Abstract
Objective
To explore the effectiveness of prostate biopsy density in predicting prostate cancer under cognitive and systematic biopsy mode in multi-parametric magnetic resonance imaging (mpMRI).
Methods
A retrospective analysis was conducted on clinical data of 204 patients who were suspected of having prostate cancer with prostate-specific antigen (PSA) levels less than 50 ng mL−1 and underwent cognitive and systematic biopsy through the perineal approach in our hospital from 2022 to 2023. Univariate and multivariate logistic regression analyses were used to evaluate the odds ratios of prostate biopsy density and relevant clinical indicators. Logistic regression analysis was performed to establish a predictive model combining indicators with predictive value. The predictive value of each indicator and the new model was evaluated using receiver operating characteristic (ROC) curves and the area under the curve (AUC).
Results
The detection rate of prostate cancer in the study population was 32.35%. Multivariate analysis showed that age, PSAD, PI-RADS 2.1 score, and prostate biopsy density were independent predictors of prostate cancer. The ROC curve analysis revealed an AUC of 0.707 (95% CI 0.625–0.790) for biopsy density, with a cutoff value of approximately 0.22 needle mL−1. The best predictive model consisted of age, PSAD, PI-RADS 2.1 score, and biopsy density, with an AUC of 0.857.
Conclusion
Biopsy density is associated with the detection of prostate cancer, with a critical value of 0.22 needle mL−1. Combining biopsy density with other clinical indicators can significantly improve the ability to predict prostate cancer and avoid unnecessary prostate biopsy cores.
Introduction
Prostate cancer (PCa) is the most common malignancy of the male genitourinary system worldwide, and its diagnosis relies on transrectal ultrasound-guided prostate biopsy. Since the introduction of the transrectal ultrasound-guided 6-core biopsy method in 1989,Citation1 transrectal ultrasound-guided prostate biopsy has been considered the standard approach for obtaining prostate tissue for pathological examination. Since then, additional biopsy cores have been usedCitation2,Citation3 to improve the accuracy of prostate cancer detection. It is generally believed that 8–12 biopsy cores increase the accuracy without significant additional adverse effects. However, there is still considerable controversy regarding the optimal number of biopsy cores to be taken.
Evidence from systematic reviews and clinical studies suggests that transrectal ultrasound-guided systematic biopsy has a false-negative rate of approximately 21% and potential complications in diagnosing prostate cancer.Citation4 On the other hand, multiparametric magnetic resonance imaging (mpMRI)-guided targeted biopsy has not only improved the detection rate of clinically significant prostate cancer (csPCa) but also has shown a correlation between lesions detected on mpMRI and tumor location in radical prostatectomy specimens.Citation5 mpMRI has become an accurate method for detecting prostate cancer, but targeted biopsy alone may miss approximately 10% of clinically significant prostate cancer.Citation6 In addition, systematic biopsy can determine the extent of the lesion to better guide diagnosis and treatment. Overall, combined biopsy remains the primary choice for prostate biopsy techniques.Citation7
Currently, the commonly used approach for prostate biopsy involves a combination of systematic biopsy with 10–12 cores and targeted biopsy with additional cores. Previous studies have found that increasing the number of biopsy cores can improve the rate of positive biopsies in patients with larger prostate volumes.Citation8 A studyCitation9 proposed the concept of biopsy density, defined as the number of cores per prostate volume, as a comprehensive indicator to guide the strategy for selecting the number of biopsy cores per milliliter of prostate tissue.
For biopsy operators, the ability to accurately identify abnormal prostate lesions by preoperative MRI and intraoperative ultrasound, as well as to determine the number of puncture needles to be performed, is the only protocol that can be subjectively decided or improved to improve diagnostic accuracy. How to determine the number of puncture needles is particularly important. The purpose of this study is to explore whether the number of puncture needles can be determined according to the biopsy density. There is currently no research on the application of prostate biopsy density in predicting prostate cancer under the cognitive and systematic biopsy. Therefore, this study aims to explore the value of prostate biopsy density in diagnosing prostate cancer under the cognitive and systematic biopsy to provide better guidance for clinical treatment.
