Abstract
The use of third-generation aromatase inhibitors (AIs), such as anastrozole and letrozole, as initial adjuvant hormonal therapy in postmenopausal women (PMW) with hormone receptor-positive (HR+) breast cancer offers a significant benefit over tamoxifen for reducing recurrence risk. Clinical studies, including the Arimidex Tamoxifen Alone or in Combination (ATAC) and the Breast International Group (BIG) 1–98 trials, have proven that both anastrozole and letrozole are, respectively, superior to tamoxifen in improving disease-free survival. Although differing in design, objectives, and follow-up time, these trials offer some insight into the comparative clinical efficacy of these two nonsteroidal AIs. In particular, results from BIG 1–98 show that letrozole significantly reduces early distant metastatic (DM) events, which constitute the majority of early recurrence events. Subsequently, there is a beneficial overall survival effect emerging in the trial, whereas survival is unchanged with anastrozole after 100 months of follow-up in ATAC. Significant differences in the potency of these two drugs, vis-à-vis their degree of aromatase inhibition, have been observed in comparative trials and show that letrozole causes a more complete suppression of estrogen levels than does anastrozole. Whether this difference in potency is relevant to reductions in DM events during adjuvant therapy remains unclear. The Femara Anastrozole Clinical Evaluation trial is addressing this issue in a more unequivocal manner by comparing initial adjuvant treatment with anastrozole or letrozole in a population of breast cancer patients at high risk of recurrence: PMW with HR+ disease and axillary lymph node involvement.
Introduction
Adjuvant hormonal therapy remains an integral component of treatment for patients with hormone receptor-positive (HR+) early breast cancer, and antagonism of estrogen (E2) has been the foundation of treatments to reduce the risk of breast cancer recurrence. Most notable in this respect is the selective estrogen receptor (ER) modulator tamoxifen, which has been in use since the 1970s and has been proven to significantly reduce both breast cancer recurrence and death from breast cancer in patients with HR+, successfully resected disease.Citation1 Tamoxifen acts via a competitive inhibition of E2 binding to the ER, thereby inhibiting the proliferative action of E2 in breast cancer cells. The drug also may act as an estrogen agonist in selected tissues such as the endometrium, and has been associated with a number of adverse effects, including uterine cancer and thromboembolic events.Citation2,Citation3 In postmenopausal women (PMW), although ovarian E2 production has ceased, significant plasma levels of E2 may still exist via peripheral conversion of androgens to E2, a process entirely dependent on the activity of the enzyme aromatase.Citation4,Citation5 Therefore, aromatase has been a target of breast cancer therapy for PMW, and three generations of aromatase inhibitors (AIs) have been developed over the years.Citation4–Citation6 AIs are characterized into two types: steroidal aromatase inactivators, which bind irreversibly to the androstenedione binding site of aromatase, and nonsteroidal AIs, which bind in a reversible fashion to the heme component of aromatase.Citation4–Citation6 First-generation non-steroidal AIs such as aminoglutethimide became available as early as the 1970s but were of limited use because of their nonselectivity and corresponding side-effect profiles, which included adrenal insufficiency, nausea, rash, somnolence, and blood dyscrasias.Citation5,Citation6 The second-generation nonsteroidal drug fadrozole also was suboptimal because of its effects on adrenal and mineralocorticoid hormone suppression.Citation5 The third-generation nonsteroidal AIs anastrozole and letrozole, as well as the steroidal aromatase inactivator exemestane, were approved for clinical use in the 1990s. All three of these drugs are highly selective for aromatase and show good oral bioavailability.Citation4,Citation5
In major clinical studies comparing initial adjuvant therapy between the third-generation AIs anastrozole or letrozole with tamoxifen, a significant benefit of AI treatment in reducing disease recurrence and improving disease-free survival (DFS) has been observed.Citation7–Citation12 Another trial has examined the efficacy of a switch to exemestane after 2 to 3 years of prior treatment with tamoxifen relative to continued tamoxifen treatment (switch adjuvant therapy); this trial also showed a benefit of AI therapy versus continued tamoxifen.Citation13 Yet another trial has examined the use of letrozole relative to placebo after about 5 years of tamoxifen therapy (extended adjuvant therapy).Citation14 These pivotal trials have led to the approval of AIs for use in these different treatment settings (ie, initial, switch, and extended adjuvant therapy). The nonsteroidal AIs anastrozole and letrozole, in particular, have been approved for use in the initial adjuvant setting and are significantly more effective than tamoxifen in improving DFS. Both drugs are also well tolerated, with predictable adverse event profiles that closely mimic changes associated with menopause, such as bone loss and arthralgias, but with lower incidences of other serious events, such as thromboembolic and gynecologic events, compared with tamoxifen.Citation8–Citation10 Accordingly, it has been generally assumed that these two nonsteroidal AIs are largely interchangeable with regard to efficacy and tolerability relative to tamoxifen; however, evidence suggests greater potency with letrozole compared with anastrozole.Citation15,Citation16 This review discusses the evidence for a greater potency of letrozole in aromatase inhibition, the apparent clinical correlates of this inhibition as gleaned from pivotal trials, and the design and undertaking of the Femara Anastrozole Clinical Evaluation (FACE) trial, specifically designed to resolve this issue unequivocally.
