Abstract
Advanced or metastatic gastric cancer constitutes the majority of patients in clinical practice. In North America, about 70% of cases are advanced or metastatic when diagnosed, which is higher than the 50% reported in Japan. This difference in presentation is reflected in 5-year overall survival, which is about 20% in North America and 40%–60% in Japan. Despite numerous efforts of randomized studies on advanced gastric cancer, no globally accepted standard regimen has yet been established. Systemic chemotherapy provides palliation and prolongs survival, but the prognosis remains poor. Several monotherapies and combined regimens are currently available and vary around the world. Additionally, several molecular targeting agents are under evaluation in international randomized studies. Human epidermal growth factor receptor-2 (HER-2) is overexpressed or amplified in approximately 22% of patients with gastric cancer. Trastuzumab, a recombinant humanized anti-HER-2 monoclonal antibody, is the first biological therapy that has showed a survival improvement by nearly three months (reduced risk of death by 26%). Therefore, trastuzumab in combination with cisplatin is a reasonable treatment option for patients with advanced gastric cancer who are HER-2 positive. This paper will focus on trastuzumab, its chemical and pharmacological characteristics, and the relevant efficacy, safety, and tolerability studies.
Keywords:
Management issues in stomach cancer
Gastric cancer is a cause of considerable morbidity and mortality the second leading cause of cancer-related death, and the third most common malignancy worldwide.Citation1 In the US, it was estimated that there would be 21,000 new cases and 10,570 deaths from gastric cancer in 2010.Citation1 In Japan, there are more than 100,000 new cases diagnosed and 50,000 who die annually.Citation2 In Western countries, most newly diagnosed cases (73%) will present in the advanced stages when gastric cancer is incurable, having a median survival of less than 1 year. Although 52% of gastric cancer cases in Japan are diagnosed at an early stage, this fact may be related to mass screening.Citation3,Citation4
Fluoropyrimidines, platinums, taxanes, and irinotecan are considered the main active cytotoxic agents for advanced gastric cancer. Palliative chemotherapy for advanced or metastatic gastric cancer offers significant advantages in selected fit patients, including increased survival, symptom control, and quality of life, compared with best supportive care alone.Citation5–Citation9 Systematic review showed that the overall survival benefit of chemotherapy over best supportive care was six months (hazards ratio [HR] 0.39, 95% confidence interval [CI]: 0.28–0.52). A three-drug regimen including 5-flurouracil, anthracyclines, and cisplatin has showed the best survival results, with an HR of 0.83 (95% CI: 0.74–0.93) over single-agent 5-flurouracil-based chemotherapy. ECF (epirubicin + cisplatin + infusional 5-fluoruracil) response rates are 40%, with a median survival of 9.4 months and 40% of patients alive at 1 year.Citation10 When oxaliplatin is substituted for cisplatin, and capecitabine for 5-flurouracil (EOX), response rates are 48%, median survival 11.2 months, and 1-year survival 48%. Interestingly, thromboembolic events were significantly higher in cisplatin groups than in oxaliplatin groups (15.1 versus 7.6%, P < 0.001).Citation11
Docetaxel combined with cisplatin ± 5-fluorouracil (DCF) has better response rates, longer progression-free survival, and a small survival advantage compared with cisplatin + 5-fluorouracil (9.2 versus 8.6 months, P = 0.02).Citation12,Citation13 However, in patients older than 65 years, increased toxicity of neutropenic infection and diarrhea has been seen. Irinotecan is also an active drug for advanced gastric cancer, although no improvement in survival has been demonstrated in randomized trials.Citation14–Citation16 It may be a more appropriate choice than cisplatin + 5-fluorouracil, given its better tolerance. DCF and 5-fluorouracil plus irinotecan regimens have not been directly compared against ECF.