Patients and Methods
Patients
A retrospective analysis was conducted on the clinical data of 204 patients who underwent prostate biopsy in our hospital from 2022 to 2023. The criteria for prostate biopsy were PSA level >4 ng mL−1 and/or abnormal findings on digital rectal examination (DRE), transrectal ultrasound (TRUS), or prostatic multiparameter magnetic resonance imaging (mpMRI). All patients underwent transrectal ultrasound-guided prostate biopsy and had pathological diagnosis results. Patients with previous history of prostate cancer, hormonal manipulation, documented urinary tract infection, acute or chronic bacterial prostatitis, previous prostate surgery, recent 5-alpha-reductase inhibitors use and any condition that may affect serum PSA level were not included. The prostate mpMRI examination was completed for all patients before surgery, and the imaging diagnosis report was reviewed independently by two radiologists, and reviewed by a third senior radiologist in case of disagreement. This study was approved by the Ethics Committees of Ningxia Medical University General Hospital. This study was conducted in accordance with the Declaration of Helsinki. All patients enrolled in the study provided informed consent.
Methods
All biopsies were performed by 2 experienced urologists. All patients underwent ultrasound-guided transperineal prostate biopsy using an ultrasound system and an 18G automatic biopsy gun with a 10+X core approach (X means the additional number of biopsy needles under cognitive fusion, The number of X is usually 1–3 in our study). Procedure: Preoperatively, the perineal area was prepared, and patients assumed the lithotomy position. The puncture site was disinfected and draped, and local anesthesia was achieved using lidocaine. After inserting the ultrasound probe, lidocaine was injected bilaterally at the base of the prostate for nerve block anesthesia. Once successful anesthesia was confirmed, free-hand biopsy was performed using an automatic biopsy gun with 10 cores: 3 cores from each side of the peripheral zone, 1 core from each transition zone, and 1 core from each apex. Additional cores were taken at abnormal nodules identified on preoperative MRI and real-time ultrasound imaging. It is generally believed that the abnormal part of the peripheral zone often occurs asymmetrically on MRI, with low signal on T2 sequence and high signal on DWI, and can often be distinguished from the normal shape. According to this abnormal change, additional biopsy is performed in combination with real-time ultrasound. In our studies, additional biopsies with 1 to 3 needle counts are usually performed. After the procedure, disinfection and compression were applied, and the perineum was bandaged. The biopsy specimens were placed in formalin solution for pathological examination, and the pathological sections were examined by two independent pathologists.
Data Collection and Analysis Data
Analysis was performed using IBM SPSS (Version 22.0) and MedCalc(Version 22.021). Our study collected data including age, PSA, fPSA, prostate volume (PV), number of biopsy cores, PSAD, prostate imaging reporting and data system (PI-RADS) 2.1 score. Data were presented as mean± standard deviation (s.d.) or median (interquartile range [IQR]) for continuous proportional and categorical variables, respectively. The Student’s t-test for independent samples was used in order to compare the two groups of patients with cancer (positive biopsy) and those without (negative biopsy). Pearson’s Chi-square test or Fisher’s exact test was used to compare PI-RADS 2.1 score between the groups. Binary logistic regression was used to identify risk factors for positive biopsy results. Area under the receiver operating characteristic (ROC) curves (AUCs) were used to calculate the efficacy of each indicator for positive biopsy results, and the AUCs of each clinical indicator were compared using the Z-test. The threshold value of biopsy density was determined using the Youden index. A p-value <0.05 was considered statistically significant.
Results
General Information
Among the 204 patients, mean patient age was 67 years old (IQR was 62.00–72.25), and the mean PSA level was 12.7 ng mL−1 (IQR was 7.99–19.30). Sixty-six patients (32.35%) were diagnosed with prostate cancer. provides a detailed summary of the comparison of general information, serology results, and prostate MRI parameters between the non-PCa and PCa groups. There were significant differences between the non-PCa group and the PCa group in terms of patient age, tPSA, F/T ratio, PSAD, PV, biopsy density, and PI-RADS 2.1 score. The biopsy density was significantly higher in the PCa group compared to the non-PCa group (0.245 vs 0.187; P = 0.001).
