Abstract
Anaplastic lymphoma kinase (ALK) gene fusions occur in 3%–7% of non-small-cell lung cancer (NSCLC) cases. Ceritinib, a once-daily, oral ALK inhibitor, has activity against crizotinib-resistant and crizotinib-naïve NSCLC, including brain metastases. Ceritinib (Zykadia™) was granted accelerated approval by the US Food and Drug Administration in 2014 for treating crizotinib-resistant ALK-positive NSCLC. Adverse events (AEs), particularly gastrointestinal (GI) AEs, are commonly experienced at the recommended dose of 750 mg/d and ∼38% of patients require dose interruption or reduction for GI AEs. This case study details our experience with the use of proactive GI AE management regimens in patients treated with ceritinib (750 mg/d) across two study sites. Proactive Regimens A and B were implemented in patients with metastatic ALK-positive NSCLC treated with ceritinib to manage drug-related GI AEs. Regimen A comprised ondansetron and diphenoxylate/atropine or loperamide, taken 30 minutes prior to ceritinib dose. Regimen B included dicyclomine (taken with the first ceritinib dose), ondansetron (taken 30 minutes prior to ceritinib dose for the first seven doses), and loperamide (taken as needed with the onset of diarrhea). The proactive medications were tapered off depending on patient tolerability to ceritinib. Nine patient cases are presented. Starting Regimens A or B before the first dose of ceritinib, or as soon as GI symptoms were encountered, prevented the need for dose reduction due to GI toxicity in eight of the nine patients. Using these regimens, 78% of patients were able to remain on 750 mg/d fasting. Two patients received 23 months and 16 months of therapy and remain on ceritinib 750 mg/d and 600 mg/d, respectively. Although not currently recommended or implemented in clinical studies, based on the patients evaluated here, upfront or proactive treatment plans that address AEs early on can allow the majority of patients to remain on the approved 750 mg/d ceritinib dose.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Anaplastic lymphoma kinase (ALK) gene fusions and mutations are implicated in the pathogenesis of several human cancers.Citation1 The fusion of echinoderm microtubule-associated protein-like 4 (EML4) with ALK is the most frequent of known ALK rearrangements resulting in constitutive oncogenic ALK activation. ALK gene rearrangement occurs in 3%–7% of non-small-cell lung cancer (NSCLC) cases,Citation2,Citation3 with higher prevalence in younger patients with adenocarcinoma who are never- or light smokers.Citation4
The ALK protein is thus a therapeutic target for the treatment of ALK-rearranged (ALK-positive) NSCLC, and a number of ALK inhibitors are currently under clinical development. Crizotinib (XALKORI®), an oral first-in-class dual ALK and c-MET inhibitor, was approved by the US Food and Drug Administration (FDA) in 2011 to treat metastatic ALK-positive NSCLC in any line, having demonstrated clinical efficacy.Citation5 In clinical trials, crizotinib achieved an overall response rate (ORR) of 60% and a median progression-free survival (PFS) of 8–10 months.Citation6,Citation7 However, patients ultimately acquire resistance within 1–2 years of therapy, resulting in disease progression, thus limiting its long-term application.Citation8,Citation9
Next-generation ALK inhibitors that overcome crizotinib resistance have been developed. Ceritinib (ZYKADIA™) is a once-daily oral, small-molecule, ATP-competitive ALK inhibitor that is 20 times more potent against ALK than crizotinib in preclinical studies, with activity against common secondary ALK mutations that confer resistance to crizotinib.Citation10 Ceritinib was granted accelerated approval by the US FDA in 2014 for treating metastatic, ALK-positive NSCLC that has progressed on or is intolerant to crizotinib, based on response rate and duration of response.Citation11 In a Phase I study, 246 ALK inhibitor-treated and ALK inhibitor-naïve patients with NSCLC achieved ORRs of 56% and 72%, respectively, with a median duration of response of 9.7 months. Median PFS was 6.9 months in prior ALK inhibitor-treated patients and 18.4 months in ALK inhibitor-naïve patients.Citation12 Ceritinib dosed at 750 mg/d also demonstrated antitumor activity in patients with ALK-positive NSCLC, including brain metastases, with durable intracranial responses (overall intracranial response rate [OIRR]: 34.5%) observed in either previously untreated (OIRR: 60.0%) or crizotinib-treated patients (OIRR: 29.2%).Citation13