Tegafur, a 5-fluorouracil prodrug, is mainly used in Japan. Phase III trials have demonstrated that tegafur is not inferior to 5-fluorouracil in overall survival, and it is associated with higher response rates, longer progression-free survival, longer time to treatment failure, and longer nonhospitalized survival.Citation17 Tegafur combined with irinotecan was not significantly better compared with tegafur alone.Citation18 However, when it was combined with cisplatin, significantly longer survival was seen than for tegafur alone, with acceptable toxicities. This regimen is standard therapy for metastatic gastric cancer in Japan.Citation19
Although a large number of chemotherapy regimens have been proven in Phase III studies, there is no internationally accepted standard of care. Monotherapy with 5-fluorouracil or doublets with 5-fluorouracil and cisplatin, irinotecan, or an anthracycline, are reasonable options for patients who are not candidates for ECF. DCF may be recommended in very fit selected patients. Additionally, a preliminary updated meta-analysis suggested that chemotherapy combinations including irinotecan, oxaliplatin, docetaxel, or oral 5-fluouoracil prodrugs, are alternative treatment options to cisplatin/5-fluorouracil or cisplatin/5-fluorouracil/anthracycline-combinations, but do not provide significant advantages in overall survival.Citation20 Median time to progression after first-line chemotherapy for metastatic gastric cancer is typically 3–5 months. No second-line regimen has been established, and historically few patients are considered for second-line treatment (20%). However, recent data from a randomized trial showed that 39%–48% of patients in a sequence of chemotherapy including ECF followed by FOLFIRI (leucovorin + 5-fluorouracil + irinotecan) and the reverse sequence, received a second-line chemotherapy.Citation21 So far, there are only preliminary data from a Phase III study in 40 patients that compared irinotecan monotherapy versus best supportive care in second line, demonstrating that irinotecan significantly prolongs overall survival (by 50.5 days) and improves tumor-related symptoms.Citation22 Some Phase II studies has been published; taxanes and irinotecan are the most commonly used drugs in this setting, as monotherapy or in combined chemotherapy. Responses vary from 0% to 50%, and time to progression and overall survival has been reported to be 3–6 and 6–9 months, respectively. Predictors of response have been described, including performance status, locally advanced rather than metastatic disease, and previous response to first-line therapy.Citation23–Citation26
Molecular targeting agents, alone or in combination with chemotherapy, are being tested in the second-line setting.Citation27–Citation29 Despite the benefits of palliative chemotherapy, and the diversity of chemotherapy regimens, the prognosis of advanced gastric cancer remains poor, with a median overall survival of 7–10 months. An increased understanding of molecular pathways has provided novel targets to treat cancer patients. Several molecularly targeted agents are under evaluation in patients with advanced gastric cancer.Citation30,Citation31
Bevacizumab combined with chemotherapy (cisplatin and irinotecan; oxaliplatin and docetaxel or 5-fluorouracil; DCF) has shown promising results in Phase II studies including treated and untreated patients (response rates 63%–71%). However, the main concern arising from these trials is toxicity.Citation32–Citation35
A recent update of a Phase III trial including 774 patients with advanced gastric cancer who received combined chemotherapy (capecitabine/5-fluorouracil + cisplatin) with bevacizumab or placebo did not show significant differences in overall survival (12.1 versus 10.1 months, respectively), although there were significant improvements in progression-free survival and overall response rate, with an acceptable safety profile favoring the bevacizumab arm.
Sunitinib has demonstrated disease control rates of 40%.Citation36 Additionally, preliminary results showed encouraging response rates when sunitinib is combined with chemotherapy, such as tegafur, capecitabine + cisplatin, or capecitabine + oxaliplatin.Citation37–Citation39 Sorafenib in combination with docetaxel and cisplatin showed an encouraging efficacy profile, with tolerable toxicity (40% partial response rate).Citation40
Cetuximab showed poor response rates (5%) in pretreated patients.Citation41 However, in combination with 5-fluorouracil and oxaliplatin or irinotecan, a relative risk of 52%–65% was seen in previously untreated patients.Citation42–Citation44 Cetuximab in combination with docetaxel and cisplatin has a response rate of 42%.Citation45
Addition of matuzumab to EOX did not improve tumor response or survival in patients with advanced gastric cancer.Citation46 Most clinical trials using epidermal growth factor receptor tyrosine kinase inhibitors in gastric cancer have shown minimal efficacy. Erlotinib had a response rate of 10% in previously untreated patients,Citation47 and gefitinib had an 18% stable disease rate in previously treated patients.Citation48 Preliminary results with lapatinib showed a 7% and 20% partial response and stable disease rate, respectively, in untreated patients.Citation49 A preliminary report of a Phase II study showed that in combination with capecitabine, a partial response and stable disease were seen in 24% and 34%, respectively.Citation50 The EOX regimen with or without panitumumab (REAL-3 study) is ongoing,Citation51 with Phase I trials of the combination showing a response rate of 65%.Citation52