Table 1 Comparison of General Information Between the Non-PCa and PCa Groups
Univariate and Multivariable Logistic Analysis of Risk Factors for PCa
Univariate logistic analysis was performed on the preoperative clinical data to determine the risk factors for PCa. The results showed that age, tPSA, F/T ratio, PSAD, PV, biopsy density, and PI-RADS 2.1 score were all significant risk factors for PCa (P<0.05), as shown in .
Table 2 Univariate Logistic Regression Analysis of Risk Factors for PCa Diagnosis
After performing univariate logistic regression analysis on all clinical parameters, the statistically significant clinical parameters were included in the multivariable binary logistic regression analysis. To avoid the interaction of data in the regression, tPSA and prostate volume were excluded in this study. The multivariable logistic regression analysis results showed that age, PSAD, biopsy density, and PI-RADS 2.1 score were independent predictors of positive biopsy results for PCa, whereas the F/T ratio (P = 0.059) was excluded. Finally, the following variables were included in the multivariable regression model: age, PSAD, biopsy density, and PI-RADS 2.1 score. shows that age, PSAD, biopsy density, and PI-RADS 2.1 score were independent predictors of PCa (P < 0.05).
Table 3 Multivariable Logistic Regression Analysis of Risk Factors for PCa Diagnosis
ROC Analysis of Biopsy Density Alone and in Combination with Other Clinical Indicators
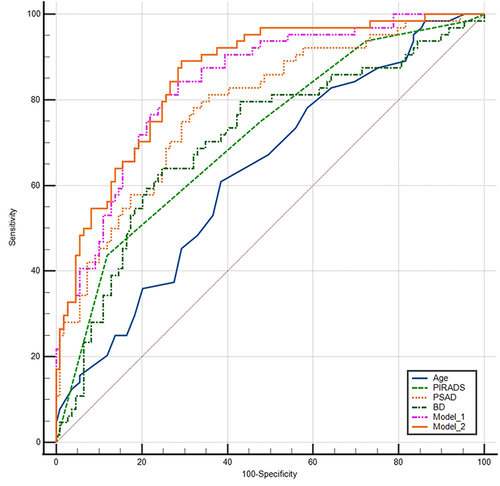
Using binary multivariable logistic regression analysis, we first created a predictive model 1 combining age, PSAD, and PI-RADS 2.1 score. To assess the contribution of biopsy density to this model, we added biopsy density to create model 2. ROC analysis showed that all indicators and both models had significant diagnostic value for prostate cancer ( and ). The AUC of biopsy density was 0.707, with a 95% confidence interval of 0.625–0.790. The AUC of PSAD (0.783) was the largest among the single indicators. Model 1, which combined age, PSAD, and PI-RADS 2.1 score, had better predictive value for PCa compared to each single indicator. Model 2, which added biopsy density, had a larger AUC (0.857) compared to model 1, indicating that the addition of biopsy density improved the predictive value of the model. The AUCs of both model 1 and model 2 were significantly higher than that of PSAD alone, but there was no statistically significant difference in predictive value between model 1 and model 2.
Table 4 ROC Analysis of Various Indicators and Two Predictive Models
Partial Cutoff Values of Biopsy Density
We have listed the partially truncated values of biopsy density and their characteristics (). Using the Youden index, a cutoff value of approximately 0.22 needle mL−1 was determined for biopsy density. Under this threshold, the sum of the corresponding specificity and sensitivity reaches the maximum. The Youden index was 0.38, with a sensitivity of 0.636 and specificity of 0.744. At this cutoff value, the positive predictive value was 56% and the negative predictive value was 81.395%. However, in our study population, as the biopsy density increased, the positive predictive value did not increase significantly, indicating that biopsy density is only a relevant factor and not a determining factor.