Ceritinib is recommended at a dose of 750 mg/d, administered orally on an empty stomach (not within 2 hours of a meal).Citation14 A food effect studyCitation14 in healthy volunteers found that dosing with a high-fat meal increases ceritinib systemic exposure (peak plasma levels) by 43%, compared with the fasted state. Adverse events (AEs) are commonly experienced at this dose level and ∼60% of patients initiating treatment at 750 mg/d require at least one dose reduction, with a median time to first dose reduction of 7 weeks. The majority of patients (96%; 14% severe cases) in the Phase I study experienced gastrointestinal (GI) AEs, including diarrhea, nausea, vomiting, and abdominal pain, when taking the recommended ceritinib dose. In clinical studies, GI AEs are one of the most common reasons for dose modification (38% of patients).Citation14
Current recommendations for the management of GI AEs are reactive and include 1) symptomatic treatment with antiemetic or antidiarrheal therapy or 2) treatment interruption and dose reduction in severe cases.Citation14 Maintaining the prescribed dose is important for preserving efficacious systemic drug exposure. During crizotinib therapy, most relapses in patients with ALK-positive NSCLC occur in the brainCitation15,Citation16; therefore, the dose of ceritinib – and consequent systemic and intracranial exposure – may affect the central nervous system (CNS) concentration, which is critical for the control of CNS metastases. As such, upfront proactive treatment regimens to address nausea (eg, ondansetron [Zofran]), diarrhea (eg, loperamide [Imodium]), and abdominal pain (eg, dicyclomine [Bentyl]) could be implemented in clinics to help ensure optimum ceritinib exposure and efficacy. Here, we report a case series of nine patients treated with ceritinib (750 mg/d) for whom proactive GI AE management strategies were implemented.
Patients and methods
All patients had metastatic ALK positive NSCLC and were participants in ceritinib clinical trials. The study protocols allowed investigators to use both reactive and proactive medications to help deal with the normal GI side effects (NCT01283516, NCT01947608, NCT01685060, and NCT01828112). All clinical studies involved were conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonization. All protocols were approved by relevant Institutional Review Boards and Ethics Committees. Written informed consent was obtained from all the patients before screening. All patients were initially dosed orally with ceritinib (750 mg/d) fasting. Patients were encouraged to maintain dosage at the same time of day for the duration of the study.
Proactive regimens
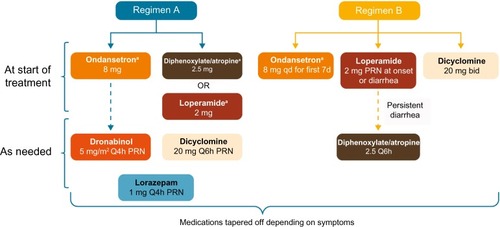
Between the two study sites, two proactive regimens were implemented in patients with metastatic ALK-positive NSCLC treated with ceritinib to manage drug-related GI AEs (). The use of these regimens was consistent with the protocol recommendations. Regimen A comprised ondansetron 8 mg, along with either diphenoxylate and atropine 2.5 mg or loperamide 2 mg, to be taken orally 30 minutes prior to the ceritinib dose. Additional agents were administered for persistent symptoms. Regimen B included dicyclomine 20 mg twice daily (to be taken orally starting with the first ceritinib dose), ondansetron 8 mg (to be taken orally 30 minutes prior to ceritinib dose for the first seven doses), and loperamide 2 mg (to be taken orally as needed with the onset of diarrhea; two tablets at onset and one tablet with every loose stool). If diarrhea persisted with loperamide, then diphenoxylate and atropine (Lomotil) 2.5 mg was prescribed, to be taken every 6 hours as needed. For both regimens, the proactive medications were tapered off, depending on clinically judged patient tolerability to ceritinib. For instance, for Regimen A, if patients reported lack of nausea but ongoing diarrhea, prophylactic antidiarrheal medication was continued, while antinausea medication use was changed from prophylactic to as-needed (PRN) basis. For Regimen B, patients remained on the prophylactic regimen for two cycles. After two cycles, the following were recommended:
Stop the dicyclomine at Week 1 of Cycle 3. If Grade 1 cramping occurs, use PRN, and restart dose if Grade 2 or greater.
Stop the ondansetron at Week 2 of Cycle 3. If Grade 1 nausea or vomiting is seen, use PRN, and restart dose if Grade 2 or greater.