Pharmacology and pharmacokinetics
Human epidermal growth factor receptor (HER)-2/neu (c-erbB-2) is a transmembrane tyrosine kinase receptor, and a member of the HER family (HER-1, 2, 3, and 4). HER-2 functions as an oncogene. Gene amplification induces protein overexpression in cell membranes, and regulates signal transduction in cellular processes, including proliferation, differentiation, and cell survival.Citation53,Citation54 Aberrant HER-2 expression or function has been implicated in breast and gastric carcinogenesis, and is evident in other cancer types, including ovarian, salivary gland, and prostate and lung cancers.Citation53,Citation55
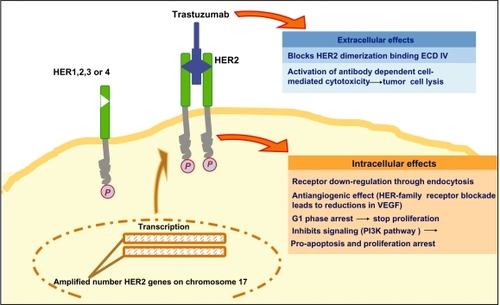
Trastuzumab is a recombinant humanized anti-HER-2 MAb (rhuMAb HER-2), directed against the HER-2 extracellular domain.Citation55 Several trastuzumab antitumor activities have been describedCitation53,Citation56 (see ):
Blocking of HER-2 receptor cleavage and inhibiting dimerization
Increasing receptor destruction by endocytosis
Inhibiting intracellular pathway signaling such as phosphoinositide 3-kinase signaling; may indirectly modulate proangiogenic and antiangiogenic factors, such as vascular endothelial growth factor production
Inducing G1 phase arrest by inducing the cyclin-dependent kinase inhibitor, p27 Kip1
Cytostatic and cytotoxic activity due to immune system recruitment by antibody-dependent cell-mediated cytotoxicity.
Figure 1 Antitumor effects of trastuzumab.
Abbreviations: ECD, extracellular domain; VEGF, vascular endothelial growth factor; PI3K, phosphoinositide 3-kinase.
The pharmacokinetics and pharmacodynamics of trastuzumab are not completely understood. Most studies have been done in breast cancer patients. Trastuzumab has a low systemic clearance (5.15 ± 2.45 mL/kg/day) and a low volume of distribution (44 mL/kg). The half-life may vary from 1.1 to 28 days with infusions of 10 mg and 500 mg, respectively. Half-lives of 2.7, 3.1, 8.8, and 10.4 days were found after single doses of 1, 2, 4, and 8 mg/kg, respectively, suggesting that, after trastuzumab receptors become saturated, total body clearance becomes linear. Doses of 4 mg/kg and 8 mg/kg are enough to achieve and maintain the serum minimum concentrations (10 μg/mL) needed for antiproliferative effects and antibody-dependent cell-mediated cytotoxicity.Citation57 Using a loading dose of 4 mg/kg followed by 2 mg/kg/week, trastuzumab achieves and maintains serum minimum concentrations greater than 20 μg/mL.Citation58
No drug interactions have been reported. No monoclonal antibody has been found to interact with major molecular pharmacokinetic determinants, such as enzymes, drug transporters, or orphan nuclear receptors. Using an initial dose of 4 mg/kg followed by 2 mg/kg/week, in combination with chemotherapy, the mean peak serum trastuzumab concentration is similar to that in patients receiving trastuzumab alone (101.0 μg/mL at week 8 and 53.4 μg/mL of mean minimum concentration).Citation58,Citation59 Elimination pathways are not yet defined, and the clinical relevance of trastuzumab kinetic variability is unknown. However, trastuzumab elimination seems to depend on high serum levels of circulating HER-2 extracellular domains, which can be cleaved from the surfaces of cancer cells by matrix metalloproteinase and released into the serum. Patients with high HER-2 extracellular domain levels tend to have a shorter trastuzumab half-life and lower minimum concentrations. Patients with four or more metastatic sites have faster clearance, independent of HER-2 extracellular domain levels. This is influenced little by trastuzumab exposure, and trastuzumab steady-state plasma levels are normally much higher than those of HER-2 extracellular domain levels.Citation58,Citation59
Efficacy studies in gastric cancer
Overexpression and amplification of HER-2/ErbB2 in gastric cancer vary widely, from 6% to 45%.Citation59–Citation64 The largest report screened 3883 patients with advanced gastric cancer from 24 countries, and found 22.1% HER-2-positivity rates in 3807 evaluated patients. Immunohistochemical and fluorescence in situ hybridization concordance was 87.3%. HER-2 positivity was higher in gastroesophageal junction cancers than in gastric cancers (33.2% versus 20.9%, P < 0.001), and higher in intestinal cancers than in diffuse/mixed cancers (32.2% versus 6.1%/20.4%, P < 0.001). No differences were seen between European and Asian countries.Citation65
Early studies in HER-2-positive gastric cancer cell lines, showed the growth inhibitory effects of trastuzumab. Additionally, when combined with doxorubicin, cisplatin, or paclitaxel, it demonstrated increased cytotoxicity, suggesting that a regimen based on these combinations could be considered for gastric cancerCitation66 (see ).