Initial adjuvant therapy: clinical evidence for efficacy differences between anastrozole and letrozole
The Arimidex Tamoxifen Alone or in Combination (ATAC) trial
The ATAC trial was originally designed to determine the efficacy and safety of initial adjuvant anastrozole (n = 3125), tamoxifen (n = 3116), or the two agents in combination (n = 3125) in PMW with early breast cancer.Citation17 The trial was funded and implemented by the manufacturer of anastrozole. At the earliest analysis (median 33-month follow-up), it was determined that the combination arm was not significantly different from tamoxifen alone, and the arm was discontinued with no further follow-up of this population of patients (n = 3125). DFS was defined in the trial as the time to local or distant recurrence, a new primary breast cancer, or death from any cause.Citation17 Importantly, when the ATAC trial was initiated, hormone receptor-negative (HR−) or HR-unknown patients were not excluded from the trial; thus, only about 84% of the trial population was HR+ (n = 5216). In this clinically important population, the hazard ratio (HR) for DFS was significantly greater in the anastrozole group (HR 0.78; 95% confidence interval [CI]: 0.65–0.93; P = 0.002).Citation17 At this first follow-up, a sufficient number of distant metastatic (DM) events had not occurred (n = 615 of 704 required); thus, the incidence of DM recurrence was not reported. Contralateral breast cancer (CLBC), however, was significantly reduced in the HR+ population (odds ratio [OR] 0.29; 95% CI: 0.13–0.64; P = 0.002).Citation17 The difference in deaths between anastrozole and tamoxifen was small (n = 200 vs 203 deaths, respectively), and thus an overall survival (OS) HR was not reported.
Subsequent analysis of the ATAC trial at a median 68-month (5.7 years) follow-up confirmed the earlier findings, with a significant reduction in recurrence events in HR+ patients ().Citation8 In this first analysis of DM events, a total of 699 had occurred (n = 324 anastrozole vs 375 tamoxifen), and there was a significant improvement in the endpoint of time to distant recurrence (TTDR) with anastrozole relative to tamoxifen in the intent-to-treat (ITT) population (HR 0.86; 95% CI: 0.74–0.99; P = 0.04); however, in the HR+ population, TTDR was not significantly improved ().Citation8 Distant DFS (DDFS) was not a defined endpoint in the ATAC trial; however, as reported in the package insert, the difference in DDFS events was 370 and 394 in the respective treatment groups in the HR+ population (HR 0.93; 95% CI: 0.80–1.07).Citation18 As with the 33-month analysis, significant improvement in CLBC was observed in the HR+ population. There were 831 deaths in the study at this early follow-up, and the HR for OS was not statistically different in either the ITT (HR 0.97; 95% CI: 0.85–1.12) or HR+ () populations, despite a 12% reduction in breast cancer-related deaths (HR 0.88; 95% CI: 0.74–1.05; P = 0.2).Citation8,Citation18
Table 1 A comparison of key efficacy outcomes in the ATAC and BIG 1–98 trialsCitation7–Citation12
Results of the ATAC trial have been reported up to the 100-month (8.3 year) follow-up, at which time patients had received a mean of 4.11 and 3.97 years of anastrozole and tamoxifen treatment, respectively, with a high rate of compliance with randomized treatment (88% and 87% of allocated treatment received before recurrence, respectively).Citation7 In the HR+ population, significant improvement was observed in the endpoints of DFS (), time to recurrence (TTR; HR 0.76; 95% CI: 0.67–0.87; P = 0.0001), and CLBC with anastrozole relative to tamoxifen (HR 0.60; 95% CI: 0.42–0.85; P = 0.004).Citation7 Of note, there were 52 fewer TTDR events with anastrozole relative to tamoxifen at this time point, and this reached significance in the HR+ population (305 vs 357, ). All cause death (472 vs 477 events, ), death after recurrence (245 vs 269 events, HR 0.90; P = 0.2), and deaths without recurrence (227 vs 208 events, HR 1.05; P = 0.6) were not significantly different in the HR+ population.