Table 5 Different Cutoff Values of Biopsy Density
Discussion
Prostate cancer is one of the most common malignancies in men,Citation10 and prostate biopsy has long been considered the gold standard for its diagnosis. Prostate-specific antigen (PSA) has been widely used as a screening tool for prostate cancer. However, PSA is an organ-specific but not cancer-specific serum marker. As a result, increased PSA levels are not exclusive to prostate cancer but can also occur in prostate enlargement, inflammation, or other non-malignant conditions. This lack of specificity of PSA leads to an overdiagnosis of prostate cancer and unnecessary biopsies. In recent years, pre-biopsy MRI has become particularly important for early detection of prostate cancer.Citation11 With the advancements in imaging technology, targeted biopsies guided by multiparametric MRI have been shown to detect many tumors with a Gleason score of 7 or higher.Citation12 However, for patients with a high clinical suspicion of prostate cancer but no visible lesions on mpMRI, systematic biopsy of the prostate is still necessary. Baco et alCitation6 published the results of a randomized controlled trial in the European Urology journal, suggesting that the detection rates of clinically significant prostate cancer with a 2-core targeted biopsy and a 12-core systematic biopsy were comparable. However, targeted biopsy alone may miss approximately 10% of clinically significant prostate cancer. Therefore, finding new parameters or adjusting existing ones to improve the accuracy of PCa prediction models has been widely explored.
Previous transperineal prostate biopsies were limited by the systemic saturation of the puncture biopsy method, which not only consumed a significant amount of time, but also incurred considerable expenses in terms of equipment and pathological examination, while simultaneously increasing the risk of puncture-related complications.Citation13 However, TPB can now be performed under local anesthesia without the need for general anesthesia.Citation14 TPB through the perineal approach does not require bowel preparation before the procedure. The incidence of complications such as urethral damage, infection, and bleeding is relatively low compared to the transrectal approach.Citation15,Citation16 Compared to transrectal biopsy (TRB), transperineal biopsy under ultrasound guidance allows for more precise targeting, enabling a more comprehensive sampling of all prostate regions, especially lesions in the apex and anterior regions.Citation17 Therefore, transperineal biopsy often achieves a higher positive rate.Citation18 It has been reported that TPB is better integrated with MRI compared to TRP.Citation19–21
A previous studyCitation9 proposed the concept of biopsy density, defined as the number of cores divided by the prostate volume, as a comprehensive indicator to guide the selection of the number of biopsy cores per milliliter of prostate tissue. Previous studies on biopsy density mainly focused on systematic biopsy, and the conclusions were mostly based on the increase in biopsy density and its association with increased cancer detection rates. However, with the widespread use of mpMRI, the current recommendation is to combine systematic and targeted biopsies.Citation22 In this approach, the precise determination of the number of our puncture needles has emerged as a novel challenge, with clinical practice aspiring to achieve the least number of missed diagnoses using the minimal number of needles. Consequently, we have conducted an investigation into the contribution of biopsy density to the cancer detection rate.
Our study used binary logistic regression analysis to suggest an association between biopsy density and positive biopsy results. The results of the univariate regression analysis showed that age, PI-RADS 2.1 score, tPSA, F/T ratio, PV, PSAD, and biopsy density were all risk factors for prostate cancer detected by ultrasound-guided biopsy. However, simple univariate analysis only provides a preliminary understanding of the relationship between variables and the risk of prostate cancer development. It does not consider the combined effects of variables. Therefore, the statistically significant clinical parameters from the preliminary screening were included in the multivariable regression analysis. The results showed that age, PSAD, biopsy density, and PI-RADS 2.1 score were independent risk factors for PCa. The ROC analysis revealed a cutoff value of approximately 0.22 needle mL−1 for biopsy density, with a sensitivity of 0.636, specificity of 0.744, positive predictive value of 56%, and negative predictive value of 81.395%. Stone et alCitation9 found that a biopsy density greater than 1.5 cores per milliliter could increase the detection rate of prostate cancer through transperineal mapping biopsy. The critical value of biopsy density (BD) obtained in our study is significantly lower than this threshold, considering the lower PSA levels and excessive number of cores used in the patients included in their study. Additionally, we believe that with the widespread use of cognitive fusion with multi-parametric magnetic resonance imaging (mpMRI), a lower biopsy density is sufficient to achieve a higher specificity in detection rates. Furthermore, in our study cohort, as biopsy density increased, the positive predictive value did not significantly improve. We consider biopsy density itself to be a related factor rather than a determining factor in disease occurrence.
