Abstract
Purpose
In view of the documented toxicity of continuous daily radiosensitizer doses of temozolomide concomitant with radiation in the treatment of glioblastoma multiforme, we aimed to compare it with a different schedule of abbreviated radiosensitizer dosing.
Patients and methods
This was a randomized prospective study comparing toxicity and survival in 60 Egyptian patients with glioblastoma multiforme. Patients in arm I received temozolomide at a dose of 75 mg/m2 daily with radiotherapy for 42 days, starting 4 weeks after surgery and reaching to a total radiation dose of 60 Gy/30 Fractions/6 weeks, while patients in arm II received temozolomide at a dose of 75 mg/m2 concomitantly with the same radiotherapy schedule daily in the first and last weeks of the same radiotherapy program.
Results
Common grade 1–2 adverse events were malaise in 28 patients (46.7%), followed by alopecia (40%) and nausea (26.7%). Grade 3–4 convulsion and decreased level of consciousness was seen in only four patients who were all from arm I. The median progression-free survival (PFS) for the entire study population was 10.6 months (95% confidence interval [CI] 7.3–14), and PFS at 12 months was 32%. The median PFS in arm I was 8.8 months (95% CI 5.9–11.7) and in arm II 11.5 months (95% CI 8.9–14.2), and PFS at 12 months for both arms was 32% and 30% respectively (P=0.571). The median overall survival (OS) of the whole group of patients was 14.2 months (95% CI 13–15.5), and OS was 70% at 12 months and 25% at 18 months. The median OS for patients in arm I was 12.3 months (95% CI 7.7–16.9), whereas in arm II it was 14.3 months (95% CI 14–14.7) (P=0.83).
Conclusion
Reduced radiosensitizer dosing of temozolomide concomitant with radiotherapy in glioblastoma multiforme exhibited comparable efficacy with a classic continuous daily schedule, though with better tolerability.
Introduction
Adult glioblastoma multiforme (GBM) tumors are invariably fatal.Citation1 Complete surgical resection of these tumors with sufficient margins of adjacent normal tissue is usually not possible, due to their diffuse pattern of local spread.Citation2 Postoperative radiotherapy has been the standard for newly diagnosed GBM for years.Citation3 GBM exhibits resistance to postoperative cytotoxic therapy.
In spite of the efficacy of adjuvant carmustine/lomustine-based chemoradiotherapy (RT) in achieving modest prolongation of median survival, the survival benefit did not exceed a few months.Citation4 Glioblastoma cells have the powerful capacity of repairing RT-induced double-strand breaks, allowing the malignant cell to escape the apoptotic mechanism.Citation5
Recent studies have demonstrated that the use of temozolomide (TMZ), a methylating agent can stabilize RT-induced double-strand breaks by producing 12-base adducts in deoxyribonucleic acid (DNA), including the cytotoxic lesions O6-methylguanine (O6-meG) and 3-methyladenine.Citation5,Citation6 TMZ is an orally administered drug, readily absorbed with an excellent biodistribution, and penetrates most of target tissues, including the central nervous system, by crossing the blood–brain barrier.
TMZ has relatively low toxicity; in the body it undergoes spontaneous hydrolysis at physiological pH to form an active metabolite that produces the DNA-intercalated adducts.Citation7 Improved survival following concurrent TMZ RT has been demonstrated in about 50% of GBMs that exhibit promoter methylation of the gene of O6-meG-DNA methyltransferase (MGMT), as well as in tumors lacking MGMT-promoter methylation, suggesting that adducts and O6-meG both have an impact on treatment outcome.