Table 1 Trastuzumab in gastric cancer: Preclinical and clinical trials
Cortés-Funes et alCitation67 have reported the preliminary results of a small Phase II study of 21 patients with advanced gastric cancer and HER-2 overexpression/amplification who received trastuzumab 8 mg/kg as a loading dose in the first cycle, followed by 6 mg/kg every 21 days, and cisplatin 75 mg/m2 every 21 days. These patients showed response rates of 35% and stable disease of 17%. The therapy was well tolerated, with no Grade 4 toxicity reported.
Similarly, Egamberdiev et al,Citation68 in a small preliminary study of 16 patients with HER-2-positive advanced gastric cancer treated with trastuzumab 6 mg/kg and chemotherapy (cisplatin, 5-fluorouracil, and leucovorin) every three weeks, found an objective response rate of 55% and a median overall survival of eight months.
The ToGA study is the first randomized, controlled Phase III trial to evaluate the efficacy and safety of trastuzumab in advanced HER-2-positive gastric cancer. Five hundred and ninety-four patients received either trastuzumab in combination with 5-fluorouracil/capecitabine and cisplatin or chemotherapy alone. The chemotherapy group (control arm) received capecitabine 1000 mg/m2 twice a day for 14 days or continuous infusion of 5-fluorouracil 800 mg/m2/day on days 1–5 of each cycle, plus cisplatin 80 mg/m2 on day 1 every three weeks. The experimental arm received the same chemotherapy plus trastuzumab 8 mg/kg on day 1 of the first cycle, followed by 6 mg/kg every three weeks. The final report at 18.6 months of median follow-up for the experimental arm and 17.1 months for the control arm showed better median survival with the combination (13.8 versus 11.1 months, P = 0.0046; HR 0.74, 95% CI: 0.60–0.91) with a 26% reduction in risk of death. Progression-free survival was 6.7 versus 5.5 months (P = 0.0002), and tumor response (including complete and partial response) was 47% versus 35% (P = 0.0017) with the addition of trastuzumab versus chemotherapy alone. Toxicity, including cardiac adverse events, was similar in both groups. More than 40% of patients in both groups received second-line treatment. Subgroup analysis for survival favored trastuzumab throughout (site of tumor, performance status, fluoropyrimidine used, histology, age, region, prior gastrectomy, and number of metastatic sites). Patients with high immunohistochemical positivity for HER-2 had a trend for better survival in the preplanned analysis (16 months) with trastuzumab compared with chemotherapy alone (11.8 months).Citation69 This finding suggests that levels of HER2 protein expression predict response to trastuzumab, similar to what has been described in metastatic breast cancer. Therefore, patients with advanced gastric cancer and these tumor characteristics should be offered trastuzumab plus chemotherapy as a treatment option.
Safety and tolerability
The most common adverse events with trastuzumab are infusion-related reactions (fever, rigors, chills, nausea, dyspnea, and hypotension), present in about 40% of patients with the first dose and in 5% with subsequent doses.Citation70 Myelosuppression, nausea, and vomiting are rare, and alopecia has not been reported with monotherapy. However, quantifying the contribution of trastuzumab to these side effects is difficult when it is given in combination with chemotherapy.Citation71 In the ToGA trial, serious adverse events were reported in 32% of patients treated with trastuzumab plus chemotherapy and 28% in the chemotherapy alone group. Treatment-related mortality was 3% and 1% in the experimental and control arm, respectively.