However, the AUC of biopsy density alone (0.707) is not satisfactory and is lower than the commonly used indicator PSAD (0.783). This indicates that if there are no specific risk factors, such as high PSA levels or suspicious areas on MR images, increasing the biopsy density and the number of biopsy cores may not be a wise decision. Hypothetically, when combining the independent risk factors for prostate cancer, age, PSAD, and PI-RADS 2.1 score, adding biopsy density to the model may improve the diagnostic value for PCa. The ROC analysis results showed that both the individual indicators and the two models had significant diagnostic value for prostate cancer (). The AUC of biopsy density was 0.707, with a 95% confidence interval of 0.625–0.790. The AUC of PSAD (0.783) was the largest among the single indicators. Model 1, which combined age, PSAD, and PI-RADS 2.1 score, had better predictive value for PCa compared to each single indicator. Model 2, which added biopsy density, had a larger AUC (0.857) compared to model 1, indicating that the addition of biopsy density improved the predictive value of the model. Although the AUCs of model 1 and model 2 were significantly higher than that of PSAD alone, there was no statistically significant difference in predictive value between model 1 and model 2. However, it should be noted that the difference in predictive value between model 1 and model 2 may not have statistical significance due to the limited sample size.
There are several limitations to our study. First, it is a retrospective study and not a prospective trial with biopsy density predetermined before the biopsy. This may introduce selection bias, as the variation in core numbers was not significant in our study. We intend to conduct a randomized controlled trial based on the optimal biopsy density values obtained to validate the results of this study. Second, the number of patients with prostate cancer was relatively small, and further analysis is needed to understand the relationship between biopsy density and cancer detection. In our study, we selected a wide range of PSA levels (4–50 ng mL−1) and did not stratify the analysis based on PSA levels, PI-RADS, or prostate volume, which could be influenced by various confounding factors. Also, we implement cognitive fusion puncture instead of MRI fusion technology, which may bring errors because of individual differences of doctors.
Conclusion
Our data suggest that biopsy density is associated with positive biopsy results and the detection of prostate cancer, with a cutoff value of approximately 0.22 needle mL−1. Under the cognitive and systematic biopsy, excessively increasing biopsy density may not be a wise decision. Biopsy density can be included in a predictive model along with other clinical parameters to significantly improve the ability to predict prostate cancer and reduce unnecessary biopsy cores.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This paper is supported by Key of Ningxia Autonomous Region research and development plans (2023ZDYF1196).
Data Sharing Statement
All data generated or analysed during this study are included in this published article.
References
- Hodge -K-K, Mcneal J-E, Stamey T-A. Ultrasound guided transrectal core biopsies of the palpably abnormal prostate. J Urol. 1989;142(1):66–70. doi:10.1016/S0022-5347(17)38663-9
- Eskew L-A, Bare R-L, Mccullough D-L. Systematic 5 region prostate biopsy is superior to sextant method for diagnosing carcinoma of the prostate. J Urol. 1997;157(1):199–202; discussion 202.