Concurrent treatment with TMZ during RT has yielded the most significant improvement in survival in a fraction of patients with GBMs. The benefit of using concurrent TMZ during radiation compared to radiation alone was demonstrated in a European Organization for Research and Treatment of Cancer (EORTC)–National Cancer Institute of Canada (NCIC) trial on 573 patients following surgery.Citation8 The Neuro-oncology Working Group (NOA) of the German Cancer Society conducted a comparable randomized Phase III trial to compare a 1-week-on/1-week-off TMZ schedule at 100 mg/m2 with dose modification and involved field RT (54–60 Gy) in elderly patients with newly diagnosed anaplastic astrocytoma or GBM (NOA-08).Citation9
Several randomized trials using alternative TMZ dose schedules with radiotherapy have been proposed, in view of achieving optimum efficacy and least toxicity of the treatment regimen, as continuous daily administration of TZM induced profound lymphocytopenia with CD4 counts <200/mm,Citation3 and this was associated with an increased risk of opportunistic pneumocystis pneumonia. Similarly, steroids also lowered lymphocytic counts.Citation10 Prophylactic administration of pentamidine inhalations or trimethoprim–sulfamethoxazole during continuous daily concomitant RT is seriously considered, which is not the case during the abbreviated radiosensitizer schedule of TMZ administration.
In the present study, we aimed to explore the treatment outcome of an abbreviated schedule of radiosensitizer TMZ administration during the first and last week of RT compared to concurrent continuous administration during the whole RT course. The primary aim of this study was to analyze a toxicity profile as well as the efficacy of both regimens in terms of progression-free survival (PFS), with overall survival (OS) as the secondary end point.
Patients and methods
All recruited patients had a histological confirmation of highgrade brain glioma, were in the age range of 18–70 years, and had a Karnofsky Performance Status (KPS) of 60–100. Patients needed to have normal hematological, renal, and hepatic functions before the start of treatment. Patients of childbearing potential, including female partners of male patients, were advised to use an effective method of contraception. Excluded from the study were patients with recurrent, metastatic, or multiple malignant glioma or with any other malignancy, patients with frequent vomiting, or those who had received prior chemotherapy and/or RT for head and neck tumors. All patients gave written consent before starting treatment.
Sixty patients fulfilling the selection criteria were randomly allocated into the treatment groups at a ratio of 1:1, using a computer system with a closed envelope to one of these two treatment arms. There were 30 patients in each arm.
Patients randomized to arm I received TMZ at a dose of 75 mg/m2 daily with radiotherapy for 42 days, starting 4 weeks after surgery and reaching a total radiation dose of 60 Gy/30 fractions/6 weeks.
Patients in arm II received TMZ at a dose of 75 mg/m2 concomitantly with radiotherapy daily in the first and last weeks of radiotherapy, starting 4 weeks after surgery and reaching a total radiation dose of 60 Gy/30 fractions/6 weeks.
Fractionated conformal three-dimensional radiotherapy to a total dose of 60 Gy in 30 daily fractions of 2 Gy each was delivered. Pretreatment evaluation included a postoperative magnetic resonance imaging (MRI) brain scan as a baseline evaluation with at least one unidimensionally measurable lesion, and posttreatment evaluation required a follow-up MRI brain scan 1 month after the end of the treatment, then every 2 months to evaluate the response according to the Response Evaluation Criteria In Solid Tumors (RECIST).Citation11
Statistical analysis
Comparison of toxicity was done using Fisher’s exact test. OS and PFS were computed by the Kaplan–Meier methodCitation12 and compared by the log-rank testCitation13 and the Cox proportional-hazards model.Citation14 P-values were always two tailed, and significance was at the 0.05 level. The multivariate Cox modelCitation14 was used to study variation in OS and PFS according to major baseline characteristics (age, sex, performance status, tumor volume, and response to treatment). Statistical analyses were conducted using SPSS software, version 13.0 (IBM, Armonk, NY, USA).
Results
Between October 2009 and December 2012, 60 patients with histological confirmation of high-grade brain glioma who attended the Kasr Alainy Center of Radiation Oncology and Nuclear Medicine in Cairo University Hospital fulfilling the inclusion criteria were randomized across two treatment groups (30 patients in each arm). Baseline patient characteristics are shown in , while baseline disease characteristics are shown in .