Stop the loperamide at Week 3 of Cycle 3. If Grade 1 diarrhea occurs, use PRN, and restart if Grade 2 or greater. Safety and tolerability of ceritinib in patients were monitored according to the Common Terminology Criteria for Adverse Events v4.03.
Figure 1 Schematic representation of proactive regimens A and B.
Notes: aTaken 30 minutes before ceritinib dose. All agents to be taken orally. Patients were tapered off proactive treatment based on symptoms.
Abbreviations: bid, twice daily; d, dose; PRN, as needed; Q4h, every 4 hours; Q6h, every 6 hours; qd, once daily.
Result
Patient characteristics and treatment
A total of nine patients were included in this series. These patients were enrolled into ceritinib clinical trials across two sites and were treated by the authors, between October 2012 and March 2014. provides an overview of patient characteristics, treatment history, and proactive management strategies. Ceritinib was the second to fifth line of treatment for these patients. Eight of nine patients had previously received crizotinib and had discontinued due to disease progression or intolerance. All patients were dosed at 750 mg/d and provided upfront with a GI AE treatment regimen to proactively address the AEs that are experienced by the majority of patients taking ceritinib. Preventive regimens were also used to potentially avoid remote AE management for patients traveling long distances to clinical study sites. Patients 1–4 were started on the two-drug proactive Regimen A, while Patients 5–9 received the three-drug Regimen B. Preventive treatment was initiated at the start of ceritinib therapy.
Table 1 Patient characteristics
Treatment outcome
In eight of the nine patients starting Regimens A and B prior to initiation of ceritinib therapy, dose reductions due to GI toxicity were not required (). Patient 3 required dose reduction to 600 mg/d due to grade 3 transaminitis. Patient 4 discontinued therapy due to GI toxicities despite receiving proactive treatment. Patient 5 had a 1-week ceritinib dose interruption due to elevated creatinine levels but resumed treatment at the recommended 750 mg/d dose. In a Phase I study,Citation12 31% of patients experienced grade 1–2 constipation while on ceritinib 750 mg/d. Two patients on Regimen A experienced intermittent constipation, suspected to be due to the use of antidiarrheal medication. Proactive regimens were tapered off in two patients on Regimen A (Patients 1 and 3) and three patients on Regimen B (Patients 5, 7, and 8) as tolerability to ceritinib increased. Proactive treatment was continued in patients who experienced persistence of diarrhea/nausea in either regimen.
Using these proactive regimens, seven of the nine (78%) patients were able to remain on ceritinib 750 mg/d fasting, with a dose reduction rate for GI side effects of 12.5% (one out of the eight patients) when accounting for the one patient whose dose was reduced due to transaminitis. Patients 3 and 7 received 16 months and 23 months of therapy and remain on ceritinib to date at 600 mg/d and 750 mg/d, respectively. All other patients discontinued ceritinib due to progressive disease, comorbidity, or intolerance.
Discussion
Ceritinib is recommended at an oral, once-daily dose of 750 mg/d based on clinical efficacy results from the Phase I dose-escalation study.Citation11 The current prescribing information recommends ceritinib dose interruption and modification for patients experiencing intolerable nausea, vomiting, or diarrhea despite optimal antiemetic or antidiarrheal therapy.Citation14 In the case series presented herein, only one patient discontinued ceritinib treatment due to GI-related toxicity. A majority of patients were able to remain on ceritinib at 750 mg/d within the prescribing guidelines (ie, without food) and without the need for dose reductions or interruptions due to GI AEs.
Preclinical studies have shown that ceritinib crosses the blood–brain barrier in non-tumor-bearing rats, and in the Phase I study, patients with ALK-positive NSCLC and documented brain metastases who received ceritinib 750 mg/d had an intracranial antitumor response rate of 34.5%.Citation13
This study was limited by the relatively small number of patients treated at our institutions and a lack of comparator patients who did not receive the proactive regimen. Nonetheless, considering that 38% of patients on ceritinib require dose modification due to GI AEs,Citation14 on the basis of the nine patients evaluated here, implementation of proactive drug treatment plans, such as Regimens A and B, may significantly increase the number of patients who remain on 750 mg daily.
