%) with venous thrombosis, 28 (1.5%) with arterial occlusion, and 12 (0.6%) with combined venous and arterial thrombosis. Demographic data on patients with cancer-related thrombosis in comparison with other cases without thrombosis are shown in . Clinical presentation of thrombosis in cancer patients is outlined in , and the outcome of thrombophilic studies in cancer patients with and without thrombosis is mentioned in . The timing of vascular thrombosis relative to the initial diagnosis of cancer for patients with cancer-related thrombosis is shown in .
Table 1 Demographic data on cancer patients with and without thrombosis
Table 2 Clinical presentation of thrombosis in cancer patients
Table 3 Thrombophilic studies in cancer patients with and without thrombosis
Cancer-related thrombosis
Among patients with cancer-related thrombosis, 194 (10.4%) cases developed venous thromboembolic events in the form of deep venous thrombosis, pulmonary embolism, or a combination of deep venous thrombosis and pulmonary embolism. Thirty-six (1.9%) patients developed thrombosis at other venous sites, such as superficial phlebitis (23, 1.2%) or intra-abdominal venous occlusion (13, 0.7%). Twenty-eight (1.5%) patients had arterial vascular occlusion in the form of cerebrovascular thrombosis leading to stroke (n = 22), myocardial ischemia with anginal symptoms (n = 2), celiac artery occlusion with ischemic colitis (n = 1), and peripheral arterial occlusion with impending limb gangrene (n = 3). Twelve (0.6%) patients developed both venous and arterial thrombosis, either concurrently (six cases), or sequentially (six patients, range 1–36 months), mainly in the form of deep venous thrombosis and/or pulmonary embolism, in addition to arterial occlusion of the radial, femoral, or popliteal arteries (five, 0.3%), prior to or following massive stroke (five, 0.2%), and mesenteric artery occlusion with or without portal vein thrombosis (two, 0.1%).
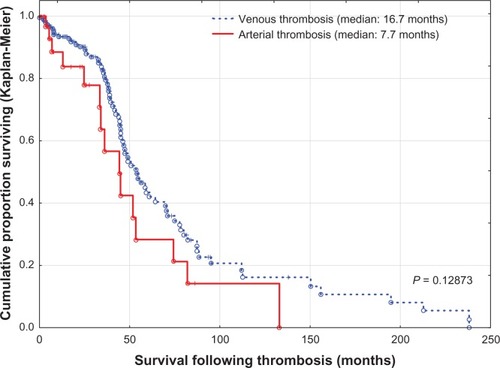
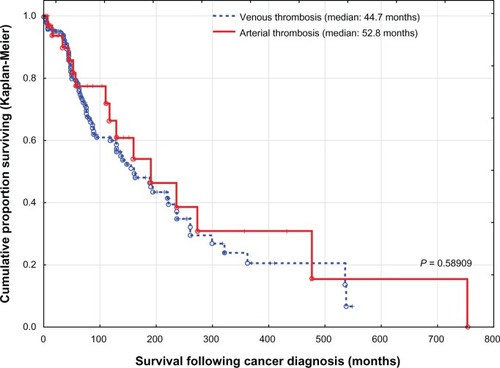
Patients with combined venous and arterial thrombosis had a mean survival (± standard deviation) following thrombosis of 22.7 ± 26.3 months versus 23.2 ± 31.1 months for arterial thrombosis and 29.8 ± 38.7 months for venous thrombosis. However, there were no differences in survival between the three groups (P = 0.31130). Due to their small number, they were included among patients with arterial thrombosis (). When cancer patients with venous thrombosis were compared with cancer patients with arterial thrombosis, there were no statistically significant differences. However, patients with venous thrombosis were relatively overweight, had mild or no atherosclerosis, and were mostly at an advanced stage of disease, while cases of arterial occlusion commonly had advanced atherosclerosis (). Survival following thrombosis and overall survival following diagnosis of cancer for both groups were also similar (, and ).