Table 1 Patient characteristics
Table 2 Tumor characteristics
All patients completed the specified course of radiation therapy. The median duration of treatment in the whole group of patients was 46 days (range 42–56 days). There was no significant difference between the mean duration of treatment between the arms (P=0.97). Deterioration of consciousness level and convulsions were observed in four patients due to increased intracranial tension during the second week of radiotherapy. Dehydrating measures and adjustments of anticonvulsants were given for 2 days, and treatment was resumed.
Safety
According to the National Cancer Institute common toxicity criteria for adverse events version 3.0,Citation15 adverse events were reported in 42 of 60 patients (70%) who started treatment. The number of patients who developed toxicity of any grade was higher among arm I patients (26 of 30, 86.7%) compared to 16 of 30 (53.3%) in arm II (P=0.11). Commonest grade 1 or 2 toxicities encountered in both groups were malaise, in 46.7% of patients, followed by alopecia (20%), and nausea (10%). Grade 1 or 2 convulsions, decreased level of consciousness, headache, and tingling were witnessed in a small percentage of arm I patients but not in arm II. Finally, grade 3–4 convulsions and decreased level of consciousness were only seen in four patients, all of whom were from arm I. The main adverse events in patients randomized across the two treatment arms are summarized in .
Table 3 Details of grade 1–2 toxicity
Efficacy
After a median follow-up of 11 months (range 6–24 months), eight of 60 patients (13.3%) were alive with disease, and 52 of 60 patients (86.7%) had died from the disease (24 patients in arm I and 28 patients in arm II).
With regard to treatment response, radiological complete response was documented in six of 60 patients (10%): four of 30 patients (13.3%) in arm I, and two of 30 patients (6.7%) in arm II. Partial remission was achieved in eight of 30 patients (26.7%) in each arm of treatment, while 36 of 60 patients (60%) showed stationary disease ().
Table 4 Initial posttreatment response
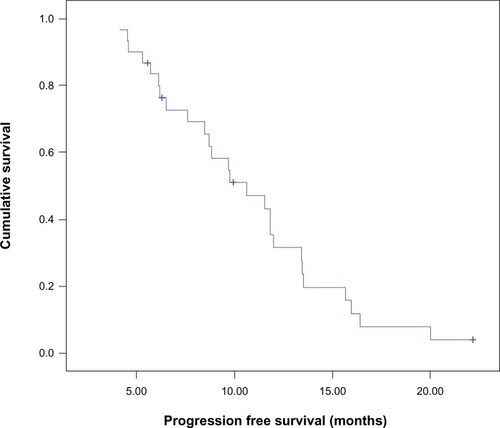
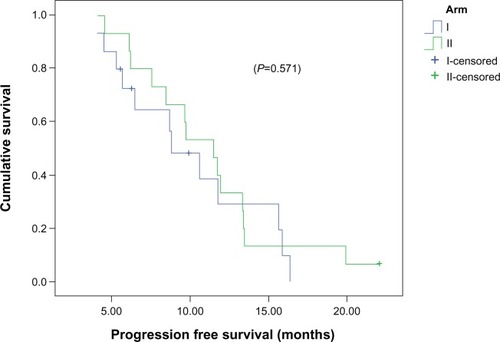
Median PFS for the entire study population was 10.6 months (95% confidence interval [CI] 7.3–14), and PFS at 12 months was 32% (). The median PFS between arm I (8.8 months, 95% CI 5.9–11.7) and arm II (11.5 months, 95% CI 8.9–14.2) was comparable (P=0.571), and PFS at 12 months was 32% and 30%, respectively ().
The impact of the different variables on PFS of the whole group of patients was evaluated. In univariate analysis, PFS was found to be longer in male patients (P<0.026), with residual tumor <10 cc either postsurgically or post-combined RT (P<0.043 and P<0.05, respectively).
The multivariate analysis on the entire patient population showed that male sex (P=0.006), postsurgical tumor volume <10 cc (P=0.01), and KPS >80% (P=0.02) were independently associated with improved PFS. The results of the univariate and multivariate analysis are summarized in .