The Breast International Group (BIG) 1–98 trial
An important distinction between the BIG 1–98 trial and the ATAC trial is that BIG 1–98 was conducted in an independent manner by the International Breast Cancer Study Group, a large cooperative group responsible for study design, data collection, management, medical review, data analysis, and reporting.Citation9 The unique design of BIG 1–98 allowed for the assessment of two adjuvant treatment strategies. The first compared initial adjuvant letrozole with tamoxifen for 5 years’ total treatment, while the second compared the use of letrozole and tamoxifen, in either order, for a 2- and 3-year treatment period, respectively, to complete a 5-year hormonal adjuvant treatment course in PMW with HR+ breast cancer.Citation9 The Primary Core Analysis (PCA) compared all patients assigned to initial adjuvant letrozole (n = 4003) or tamoxifen (n = 4007), thus including patients assigned to sequential therapy for the first 2 years, on either letrozole or tamoxifen, while the monotherapy analysis compared only those patients who were assigned to the monotherapy (tamoxifen or letrozole) arms.
The ATAC and BIG 1–98 trials also differed significantly with respect to the definition of DFS. As noted earlier, ATAC defined a DFS event as local or distant recurrence, new primary breast cancer, or death from any cause,Citation17 whereas BIG 1–98 included the occurrence of a second, nonbreast cancer in its definition and also added an additional endpoint of DFS excluding a second, nonbreast cancer, similar to ATAC.Citation9 Median follow up for the PCA was 25.8 months (2.2 years); at this time, both DFS (as defined in BIG 1–98, ) and DFS excluding a second, nonbreast cancer (HR 0.79; 95% CI: 0.68–0.92; P = 0.002) were significantly improved with letrozole relative to tamoxifen.Citation9 TTR (HR 0.72; 95% CI: 0.61–0.86; P < 0.001) also was significantly improved with letrozole, and importantly, TTDR at this early time point was improved by 27% with letrozole (184 vs 249 events, ). There was no significant difference between the treatment groups in OS (166 vs 192 events, ) at the time of this analysis.
In a subsequent, predefined analysis of BIG 1–98 in patients randomized to the monotherapy arms (n = 2463 letrozole, n = 2459 tamoxifen), median follow-up was approximately double that of the PCA, at 51 months (4.3 years).Citation10 At this time, there were 352 DFS events in the letrozole arm, compared with 418 events in the tamoxifen arm (); TTR (231 vs 291 events, HR 0.78; 95% CI: 0.65–0.92; P = 0.004) as well as TTDR (193 vs 234 events, ) remained significantly improved with letrozole over tamoxifen in this analysis.Citation10 Again, there were fewer overall deaths with letrozole versus tamoxifen (194 vs 211 events, ), but the difference did not reach significance.
The pivotal results from the first PCA at 25.8 months led to a decision by the International Breast Cancer Study Group to unblind the tamoxifen arm of the trial (with all other arms remaining blinded), inform patients of the superiority of letrozole, and allow the selective crossover of patients from tamoxifen to letrozole treatment.Citation11,Citation12 In total, 619 patients, or 25.2% from the tamoxifen arm, elected to cross over to letrozole therapy, and most patients did so between years 3 and 5 of the trial. Of note, the crossover did not significantly affect results of the 51-month monotherapy analysis; however, subsequent comparisons between the letrozole and tamoxifen arms in the PCA and monotherapy populations, as detailed below, were affected.