Clinical presentation of thrombosis
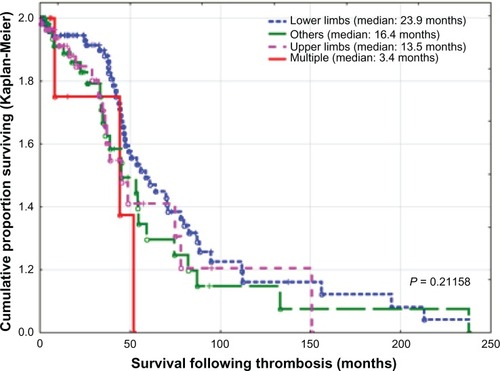
Of 104 patients with deep venous thrombosis, 59 (36.7%) had venous thrombosis involving the lower limbs, 34 (32.7%) involving the upper limbs, six (5.8%) involving both upper and lower limbs, and five (4.8%) had undetermined involvement. For patients with lower limb thrombosis, 35 (33.7%) had thrombosis at the distal (peroneal or popliteal) veins, 18 (17.3%) had thrombus extension into the proximal veins (femoral veins, 16; iliac veins, one; and inferior vena cava, one), and six (5.8%) had thrombosis at the proximal veins without distal involvement, ie, at femoral veins (n = 4) and femoral to iliac veins (n = 2). For patients with upper limb thrombosis, nine (8.7%) had thrombosis at distal veins (basilic vein), extending into brachial veins (n = 2), up to subclavian veins (n = 6), or to superior vena cava (n = 1); while 16 (15.4%) had proximal vein thrombosis without distal involvement mainly at axillary vein (n = 1), axillary to subclavian (n = 4), jugular veins (n = 7), subclavian vein to superior vena cava (n = 2), and at the superior vena cava (n = 2). There was no statistically significant difference in survival according to the site of thrombosis (P = 0.21158, ).
Ethnic background may play a role in the location of venous thromboembolism. In 259 cancer patients with thrombosis, 21 (9.3%) of 227 with Caucasian ancestry developed pulmonary embolism and 57 (25.1%) developed pulmonary embolism combined with deep venous thrombosis. In comparison, of 23 patients with African American ancestry, four (17.4%) developed pulmonary embolism and another four (17.4%) developed pulmonary embolism in combination with deep venous thrombosis. None of the nine patients from other ethnic groups had pulmonary embolism. This raises the possibility that cancer patients of African American ancestry have a greater tendency to develop pulmonary embolism than other ethnic groups. However, the numbers in this study were too small to be of statistical significance (P = 0.35347)
Of 94 patients who developed pulmonary embolism, either without concomitant deep venous thrombosis (27 cases, 28.7%) or in combination with other clotting episodes (67 patients, 71.3%), three (3.1%) died of pulmonary embolism ( and ).
Incidental venous thromboembolism was found in three patients with gonadal vein thrombosis and one case with a tiny asymptomatic pulmonary embolism. None of these patients was treated with anticoagulant therapy. Unexpected venous thromboembolism was found in 12 patients. Eleven patients presented with abdominal pain and a computed tomography scan showed mesenteric vein thrombosis (five patients) or portal vein thrombosis (five patients), and one patient had splenic vein thrombosis. Splenic vein thrombosis was also seen during surgery in one patient.
With regard to laboratory studies that may be related to thrombosis, D-dimer is suggested to be a sensitive marker. In the present study, almost all patients with acute thrombosis that were previously tested, had elevated D-dimer levels. However, it was difficult to identify a cutoff level because tests were performed at different laboratories. Elevated white blood count as well as low hemoglobin are common in cancer patients with and without thrombosis, but these parameters were not adequately investigated in the present study.
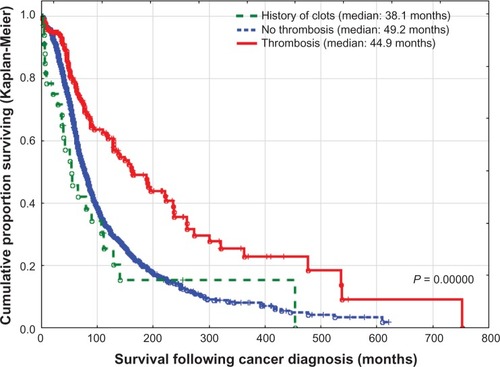
Mean survival (± standard deviation) following cancer-related thrombosis was 28.8 ± 37.5 months, with a median of 16.2 months. For the same group, the mean overall survival following initial diagnosis of cancer was 85.1 ± 109.7 months, with a median of 44.9 months. At a mean follow-up of 5.3 ± 9.29 (range 0–58.3) months in 270 patients with cancer-related thrombosis, 90 (33.3%) with thrombosis were still alive and 180 (66.7%) had died. Of the 47 (20.4%) patients who died within 3 months of venous thrombosis, 43 (91.5%) died from cancer progression, one (2.1%) secondary to therapy, one (2.1%) from pulmonary embolism, one (2.1%) from age-related medical conditions, and two (4.3%) of other causes. Incidentally, in the 37 cancer patients with a history of thrombosis, none died in the 3 months following thrombosis.