Table 5 Progression-free survival (PFS) according to different variables in univariate and multivariate analyses
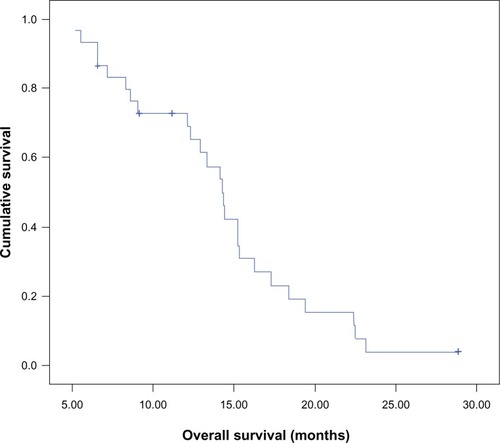
Median OS of the whole group of patients was 14.2 months (95% CI 13–15.5), and OS was 70% at 12 months and 25% at 18 months (). Median OS for patients in arm I was 12.3 months (95% CI 7.7–16.9), whereas that for patients in arm II was 14.3 months (95% CI 14–14.7), which was also comparable (P=0.83).
The univariate analysis showed that survival was significantly longer in male patients (P=0.038), either postsurgically or post-combined RT tumor residual were <10 cc (P=0.029 and P<0.03 respectively) were prognostic factors. On multivariate analysis, the previous significant prognostic factors that were detected in univariate analysis also showed significant prolonged overall survival (OAS), in addition to KPS >80% and presurgical tumor volume <30 cc ().
Table 6 Overall survival (OS) according to different variables in univariate and multivariate analysis
Discussion
For many years, differing radiotherapy schedules, doses, and techniques, and the addition of nitrosourea-based chemotherapy combinations were attempted to improve the dismal prognosis of patients with GBM.Citation3 In the late 1990s, TMZ seemed promising for the treatment of recurrent GBM.Citation16,Citation17 A pilot Phase II study showed that concomitant TMZ with conventionally fractionated radiotherapy followed by six cycles of the drug is feasible.Citation18
The final results of the EORTC–NCIC trial, published in 2009, showed that the benefits of adjuvant TMZ with radiotherapy were maintained throughout the 5 years of follow-up, with a 5-year survival rate of 9.8% versus 1.9% for those treated with radiation therapy alone. Median OS rates for RT/TMZ versus RT alone were 14.6 months and 12.1 months, respectively. PFS for patients receiving RT/TMZ compared with RT alone were 11.2% and 1.8% at 2 years, 6.0% and 1.3% at 3 years, 5.6% and 1.3% at 4 years, and 4.1% and 1.3% at 5 years.Citation8
Other trials also investigated whether continuous daily administration of TMZ during RT is necessary. They compared the results achieved with the historical standard dosing regimen (75 mg/m2 TMZ 7 days per week × 6 weeks) with TMZ 75 mg/m2/day × 5 days during only the first and last week of RT (total of 10 days). Median survival was comparable – 19 months and 21 months, respectively – with better neurological toxicity in the abbreviated-arm TMZ.Citation10 One question is the contribution of the concomitant and adjuvant drug doses, which is now being investigated in the EORTC Phase III trial on Concurrent and Adjuvant Temozolomide Chemotherapy in Non-1p/19q Deleted Anaplastic Glioma (CATNON). Our trial was not designed to answer that question, but rather to explore issues related to optimum TMZ radiosensitizer dosing. Therefore, our study was designed to randomize patients to receive RT concomitant with TMZ either daily or for 2 weeks (1st and last week of RT) without receiving postoperative adjuvant TMZ, due to economic problems and limited resources in our country.
Median PFS for the whole group of patients was 10.6 months (95% CI 9.1–12.5). There was no significant difference (P=0.571) in the median PFS between patients treated with RT and concomitant daily TMZ (8.8 months, 95% CI 5.9–11.7) and patients treated with RT and a concomitant abbreviated course of TMZ (11.5 months, 95% CI 8.9–14.2). The estimated median OS for the whole group of patients was 14.2 months (95% CI 12.1–16.4), which is comparable to data from the EORTC–NCIC trial of median survival: 14.6 months in the continuous daily radiosensitizer arm. In our study, median survival was not statistically different (P=0.83) between the schedules: patients treated with concomitant daily TMZ (12.3 months, 95% CI 7.7–16.9) and patients treated with a concomitant abbreviated course of TMZ (14.3 months, 95% CI 14–14.7).