Special attention has focused on the cardiotoxicity of trastuzumab. Sporadic cases of congestive heart failure were reported in the early trials. However, an association between impairment of left ventricular ejection fraction and trastuzumab was more evident when given in combination with chemotherapy. The pivotal study in breast cancer showed cardiac toxicity in 27% of patients when trastuzumab was combined with anthracyclines, in 13% when it was combined with paclitaxel, and in 5% with trastuzumab alone.Citation71 The ToGA trial reported cardiac event rates of 6%.Citation70
Postmarketing surveillance for trastuzumab has reported that 62 of 25,000 (0.002%) patients have had serious adverse events (hypersensitivity reactions, infusion-related reactions, and pulmonary events). Adult respiratory distress syndrome, anaphylaxis, and death within 24 hours of a trastuzumab infusion were reported. Fatal events occurred mostly in patients with pre-existing pulmonary dysfunction, so these patients should be treated with caution, and trastuzumab should be discontinued if severe infusion-related reactions occur.Citation58,Citation72 A Phase IV trial is currently underway to evaluate the efficacy and safety of trastuzumab in patients with advanced HER-2 gastric cancer.
Patient-focused perspectives
Ideally, any gain in survival should also be accompanied by an improvement in or at least stable quality of life. Assessment of quality of life with trastuzumab therapy has been studied in breast cancer patients, with favorable results. There are no available data in patients with gastric cancer as yet. Osoba et alCitation73 found that patients treated with trastuzumab plus chemotherapy had significantly better improvement in quality of life (51%) than patients treated with chemotherapy alone (36%).
More recently, Rugo et alCitation74 demonstrated that patients treated with chemotherapy and trastuzumab versus chemotherapy alone had more improvement in quality of life (51% versus 36%, respectively). Improved physical, role functioning, and fatigue were also seen in the combined therapy arm.
Discussion
Gastric cancer is an aggressive disease with a high mortality rate, and despite recent progress in diagnosis, surgical techniques, chemotherapy, and radiotherapy, the prognosis remains poor. Advanced or metastatic gastric cancer constitutes the majority of patients in Western clinical practice. Chemotherapy has been considered the standard, with the significant advantages of increased survival, symptom control, and quality of life, compared with best supportive care alone. Trastuzumab is the first monoclonal antibody that has been shown to prolong life in patients with a malignant epithelial condition. In HER-2 breast cancer patients, its impact has been considerable. For advanced gastric cancer patients, trastuzumab is the first biological therapy that has showed a survival benefit. The ToGA trial included patients selected for treatment according to molecular profile. Encouragingly, trastuzumab in combination with chemotherapy showed a median survival of 13.8 months. Trastuzumab is a reasonable treatment option for patients with HER-2-positive advanced gastric cancer, although only approximately 20% of patients would be potential candidates. Some patients with HER-2-positive disease demonstrated primary or secondary resistance. Mechanisms of resistance and potential strategies to overcome these have been extensively researched in breast cancer, including loss of phosphatase and tensin homolog protein, activating mutations in the gene encoding phosphatidylinositol kinase-3, and increased signaling through other receptors (epithelial growth factor receptor and insulin-like growth factor-1 receptor). Lapatinib, the dual HER-2/epithelial growth factor receptor inhibitor, prolongs time to progression in patients with trastuzumab-resistant HER-2-positive breast cancer,Citation75,Citation76 and warrants evaluation in trastuzumab-resistant HER-2-positive gastric cancer.
Two Phase II trials, one combining chemotherapy (capecitabine and oxaliplatin) and targeted therapy (bevacizumab and trastuzumab), and the other with trastuzumab in combination with tegafur and cisplatin in advanced gastric cancer are planned. Additionally, two Phase II studies evaluating lapatinib in combination with capecitabine and weekly paclitaxel in first-line and second-line settings, respectively, in advanced gastric cancer are open to recruitment.
Further research is needed to evaluate the efficacy of trastuzumab as monotherapy, maintenance treatment, and second-line therapy for advanced gastric cancer. Moreover, trastuzumab should be integrated into curative treatment trials for patients with gastric cancer, such as perioperative or postoperative therapy.