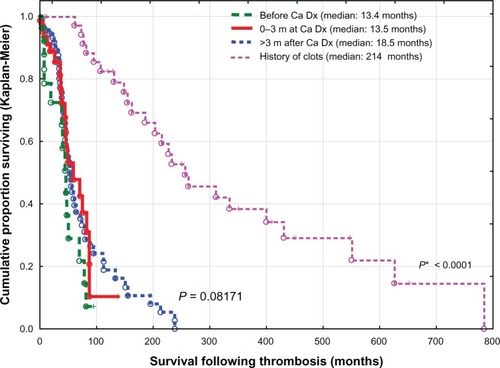
The worst survival rate was found in cancer patients who developed thrombosis within 3 months prior to or after diagnosis of cancer (median 13.5 months) or more than 3 months after diagnosis of cancer (median 18.5 months), with no statistically significant difference found between both groups (P = 0.16946), versus cancer cases with a history of thrombosis (a median of 214 months), (P < 0.0001), ().
Figure 5 Time elapsed from diagnosis of cancer to onset of thrombosis and impact on survival.
Notes: P, differences between cancer patients with thrombosis up to 3 months prior to diagnosis, 0–3 months at diagnosis, and over 3 months after histologic diagnosis; P*, significant differences in survival between the above three groups and cases with a history of thrombosis unrelated to cancer.
Abbreviations: Ca, cancer; Dx, diagnosis; m, months.
Thrombosis versus no thrombosis
When patients with cancer-related thrombosis were compared with those without thrombosis, there were no differences with regard to gender or smoking status. However, patients with thrombosis were predominantly elderly, of Caucasian ancestry, relatively obese, with lung, genitourinary, gynecologic, gastrointestinal primaries, and were in advanced stages of the disease (). On multivariate logistic regression analysis of cancer patients with and without thrombosis, after adjustment for the above-mentioned risk factors, only advanced stage of malignancy and presence of severe peripheral vascular disease were important risk factors for thrombosis, while patients with cachexia and marked weight loss were less likely to develop thrombosis (). However, the number of patients with lower BMI was relatively small (61 cases), compared with patients who were overweight (1024 patients). With regard to overall survival following initial diagnosis of cancer, the median survival was 44.9 months for patients with cancer-related thrombosis compared with 49.2 months for those without thrombosis (P < 0.0001, ). However, these groups were not comparable, especially in regard to age, ethnic background, prevalence of atherosclerosis, BMI, primary tumor site, or stage of malignancy ( and ).
Table 4 Risk of developing vascular thrombosis in cancer patients (univariate and multivariate logistic regression analysis of those with and without thrombosis)
Cancer patients with a history of thrombosis unrelated to malignancy had different survival times following initial diagnosis of cancer compared with cases with cancer-related thrombosis and cases without thrombosis, (P < 0.0001) with a significantly worse prognosis than those without thrombosis (P = 0.00999, ).
Role of thrombophilic testing
In total, 166 patients had one or more genetic tests aimed at detecting gene mutation for factor V Leiden, prothrombin-20210G>A, MTHFR-A1298c, and MTHFR-c677t genes or their combination. There were no differences between patients with venous or arterial thrombosis or between cancer patients with and without thrombosis (). The only differences were among patients of Caucasian ancestry versus other ethnic groups. The incidence of thrombosis was much higher in Caucasian cancer patients (259 of 1481, 17.5%), compared with other ethnic groups (35 of 276, 11.3%, P = 0.0069). In the present study, the prevalence of gene mutation for factor V Leiden, prothrombin-20210G>A, MTHFR-A1298c, and MTHFR-c677t among cancer patients of Caucasian ancestry with thrombosis was much higher (9.7%, 6.3%, 54.2%, and 56.5%, respectively) compared with those previously published for the Caucasian population without cancer (4.1%–8.5%, 1%–3.9%, 31.5%–40%, and 25.3%–40%, respectively), and also compared with African and Asian populations, where factor V Leiden and prothrombin-20210G>A mutations are extremely rare or nonexistent.Citation5–Citation7 When abnormal genes were added (cumulative gene mutation), of 136 cancer patients with Caucasian ancestry, 43.7% had one gene mutation and 44.9% had two or more gene mutations, with an overall quantitative gene mutation rate of 87.5%, compared with 36.4%, 18.2%, and 54.5%, respectively, for 11 patients of other ethnic backgrounds (P = 0.01021).