In the EORTC–NCIC trial, treatment was generally well tolerated, with most notable toxicities being myelosuppression, thromboembolic events, fatigue, pneumonia, nausea, vomiting, rash, constipation, and arthralgias. The dose-limiting toxicity of TMZ was myelosuppression, with grade 3 or 4 hematologic toxic effects encountered in 14% of patients during adjuvant TMZ therapy.
In our study, the total number of patients who developed toxicity of any grade was 42 (70%). The number of patients who developed toxicity of any grade was higher among arm I patients (26, 86.7%) compared to 16 patients (53.3%) in arm II (P=0.11). Four patients in arm I developed at least one toxicity of grade 3 or 4 – two patients with decreased level of consciousness and two others with convulsions – while no patients in arm II developed grade 3 or 4 toxicity (P=0.224), demonstrating better tolerance for the abbreviated course of radiosensitizer TMZ.
In conclusion, a continuous daily and abbreviated course of TMZ in combination with RT exhibits comparable efficacy in terms of PFS and OS; however, tolerability was rather improved in the abbreviated course of TMZ radiosensitizer in GBM.
Disclosure
The authors report no conflicts of interest in this work.
References
- WenPYKesariSMalignant gliomas in adultsN Engl J Med200835949250718669428
- StummerWPichlmeierUMeinelTWiestlerODZanellaFReulenHJFluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trialLancet Oncol2006739240116648043
- LaperriereNZurawLCairncrossGRadiotherapy for newly diagnosed malignant glioma in adults: a systematic reviewRadiother Oncol20026425927312242114
- WalkerMDGreenSBByarDPRandomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgeryN Engl J Med1980303132313297001230
- TrivediRNAlmeidaKHFornsaglioJLSchamusSSobolRWThe role of base excision repair in the sensitivity and resistance to temozolomide-mediated cell deathCancer Res2005656394640016024643
- TentoriLGrazianiGPharmacological strategies to increase the anti-tumor activity of methylating agentsCurr Med Chem200291285130112052167
- StevensMENewlandsESFrom triazines and triazenes to temozolomideEur J Cancer199329A104510478499135
- StuppRHegiMEMasonWPEffects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trialLancet200910459466
- WickWEngelCCombsSENOA-08 randomized phase III trial of 1-week-on/1-week-off temozolomide versus involved-field radiotherapy in elderly (older than age 65) patients with newly diagnosed anaplastic astrocytoma or glioblastoma (Methusalem)J Clin Oncol201028SupplLBA2001
- D’AgostinoGBalducciMAnileCAn analysis of two different schedules of radiochemotherapy with concomitant temozolomide in high grade gliomasJ Clin Oncol200725Suppl203517513807
- TherassePArbuckSGEisenhauerEANew guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of CanadaJ Natl Cancer Inst20009220521610655437
- KaplanELMeierPNonparametric estimation from incomplete observationsJ Am Stat Assoc195853457481
- MantelNEvaluation of survival data and two new rank order statistics arising in its considerationCancer Chemother Rep1966501631705910392
- CoxDRRegression models and life tablesJ R Stat Soc Series B Stat Methodol197234187220
- Cancer Therapy Evaluation ProgramCommon terminology criteria for adverse events v3.0 (CTCAE)2006 Available from: http://www.eortc.be/services/doc/ctc/ctcaev3.pdfAccessed February 28, 2013
- NewlandsESStevensMFWedgeSRWheelhouseRTBrockCTemozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trialsCancer Treat Rev19972335619189180
- BrockCSNewlandsESWedgeSRPhase I trial of temozolomide using an extended continuous oral scheduleCancer Res199858436343679766665
- StuppRDietrichPYOstermann KraljevicSPromising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomideJ Clin Oncol2002201375138211870182