In late 2008, extended follow-up for patients randomized to the monotherapy arms (n = 2463 letrozole, n = 2459 tamoxifen), at a median follow-up of 76 months (6.3 years), was reported. All patients had completed protocol treatment at the time of this predefined, 10-year analysis.Citation11 In the ITT population, despite the above-noted crossover of about a quarter of patients, DFS was significantly improved in the letrozole arm (509 events) compared with the tamoxifen arm (565 events) (). Specifically, letrozole monotherapy resulted in fewer breast cancer events (321 vs 363) and fewer second, nonbreast malignancies (101 vs 115). Similar improvements within the letrozole arm occurred for TTDR compared with the tamoxifen arm (257 vs 298, ). Importantly, there was a pronounced trend in favor of letrozole in OS compared with tamoxifen. There were 40 fewer deaths in the letrozole treatment arm versus the tamoxifen arm (303 vs 343, ). In addition, the number of deaths without a prior cancer event was the same in both treatment groups (87 vs 87). As the crossover likely affected the comparison between the letrozole and tamoxifen groups, an exploratory analysis censoring events at the time of crossover was conducted. This analysis showed a greater and significant benefit of letrozole in all endpoints, including DFS, OS, and TTDR ().Citation11 Additionally, an analysis called the inverse probability of censoring weighting was performed to handle bias created by censoring events and assess the true benefits of each treatment regimen. The results showed significant benefits with letrozole in DFS (HR 0.85; 95% CI: 0.76–0.96) and OS (HR 0.83; 95% CI: 0.71–0.97), with HRs residing between the results of the ITT and censored analyses.Citation11 These data show an emerging survival benefit for patients treated with letrozole monotherapy over time.
Results presented in early 2009 also have detailed findings in the larger PCA population of BIG 1–98 (N = 8010) at the predefined, 10-year update (median 60.5 month follow-up).Citation12 As with the monotherapy analysis, despite the crossover of patients from tamoxifen to letrozole, there was a significant benefit of letrozole over tamoxifen in DFS (585 vs 664 events, ), TTR (368 vs 441 events, HR 0.82; 95% CI: 0.71–0.94; P = 0.004), and TTDR (287 vs 357 events, ), and a strong trend in OS favoring letrozole (330 vs 374 events, ). In the exploratory censored analysis, the benefit of letrozole over tamoxifen was again greater and significant for all endpoints, including DFS, TTR, TTDR, and OS ().Citation12 Results from the PCA population thus confirmed the findings of the monotherapy analysis, showing the emergence of a survival benefit over time with letrozole treatment.
ATAC and BIG 1–98: key efficacy differences
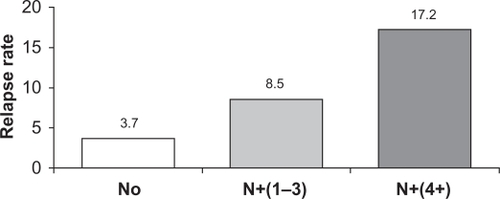
Several important subgroups of breast cancer patients can be identified. One of the most important subgroups comprises patients with lymph node involvement (ie, node positive [N+]), as they typically are more prone to early recurrence compared with node-negative (N0) patients ().Citation19,Citation20 Results of both the PCA (HR 0.71; 95% CI: 0.59–0.85; P < 0.001) and the monotherapy analysis of BIG 1–98 (HR 0.77; 95% CI: 0.64–0.92; P = 0.004) confirmed a significant benefit of letrozole over tamoxifen for DFS in the subgroup of N+ patients (n = 3311 and n = 2067, respectively). There also was a significant benefit of letrozole for patients with prior chemotherapy (PCA: n = 2024, HR 0.70; P = 0.01; monotherapy analysis: n = 1232, HR 0.74; P = 0.03) and in those with tumor size >2 cm (PCA: n = 2973, HR 0.76; P = 0.004; monotherapy analysis: n = 1858, HR 0.79; P = 0.01).Citation9,Citation10 The updated results at 60.5 months from the PCA cohort also confirm a significant benefit of letrozole over tamoxifen in the subgroup of N+ patients (n = 3313) for both DFS (HR 0.79; 95% CI: 0.68–0.91) and OS (HR 0.81; 95% CI: 0.67–0.97).Citation12 In the ATAC trial, the DFS benefit also favored anastrozole in these important high-risk patient subgroups (N+: HR 0.84, tumors >2 cm: HR 0.74, prior chemotherapy: HR 0.89; P values were not reported at the 100-month analysis), and anastrozole benefits were maintained regardless of the use of prior chemotherapy or the type of chemotherapy.Citation7,Citation17,Citation21