With a better understanding of gastric cancer epidemiology and an ability to categorize it into distinct clinical and pathologic entities, improvement in gastric cancer therapy is expected. Moreover, by improving our knowledge of gastric cancer biology and signaling pathways, integration of targeted therapies has become possible and promising. Better selection of patients for a particular therapy will significantly improve treatment paradigms for this deadly disease, with the possibility of also improving patient survival. Several clinical trials with targeted therapies, including bevacizumab, cetuximab, panitumumab, and lapatinib in combination with conventional chemotherapy regimens, are currently ongoing, which may help to increase our armamentarium against gastric cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
- American Cancer SocietyCancer Facts and Figures 2010Atlanta, GAAmerican Cancer Society2010
- SuganoKGastric cancer: Pathogenesis, screening, and treatmentGastrointest Endosc Clin N Am200818351352218674700
- InoueMTsuganeSEpidemiology of gastric cancer in JapanPostgrad Med J20058195741942415998815
- FoukakisTLundellLGubanskiMAdvances in the treatment of patients with gastric adenocarcinomaActa Oncol200746327728517450463
- MuradAMSantiagoFFPetroianuAModified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancerCancer199372137418508427
- PyrhönenSKuitunenTNyandotoPRandomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancerBr J Cancer19957115875917533517
- GlimeliusBEkstromKHoffmanKRandomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancerAnn Oncol1997821631689093725
- JanungerKGHafströmLGlimeliusBChemotherapy in gastric cancer: A review and updated meta-analysisEur J Surg20021681159760812699095
- WagnerADGrotheWHaertingJChemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate dataJ Clin Oncol200624182903290916782930
- RossPNicolsonMCunninghamDProspective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancerJ Clin Oncol20022081996200411956258
- CunninghamDStarlingNRaoSUpper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United KingdomCapecitabine and oxaliplatin for advanced esophagogastric cancerN Engl J Med20083581364618172173
- Van CutsemEMoiseyenkoVMTjulandinSPhase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A Report of the V325 Study GroupJ Clin Oncol200624314991499717075117
- AjaniJAMoiseyenkoVMTjulandinSClinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: The V-325 Study GroupJ Clin Oncol200725223205320917664467
- BoucheORaoulJLBonnetainFRandomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: A Federation Francophone de Cancerologie Digestive Group Study – FFCD 9803J Clin Oncol200422214319432815514373
- MoehlerMEimermacherASieblerJRandomized phase II evaluation of irinotecan plus high-dose 5-fluorouracil and leucovorin (ILF) versus 5-fluorouracil, leucovorin, and etoposide (ELF) in untreated metastatic gastric cancerBr J Cancer200592122122212815942629
- DankMZaluskiJValvereVRandomized phase III trial of irinotecan (CPT 11) 5-FU/folinic acid (FA) vs CDDP–5-FU in first line advanced gastric cancer patientsJ Clin Oncol200523Suppl 16S4003
- BokuNYamamotoSShiraoKRandomized phase III study of 5-fluorouracil (5-FU) alone versus combination of irinotecan and cisplatin (CP) versus S-1 alone in advanced gastric cancer (JCOG9912)J Clin Oncol200725Suppl 18 SLBA4513T.
- ImamuraHIIishiHTsuburayaARandomized phase III study of irinotecan plus S-1 (IRIS) versus S-1 alone as first-line treatment for advanced gastric cancer (GC0301/TOP-002)Poster A5 presented at the ASCO Gastrointestinal Cancers SymposiumSan Francisco, CAJanuary 15–17, 2008
- KoizumiWNaraharaHHaraTS-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trialLancet Oncol20089321522118282805
- WagnerADUnerzagtSGrotheWNovel chemotherapy combinations in advanced gastric cancer: An updated meta-analysisAnn Oncol201021Suppl 8S740
- GuimbaudRLouvetCBonnetainFFinal results of the intergroup FFCD-GERCOR-FNCLCC 03-07 phase III study comparing two sequences of chemotherapy in advanced gastric cancersAnn Oncol201021Suppl 8S8010
- Thuss-PatiencePCKretzschmarADeistTIrinotecan versus best supportive care (BSC) as second-line therapy in gastric cancer: A randomized phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO)J Clin Oncol200927Suppl 5S4540
- ShimoyamaRYasuiHBokuNWeekly paclitaxel for heavily treated advanced or recurrent gastric cancer refractory to fluorouracil, irinotecan, and cisplatinGastric Cancer200912420621120047125
- TakahariDShimadaYTakeshitaSSecond-line chemotherapy with irinotecan plus cisplatin after the failure of S-1 monotherapy for advanced gastric cancerGastric Cancer201013318619020820988
- WilsonDHillerLGehJIReview of second-line chemotherapy for advanced gastric adenocarcinomaClin Oncol (R Coll Radiol)2005172819015830569
- BokuNChemotherapy for metastatic gastric cancer in JapanInt J Clin Oncol200813648348719093174
- BangYJKangYKKangWKPhase II study of sunitinib as second-line treatment for advanced gastric cancerInvest New Drugs2010512 [Epub ahead of print].