Discussion
Incidence of thrombosis in cancer patients
Large population-based studies have shown that cancer patients have a 4–7-fold increased risk of developing venous thromboembolism compared with those without cancer.Citation8,Citation9 The rate of cancer-related thrombosis varies widely between published reports, from 0.6% to more than 18% depending on the type of study population, duration of follow-up, definition of thromboembolic events, and active versus passive ascertainment.Citation9–Citation13 The actual incidence may be even higher, exceeding 50% in autopsy series.Citation13,Citation14 In a large cohort study of neutropenic cancer patients, venous thromboembolism was reported in 5.4% and arterial thrombosis in 1.5% of patients.Citation15 In the present study, there was a 10.4% incidence of venous thromboembolism, a 1.9% incidence of other venous thrombosis, a 1.5% incidence of arterial thrombosis, and a 0.6% incidence of combined venous and arterial thrombosis.
Clinical presentation and risk factors
Venous and arterial thrombosis in cancer patients are clinical manifestations of a multifactorial systemic disease and related to several risk factors, particularly with regard to patient characteristics, cancer characteristics, and type of therapy administered.
Elderly patients are much more prone to vascular thrombosis. In the present study, patients with thrombosis were predominantly elderly, unlike those without thrombosis. However, this difference was not statistically significant.
The incidence of vascular thrombosis in the general population varies greatly according to ethnic background, with an adjusted standardized incidence rate of 141 per 100,000 for African Americans, 104 for Caucasians, 55 for Hispanics, 33.1 for Native American Indians, and a similar rate for Pacific Islanders.Citation7 For the Caucasian population, genetics account for up to 60% of the risk of thrombosis.Citation6 For those with African American ancestry, the risk may be related to sickle cell trait, compound heterozygous hemoglobinopathy, or other unidentified genes.Citation6 For Native American Indians, who genetically have Asian ancestry,Citation16 and those of direct Asian ancestry, the most representative risk factor is deficiency in natural coagulation inhibitors.Citation6 In the present study, Caucasians accounted for the majority of cancer patients with thrombosis. Further, although single thrombophilia testing was not helpful in predicting patients with thrombosis, the cumulative gene mutation rate was significantly higher in Caucasian patients compared with other ethnic groups, supporting the possibility of genetic factors being involved in thrombus formation among cancer patients of Caucasian ancestry.
Several large prospective studies have correlated obesity with an increased risk of postoperative venous thromboembolism and subsequent death.Citation17 In the present study, the majority of cancer patients with thrombosis were relatively overweight compared with cases without thrombosis. Obesity was not a major risk factor for thrombosis in multivariate regression analysis. However, patients with weight loss and a lower BMI were less prone to thrombosis.
The rate of thrombosis in cancer patients differs greatly according to the primary site, with the highest prevalence in patients with cancer of the liver, pancreas (about 4%), ovary, brain, kidney, stomach, lung, and lymphatic system, compared with bladder and head and neck cancers (about 1%).Citation9,Citation18 In the present analysis, a similar trend was also noticed, although with different percentages.
Metastatic disease at the time of initial diagnosis is associated with a 1.4–21.5-fold higher risk of thrombosis compared with localized malignancies, and the incidence of thrombosis increases with advancing stages of cancer.Citation19,Citation20 In the present study, the stage of cancer was the most important risk factor for cancer-related thrombosis in regression analysis (P ≤ 0.001).