GI AEs experienced on tyrosine kinase inhibitor therapy can be distressing for patients and if persistent, may affect their quality of life.Citation17 In our experience, the initial 1–2 weeks of treatment are often the most difficult time for patients, and therefore addressing potential AEs at this stage may help prevent deviations from the recommended 750 mg daily dose. Adherence to therapy is a pertinent issue for cancer patients, and it is estimated that up to 80% of patients do not take oral antineoplastic agents according to their prescription.Citation18 These two regimens allow for the initiation of supportive care with the first dose and are then tapered off based upon symptoms, to minimize the duration of needed treatment. Patient education has been reported to increase patient adherence to oral anticancer agents.Citation18 Therefore, preparing patients and physicians to expect adverse reactions is valuable; dispensing the supportive medication prior to leaving the office and taking the first dose is essential; and presenting proactive AE treatment options upfront as part of a treatment package could help maximize patient drug exposure as they start a new therapy.
Acknowledgments
This study was supported by Novartis Pharmaceuticals. We thank the participating patients, their families, research coordinators, and nurses, as well as thanking Maria Alfaradhi from Articulate Science for providing editorial assistance.
Disclosure
CB has received institutional research support from Novartis Pharmaceuticals Corporation. CB and ESS have participated as advisory board members for Novartis Pharmaceuticals Corporation. The authors report no other conflicts of interest in this work.
References
- BarrecaALasorsaERieraLEuropean T-Cell Lymphoma Study GroupAnaplastic lymphoma kinase in human cancerJ Mol Endocrinol2011471R11R2321502284
- SodaMChoiYLEnomotoMIdentification of the transforming EML4-ALK fusion gene in non-small-cell lung cancerNature2007448715356156617625570
- WongDWLeungELSoKKUniversity of Hong Kong Lung Cancer Study GroupThe EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRASCancer200911581723173319170230
- ShawATSolomonBTargeting anaplastic lymphoma kinase in lung cancerClin Cancer Res20111782081208621288922
- Xalkori®New York, NYPfizer Labs, Pfizer Inc.; FDA-approved prescribing information; LAB-0441-702015 Available from: http://labeling.pfizer.com/showlabeling.aspx?id=676Accessed February 25, 2016
- ShawATKimDWNakagawaKCrizotinib versus chemotherapy in advanced ALK-positive lung cancerN Engl J Med2013368252385239423724913
- CamidgeDRBangYJKwakELActivity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 studyLancet Oncol201213101011101922954507
- KatayamaRShawATKhanTMMechanisms of acquired crizotinib resistance in ALK-rearranged lung cancersSci Transl Med20124120120ra17
- DoebeleRCPillingABAisnerDLMechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancerClin Cancer Res20121851472148222235099
- LiNMichellysPYKimSActivity of a potent and selective phase I ALK inhibitor LDK378 in naive and crizotinib-resistant preclinical tumor modelsPoster presented at: AACR-NCI-EORTC International Conference on Molecular Targets and Cancer TherapeuticsNovember 12–16 2011San Francisco, CA Abstract B232
- ShawATKimDWMehraRCeritinib in ALK-rearranged non-small-cell lung cancerN Engl J Med2014370131189119724670165
- FelipEKimDMehraREfficacy and safety of ceritinib in patients with advanced anaplastic lymphoma kinase (ALK)-rearranged (ALK+) non-small cell lung cancer (NSCLC): an update of ASCEND-1Poster presented at: European Society for Medical Oncology CongressSeptember 26–30 2014Madrid, Spain Abstract 1295P
- ShawATFelipEVansteenkisteJFEvaluation of ceritinib-treated patients (pts) with anaplastic lymphoma kinase rearranged (ALK+) non-small cell lung cancer (NSCLC) and brain metastases in the ASCEND-1 studyPoster presented at: European Society for Medical Oncology CongressSeptember 26–30 2014Madrid, Spain Abstract 1293P
- Novartis Pharmaceuticals Corporation: Zykadia™FDA-approved prescribing information; T2015-114/T2015-1152015 Available from: http://www.pharma.us.novartis.com/product/pi/pdf/zykadia.pdfAccessed February 10, 2016
- WeickhardtAJScheierBBurkeJMLocal ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancerJ Thorac Oncol20127121807181423154552
- ShawATYeapBYSolomonBJEffect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysisLancet Oncol201112111004101221933749
- CalifanoRTariqNComptonSExpert consensus on the management of adverse events from EGFR tyrosine kinase inhibitors in the UKDrugs201575121335134826187773
- PartridgeAHAvornJWangPSWinerEPAdherence to therapy with oral antineoplastic agentsJ Natl Cancer Inst200294965266111983753