- YoonDHRyu1MLeeJPhase II study of everolimus in patients with advanced gastric cancer refractory to chemotherapy including fluoropyrimidine and platinumAnn Oncol201021Suppl 8S725
- TebbuttNCSourjinaTStricklandAHATTAX2: Docetaxel plus cetuximab as second-line treatment for docetaxel refractory oesophago-gastric cancer – final results of a multicentre phase II trial by the AGITGJ Clin Oncol200826Suppl 20S15554
- ArkenauHTGastric cancer in the era of molecularly targeted agents: current drug development strategiesJ Cancer Res Clin Oncol2009135285586619363621
- OhtsuAChemotherapy for metastatic gastric cancer: Past, present, and futureJ Gastroenterol200843425626418458840
- ShahMARamanathanRKIlsonDHMulticenter phase II study of irinotecan, cisplatin and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinomaJ Clin Oncol200624335201520617114652
- EnzingerPCFidiasPMeyerhardtJPhase II study of bevacizumab and docetaxel in metastatic esophageal and gastric cancerPoster A68 presented at the ASCO Gastrointestinal Cancers SymposiumSan Francisco, CAJanuary 26–28, 2006
- CohenuramMKLacyJFOLFOX6 and bevacizumab (FOLFOX6/B) for metastatic esophageal (E), gastroesophageal (GE), and gastric (G) adenocarcinoma: A single institution’s initial clinical experiencePoster A74 presented at the ASCO Gastrointestinal Cancers SymposiumSan Francisco, CAJanuary 15–17, 2008
- HammadNPhilipPAShieldsAFA phase II study of bevacizumab, docetaxel, and oxaliplatin in gastric and gastroesophageal junction (GEJ) cancerPoster A30 presented at the ASCO Gastrointestinal Cancers SymposiumSan Francisco, CAJanuary 15–17, 2008
- BangYJKangYKangWSunitinib as second line treatment for advanced gastric cancer: Preliminary results from a phase II studyJ Clin Oncol200725Suppl 18S4603
- ParkSRLeeKOhDSunitinib (su) with cisplatin (p) and capecitabine (x) or oxaliplatin (o) and x (xelox) in advanced gastric cancer (gc) – a phase I, dose-finding studyAnn Oncol201021Suppl 8S815
- MuroKMiyataYLiMWatanabeKBokuNCisplatin (P) in patients (pts) with advanced or metastatic gastric cancer (CG)Ann Oncol201021Suppl 8S816
- Gomez-MartinCGil-MartinMMontagutCA phase I, dose-finding study of sunitinib (su) in combination with cisplatin (p) and 5-fluorouracil (5-fu) in patients (pts) with advanced gastric cancer (gc)Ann Oncol201021Suppl 8S818
- SunWPowellMEO’DwyerPPhase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203J Clin Oncol201028182947295120458043
- SteinAAl-BatranSEArnoldDCetuximab with irinotecan as salvage therapy in heavily pretreated patients with metastatic gastric cancerPoster A47 presented at the ASCO Gastrointestinal Cancers SymposiumSan Francisco, CAJanuary 15–17, 2008
- PintoCDi FabioFSienaSPhase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study)Ann Oncol200718351051717164226
- LordickFLuberBLorenzenSCetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: A phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO)Br J Cancer2010102350050520068568
- KimCLeeJLRyuMHA prospective phase II study of cetuximab in combination with XELOX (capecitabine and oxaliplatin) in patients with metastatic and/or recurrent advanced gastric cancerInvest New Drugs2009129 [Epub ahead of print].
- PintoCDi FabioFBaroneCPhase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study)Br J Cancer200910181261126819773760
- RaoSStarlingNCunninghamDMatuzumab plus epirubicin, cisplatin and capecitabine (ECX) compared with epirubicin, cisplatin and capecitabine alone as first-line treatment in patients with advanced oesophago-gastric cancer: A randomised, multicentre open-label phase II studyAnn Oncol201021112213221920497967
- DragovichTMcCoySFenoglio-PreiserCSWOG 0127 phase II trial of erlotinib in GEJ and gastric adenocarcinomaJ Clin Oncol200624304922492717050876
- DoiTKoizumiWSienaSEfficacy, tolerability and pharmacokinetics of gefitinib (ZD1839) in pretreated patients with metastatic gastric cancerProc Am Soc Clin Oncol200322 Abstr 1036.