With regard to cancer therapy, surgical intervention increases the risk of thrombosis, and may reach nearly 13-fold after major trauma requiring hospitalization and surgical intervention.Citation21 The risk of thrombosis following chemotherapy varies from 2.5% to 12.5%, depending on type of therapy and presence of other risk factors, with a higher prevalence of arterial thrombosis.Citation22 Such arterial thrombosis is much more common after treatment with antiangiogenic agents,Citation8,Citation19,Citation22 cisplatinum-based chemotherapy regimens,Citation23 anti-epidermal growth factor receptors,Citation24 and hormone therapy in patients with breast and prostate cancers.Citation25 In the present analysis, the type of therapy was an important risk factor for thrombosis, but did not reach statistical significance in the regression model.
Time elapsed from diagnosis of cancer to thrombosis is known to be an important issue. In a large population-based, case-control study, patients with cancer had a significantly increased risk of vascular thrombosis, particularly during the first 3–6 months after diagnosis of cancer.Citation8,Citation9 In the current study, most cases of thrombosis were diagnosed in a similar time frame, ie, between 3 months before and 6 months after diagnosis of cancer.
Most reports refect the incidence of venous thromboembolic events, particularly deep venous thrombosis and pulmonary embolism. It was thought that risk factors for venous thrombosis and arterial thrombosis are different, but it is now accepted that vascular thrombosis, especially in cancer patients, is a systemic disease and the risks for both venous and arterial thrombosis are similar.Citation26–Citation28 In a major placebo-controlled study of the use of rosuvastatin for 2 years, use of the active medication led to a 43% reduction in risk of venous thromboembolism compared with the control group.Citation26,Citation29 In the present analysis, the risks for both venous thrombosis and arterial thrombosis were similar, except for obesity and advanced stage of cancer, both of which potentiate the risk of venous thrombosis, while generalized and severe atherosclerosis precipitates arterial occlusion. Survival following thrombosis, overall survival after diagnosis of cancer, and 3-month mortality rates were also similar. This may confirm the concept of cancer-associated hypercoagulation as a mediator for both venous thrombosis and arterial thrombosis and underscores the importance of cardiac and cerebrovascular risk factors in patients with cancer.
Site of thrombosis
Most patients with cancer-related thrombosis present with deep venous thrombosis affecting the lower limb. Deep venous thrombosis affecting the upper extremities has been considered to be a rare condition, accounting for 1%–4% of all deep venous thromboses.Citation30 However, over the last two decades, with use of central venous catheters and implantable venous access ports, the incidence has markedly increased, with higher morbidity and mortality.Citation31 In the present study, venous thrombosis of the upper extremity accounted for 25.7% of all venous thrombotic episodes, with no difference in survival compared with other sites of thrombosis.
Role of thrombophilia
Patients with cancer have a 6–7-fold increased risk of vascular thrombosis compared with those without cancer.Citation9 Recently, it has become clear that genetic mechanisms responsible for neoplastic transformation either via the activation of oncogenes (such as RAS or MET) or inactivation of suppressor genes (such as p53 or PTEN) directly induce the expression of the genes controlling hemostasis.Citation32 Activation of coagulation confers a selective survival advantage for tumor cells, and the interplay between tumor and hemostasis persists for many years, even after successful therapy and cure.Citation32 Such a thrombophilic state in cancer presents as a systemic disorder linked to chronic, low-grade disseminated intravascular coagulation, with thrombotic episodes occurring alone or in the presence of disseminated intravascular coagulation, at different levels of expression in the majority of patients with malignancies.Citation32,Citation33 This was partly supported in a recent study on 1178 cancer patients. An elevated D-dimer level was reported in 74.2% of those patients, with subsequent shorter survival for cases having higher levels of D-dimer.Citation33
Several genetic abnormalities are currently identified to be major risk factors for vascular thrombosis, particularly factor V Leiden and prothrombin-20210G>A, and to a lesser extent, methyltetrahydrofolate reductase (MTHFR-A1298c and MTHFR-c677t). However, their role in causing thrombosis, particularly in cancer patients, is still controversial.
Other hereditary polymorphisms not yet discovered, and/or environmental factors may also contribute to thrombosis in cancer patients.Citation5 This was evident in a recent genome sequencing study in multiple myeloma patients which revealed several new oncogenic mechanisms, as well as mutation in genes involved both in blood coagulation as well as initiation of myeloma.Citation34 Thus, genetic variation may be responsible for both thrombosis and cancer development. In the present study, testing for any particular gene was not helpful in predicting patients at high risk for thrombosis. However, among cancer patients of Caucasian ancestry, the prevalence of gene mutations was much higher compared with those previously published for the general population. Further, quantitative gene mutations were much higher in cancer patients of Caucasian ancestry compared with other ethnic groups. Multiple testing for quantitative gene mutations or genomic sequencing of cancer patients may be more accurate in defining cases at higher risk of thrombosis.