- Stamatiou K, Alevizos A, Karanasiou V, et al. Impact of additional sampling in the TRUS-guided biopsy for the diagnosis of prostate cancer. Urol Int. 2007;78(4):313–317. doi:10.1159/000100834
- Martin PR, Cool DW, Fenster A, Ward AD. A comparison of prostate tumor targeting strategies using magnetic resonance imaging-targeted, transrectal ultrasound-guided fusion biopsy. Med Phys. 2018;45(3):1018–1028. doi:10.1002/mp.12769
- Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186(5):1818–1824. doi:10.1016/j.juro.2011.07.013
- Baco E, Rud E, Eri LM, et al. A randomized controlled trial to assess and compare the outcomes of two-core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12-core systematic biopsy. Europ urol. 2015;69(1):149–156. doi:10.1016/j.eururo.2015.03.041
- Borkowetz A, Hadaschik B, Platzek I, et al. Prospective comparison of transperineal magnetic resonance imaging/ultrasonography fusion biopsy and transrectal systematic biopsy in biopsy-naïve patients. BJU Int. 2017;121(1):53–60. doi:10.1111/bju.14017
- Ito K, Ohi M, Yamamoto T, et al. The diagnostic accuracy of the age-adjusted and prostate volume-adjusted biopsy method in males with prostate specific antigen levels of 4.1–10.0 ng/mL. Cancer. 2002;95(10):2112–2119. doi:10.1002/cncr.10941
- Stone NN, Crawford ED, Skouteris VM, et al. The ratio of the number of biopsy specimens to prostate volume (Biopsy Density) greater than 1.5 improves the prostate cancer detection rate in men undergoing transperineal biopsy of the prostate. J Urol. 2019;202(2):264–271. doi:10.1097/JU.0000000000000204
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. Ca Cancer J Clin. 2023;73(1):17–48.
- Gayet M, van der Aa A, Beerlage HP, Schrier BP, Mulders PF, Wijkstra H. The value of magnetic resonance imaging and ultrasonography (MRI/US)-fusion biopsy platforms in prostate cancer detection: a systematic review. BJU Int. 2015;117(3):392–400.
- Radtke JP, Kuru TH, Boxler S, et al. Comparative analysis of transperineal template saturation prostate biopsy versus magnetic resonance imaging targeted biopsy with magnetic resonance imaging-ultrasound fusion guidance. J Urol. 2014;193(1):87–94. doi:10.1016/j.juro.2014.07.098
- Miah S, Eldred-Evans D, Simmons LA, et al. Patient reported outcome measures for transperineal template prostate mapping biopsies in the PICTURE study. J Urol. 2018;200(6):1235–1240. doi:10.1016/j.juro.2018.06.033
- Hong A, Hemmingway S, Wetherell D, Dias B, Zargar H. Outpatient transperineal prostate biopsy under local anaesthesia is safe, well tolerated and feasible. ANZ J Surg. 2022;92(6):1480–1485. doi:10.1111/ans.17593
- Saito K, Washino S, Nakamura Y, et al. Transperineal ultrasound-guided prostate biopsy is safe even when patients are on combination antiplatelet and/or anticoagulation therapy. BMC Urol. 2017;17(1):53. doi:10.1186/s12894-017-0245-z
- Wang L, Wang X, Zhao W, et al. A retrospective comparison between transrectal and transperineal prostate biopsy in the detection of prostate cancer. BMC Urol. 2017;19:55–59.
- Pepe P, Aragona F. Prostate biopsy: results and advantages of the transperineal approach--twenty-year experience of a single center. World J Urol. 2013;32(2):373–377.
- Savin Z, Dekalo S, Marom R, et al. Anterior and apical samplings during transperineal image-guided prostate biopsy. Urol Oncol. 2021;40(1):5.e15–5.e21.
- Rai BP, Mayerhofer C, Somani BK, Kallidonis P, Nagele U, Tokas T. Magnetic resonance imaging/ultrasound fusion-guided transperineal versus magnetic resonance imaging/ultrasound fusion-guided transrectal prostate biopsy-a systematic review. Eur Urol Oncol. 2021;4(6):904–913.
- Koparal MY, Sözen TS, Karşıyakalı N, et al. Comparison of transperineal and transrectal targeted prostate biopsy using Mahalanobis distance matching within propensity score caliper method: a multicenter study of Turkish Urooncology Association. Prostate. 2021;82(4):425–432.
- Hsieh PF, Chang TY, Lin WC, et al. A comparative study of transperineal software-assisted magnetic resonance/ultrasound fusion biopsy and transrectal cognitive fusion biopsy of the prostate. BMC Urol. 2022;22(1):72.
- Wang L, Wang X, Zhao W, et al. Surface-projection-based transperineal cognitive fusion targeted biopsy of the prostate: an original technique with a good cancer detection rate. BMC Urol. 2019;19(1):107. doi:10.1186/s12894-019-0535-8