- IqbalSGoldmanBLenzHJA phase II SWOG study of GW572016 (lapatinib) as first line therapy in patients (pts) with advanced or metastatic gastric cancerJ Clin Oncol200725Suppl 8S4621
- LenzHZhangWKemnerAMLapatinib 1 capecitabine in advanced gastric cancer: An open-label phase II study of non ERBB2-targeted diseaseAnn Oncol201021Suppl 8S817
- OkinesAFAshleySECunninghamDEpirubicin, oxaliplatin, and capecitabine with or without panitumumab for advanced esophagogastric cancer: Dose-finding study for the prospective multicenter, randomized, phase II/III REAL-3 trialJ Clin Oncol201028253945395020679619
- RaoSStarlingNCunninghamDPhase I study of epirubicin, cisplatin and capecitabine plus matuzumab in previously untreated patients with advanced oesophagogastric cancerBr J Cancer200899686887419238629
- BaselgaJSwainSMNovel anticancer targets: Revisiting ERBB2 and discovering ERB3Nat Rev Cancer20099746347519536107
- HallaPSCameronDACurrent perspective – trastuzumabEur J Cancer2009451121819042123
- CarterPPrestaLGormanCMHumanization of an anti-p185HER2 antibody for human cancer therapyProc Natl Acad Sci U S A19928910428542891350088
- ValabregaGMontemurroFAgliettaMTrastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancerAnn Oncol200718697798417229773
- TreishISchwartzRLindleyCPharmacology and therapeutic use of trastuzumab in breast cancerAm J Health Syst Pharm200057222063207611098307
- BrunoRWashingtonCBLuJFPopulation pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancerCancer Chemother Pharmacol200556436136915868146
- MarxAHTharunLMuthJHER-2 amplification is highly homogenous in gastric cancerHum Pathol200940676977719269014
- Barros-SilvaJDLeitãoDAfonsoLAssociation of ERBB2 gene status with histopathological parameters and disease-specific survival in gastric carcinoma patientsBr J Cancer2009100348749319156142
- KimMAJungEJLeeHSEvaluation of HER-2 gene status in gastric carcinoma using immunohistochemistry, fluorescence in situ hybridization, and real-time quantitative polymerase chain reactionHum Pathol20073891386139317555797
- TannerMHollmenMJunttilaTTAmplification of HER-2 in gastric carcinoma: Association with topoisomerase II alpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumabAnn Oncol200516227327815668283
- YanoTDoiTOhtsuAComparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancerOncol Rep2006151657116328035
- YuGZChenYWangJJOverexpression of Grb2/HER2 signaling in Chinese gastric cancer: their relationship with clinicopathological parameters and prognostic significanceJ Cancer Res Clin Oncol2009135101331133919337752
- BangYChungHXuJPathological features of advanced gastric cancer (GC): Relationship to human epidermal growth factor receptor 2 (HER2) positivity in the global screening programme of the ToGA trialJ Clin Oncol200927Suppl 15S4556
- GongSJJinCJRhaSYGrowth inhibitory effects of trastuzumab and chemotherapeutic drugs in gastric cancer cell linesCancer Lett2004214221522415363548
- Cortés-FunesHRiveraFAlésIPhase II of trastuzumab and cisplatin in patients (pts) with advanced gastric cancer (AGC) with HER2/neu overexpression/amplificationJ Clin Oncol200725Suppl 18S4613
- EgamberdievDMDjuraevMDTuydjanovaKNematovONOur experience in the use of trastuzumab in patients with advanced stomach cancerAnn Oncol201021Suppl 8S839
- Van CutsemEKangYChungHToGA Trial InvestigatorsTrastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomized controlled trialLancet2010376974268769720728210
- HudisCATrastuzumab – mechanism of action and use in clinical practiceN Engl J Med20073571395117611206
- SeidmanAHudisCPierriMKCardiac dysfunction in the trastuzumab clinical trials experienceJ Clin Oncol20022051215122111870163
- GonzálezVSalgueiroEJimenoFJPost-marketing safety of antineoplasic MAb: Rituximab and trastuzumabPharmacoepidemiol Drug Saf200817771472118340626
- OsobaDSlamonDJBurchmoreMMurphyMEffects on quality of life of combined trastuzumab and chemotherapy in women with metastatic breast cancerJ Clin Oncol200220143106311312118024
- RugoHBrammerMZhangFLallaDEffect of trastuzumab on health-related quality of life in patients with HER2-positive metastatic breast cancer: Data from three clinical trialsClin Breast Cancer201010428829320705561
- CameronDCaseyMPressMA phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analysesBreast Cancer Res Treat2008112353354318188694
- GeyerCEForsterJLindquistDLapatinib plus capecitabine for HER2-positive advanced breast cancerNew Engl J Med2006355262733274317192538