Impact on survival
Survival of cancer patients following thrombotic episodes is less defined, because most reports were derived from registries with limited follow-up. Most studies have demonstrated that the presence of venous thromboembolism in patients with cancer is an independent predictor of poor survival,Citation3,Citation4,Citation18 with virtually all patients being dead within 6 months after thrombosis (1988–1990),Citation35 a one-year survival of 12% (1977–1992),Citation3 and a median survival of 13.5 months, with survival rates at 6 months and 1, 2, and 5 years of 64%, 53%, 43%, and 33%, respectively (1997–2006)Citation36 and 3-month mortality rate following thrombosis of 7.9%, potentially related to pulmonary embolism in 1.4% (2001–2010).Citation37 In the present study, conducted in the years 2005–2012, the median survival following venous thrombosis was 16.7 months, and survival rates at 6 months and 1, 2, and 5 years were 96.8%, 93.4%, 90.0%, and 43.4%, respectively. The 3-month mortality rate following venous thrombosis was 20.4%, and mortality was mainly due to progression of cancer (91.5%) or development of pulmonary embolism (2.1%). The improved survival and reduced deleterious impact of pulmonary embolism may be related to improved cancer therapy outcomes during the last two decades.
Limitations
This study, being a retrospective analysis of a limited number of patients participating in thrombophilic studies, has several limitations. It explores the outcome of a heterogeneous group of patients at different stages of malignancy who were receiving different types of cancer therapy. Further, with such variation in the incidence of thrombophilic factors, our findings may not necessarily be applicable to other populations of different ethnic composition. Consequently, unexpressed bias may exist. However, our study has significance despite these limitations. Its main strength is inclusion of all patients seen and followed closely in a single practice setting, ie, a real world situation that provides a representative picture of cancer patients with and without thrombosis. It defines the relative incidence of thrombosis among cancer patients of various ethnic groups, and shows the importance of not only venous thrombosis but also arterial occlusion as causes of higher morbidity. With the present active therapy for malignancy and the management of thrombosis, the subsequent survival of cancer patients with and without thrombosis is expected to show further improvement.
Acknowledgments
The author thanks Joseph Anigbogu, Kemi Azeez, Pratap Balusu, Glenn Bryant, Henry Gerad, Ravi Kamepalli, Abbas Khalil, Sarat Kuchipudi, Chaoyang Li, Oluremi Ojo, David Powell, Sreenivasa Chanamolu, and Abdullah Taja for patient referrals; Manar Moneer for assistance with the statistical analysis; as well as Betty Badertscher and Kathy Herold for manuscript preparation.
Disclosure
The authors report no conflicts of interest in this work.
References
- SackGHJrLevinJBellWRTrousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic featuresMedicine (Baltimore)1977561137834136
- DonatiMBFalangaAPathogenetic mechanisms of thrombosis in malignancyActa Haematol20011061–2182411549773
- SorensenHTMellemkjaerLOlsenJHBaronJAPrognosis of cancers associated with venous thromboembolismN Engl J Med2000343251846185011117976
- ChewHKWunTHarveyDZhouHWhiteRHIncidence of venous thromboembolism and its effect on survival among patients with common cancersArch Intern Med2006166445846416505267
- HadhriSRejabMBGuedriaHIfaLChattiNSkouriHFactor V Leiden, prothrombin 20210G > A, MTHFR 677C > T and 1298 A > C, and homocysteinemia in Tunisian blood donorsJ Clin Lab Anal201226316717322628232
- MargaglioneMGrandoneEPopulation genetics of venous thromboembolism. A narrative reviewThromb Haemost2011105222123120941456
- WhiteRHZhouHMurinSHarveyDEffect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996Thromb Haemost200593229830515711746
- HeitJASilversteinMDMohrDNPettersonTMO’FallonWMMeltonLJ3rdRisk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control studyArch Intern Med2000160680981510737280
- BlomJWDoggenCJOsantoSRosendaalFRMalignancies, prothrombotic mutations, and the risk of venous thrombosisJAMA2005293671572215701913
- AgnelliGGussoniGBianchiniCNadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind studyLancet Oncol2009101094394919726226
- HeitJAMohrDNSilversteinMDPettersonTMO’FallonWMMeltonLJ3rdPredictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort studyArch Intern Med2000160676176810737275
- LymanGHKhoranaAACancer, clots and consensus: new understanding of an old problemJ Clin Oncol200927294821482619752337
- RicklesFRLevineMNEpidemiology of thrombosis in cancerActa Haematol20011061–261211549771
- SvendsenEKarwinskiBPrevalence of pulmonary embolism at necropsy in patients with cancerJ Clin Pathol19894288058092475526
- KhoranaAAFrancisCWCulakovaEFisherRIKudererNMLymanGHThromboembolism in hospitalized neutropenic cancer patientsJ Clin Oncol200624348449016421425
- KayserMBrauerSSchadlichHY chromosome STR haplotypes and the genetic structure of US populations of African, European, and Hispanic ancestryGenome Res200313462463412671003
- ParkinLSweetlandSBalkwillAGreenJReevesGBeralVBody mass index, surgery, and risk of venous thromboembolism in middle-aged women: a cohort studyCirculation2012125151897190422394567
- SteinPDBeemathAMeyersFASkafESanchezJOlsonREIncidence of venous thromboembolism in patients hospitalized with cancerAm J Med20061191606816431186
- BlomJWVanderschootJPOostindierMJOsantoSvan der MeerFJRosendaalFRIncidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage studyJ Thromb Haemost20064352953516460435
- ChewHKWunTHarveyDJZhouHWhiteRHIncidence of venous thromboembolism and the impact on survival in breast cancer patientsJ Clin Oncol2007251707617194906
- HolbrookASchulmanSWittDMEvidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th edition: American College of Chest Physicians Evidence-Based Clinical Practice GuidelinesChest2012141Suppl 2e152Se184S22315259
- AgnelliGGeorgeDJKakkarAKSemuloparin for thromboprophylaxis in patients receiving chemotherapy for cancerN Engl J Med2012366760160922335737
- MooreRAAdelNRiedelEHigh incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysisJ Clin Oncol201129253466347321810688
- PetrelliFCabidduMBorgonovoKBarniSRisk of venous and arterial thromboembolic events associated with anti-EGFR agents: a meta-analysis of randomized clinical trialsAnn Oncol20122371672167922241897
- DipascoPJMisraSKoniarisLGMoffatFLJrThrombophilic state in cancer, part I: biology, incidence, and risk factorsJ Surg Oncol2011104331632221480262
- GoldhaberSZRisk factors for venous thromboembolismJ Am Coll Cardiol20105611720620709
- SpencerFAGinsbergJSChongAAlterDAThe relationship between unprovoked venous thromboembolism, age, and acute myocardial infarctionJ Thromb Haemost2008691507151318624983
- SchwarzbachCJSchaeferAEbertAStroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiologyStroke201243113029303422996958
- GlynnRJDanielsonEFonsecaFAA randomized trial of rosuvastatin in the prevention of venous thromboembolismN Engl J Med2009360181851186119329822
- KommareddyAZaroukianMHHassounaHIUpper extremity deep venous thrombosisSemin Thromb Hemost2002281899911885029
- BaarslagHJKoopmanMMReekersJAvan BeekEJDiagnosis and management of deep vein thrombosis of the upper extremity: a reviewEur Radiol20041471263127414991322
- BoccaccioCComoglioPMGenetic link between cancer and thrombosisJ Clin Oncol200927294827483319738115
- AyCDunklerDPirkerRHigh D-dimer levels are associated with poor prognosis in cancer patientsHaematologica20129781158116422371182
- ChapmanMALawrenceMSKeatsJJInitial genome sequencing and analysis of multiple myelomaNature2011471733946747221430775
- LevitanNDowlatiARemickSCRates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims dataMedicine (Baltimore)199978528529110499070
- PrestidgeTLeeSHarperPYoungLOckelfordPSurvival in patients with malignancy and venous thromboembolism by tumour subtype and thrombus locationIntern Med J2012421717421118408
- GussoniGFrassonSLa ReginaMDi MiccoPMonrealMThree-month mortality rate and clinical predictors in patients with venous thromboembolism and cancer. Findings from the RIETE registryThromb Res20131311243023141849