Figure 1 Increasing risk of early recurrence in patients with node-positive (N+) disease. The figure shows the relapse rate at 2.5 years for patients with increasing nodal involvement. Node-negative (N0) (n = 1962); N+ (1–3), 1 to 3 involved nodes (n = 1650); N+ (4+), 4 or more involved nodes.Citation17
Comparison of the ATAC and BIG 1–98 trials also yields some important findings with respect to DM events, which have been associated with a greater risk of death overall as well as death due to breast cancer.Citation22,Citation23 In a retrospective analysis comparing the 33-month results from ATAC and the 25.8-month follow-up results from BIG 1–98 (as these first analyses represent the closest follow-ups between trials), DFS efficacy of these two drugs versus tamoxifen was similar, but DM events were significantly lower with letrozole (relative risk [RR] 0.76; 95% CI: 0.63–0.92) compared with anastrozole (RR 0.87; 95% CI: 0.70–1.06).Citation24 In addition, a trend toward improved OS was observed with letrozole in this analysis (RR 0.87; 95% CI: 0.71–1.06), arising primarily from a significant reduction in deaths after a cancer event (RR 0.72; 95% CI: 0.57–0.92), whereas no such trend was observed in the ATAC trial.Citation24 A retrospective analysis, focused specifically on early recurrence events occurring in BIG 1–98 (at 2 years), found a 30% reduction in early DM events with letrozole (87 vs 125 events, 2.3% vs 3.3%).Citation25,Citation26 A similar unpublished retrospective analysis of early events in the ATAC trial revealed a comparatively smaller reduction in early DM events with anastrozole; distant recurrence at 2.5 years was reduced by 7% (133 events vs 143 events) with anastrozole relative to tamoxifen in the ITT population.Citation26,Citation27 A recently reported retrospective analysis of the HR+ subgroup (n = 5216) in ATAC at 2 years showed a 21% relative reduction in DM events (HR 0.79; 95% CI: 0.58–1.07) with anastrozole that was not significant, compared with a 32% relative reduction in overall recurrences, which was significant (HR 0.68; 95% CI: 0.52–0.88).Citation28 In an earlier report, Rugo et al used a number needed to treat (NNT) analysis to compare early recurrence results from ATAC and BIG 1–98.Citation29 In this analysis, a lower NNT would indicate the more efficacious drug in preventing a given recurrence event (meaning fewer numbers of patients needing to be treated with that agent to prevent 1 recurrence). The results showed a major advantage of letrozole over anastrozole in the prevention of DM events (NNT = 100 for letrozole vs 300 for anastrozole). By comparison, anastrozole showed a slight advantage in terms of local and regional recurrences (NNT = 279 vs 423) as well as CLBC (NNT = 156 vs 948), although it should be noted that the number of these latter recurrence events was far smaller at the early follow-up, and the study did not take into account recently reported early recurrence results from ATAC in the HR+ subgroup.Citation28,Citation29 Interestingly, both drugs were nearly identical for preventing DFS events (ie, recurrences overall), with an NNT of 77 for anastrozole versus 75 for letrozole.
Taken together, these findings suggest a meaningful benefit of letrozole over anastrozole in reducing early DM events, and the significance of this reduction should not be underestimated. In a recently published study (N = 3614 PMW with operable, estrogen receptor-positive breast cancer receiving adjuvant tamoxifen), a peak of recurrence was reported at 2 years (4.3% per annum), and DM events were found to be the predominant type of early recurrence event at this peak (3.4% per annum).Citation30 Retrospective analyses from the ATAC and BIG 1–98 trials also confirmed that DM events constitute the majority of recurrence events at 2 years.Citation25,Citation28 Prevention of DM recurrence is thus a key factor in improving survival and reducing death from breast cancer.Citation22,Citation23
In light of the survival benefit with letrozole over tamoxifen, which has been seen in the later follow-ups of both the PCA and monotherapy cohorts,Citation11,Citation12 a recent study examined the NNT to save a life with anastrozole and letrozole.Citation31 This analysis was based on data from the 68-month follow-up of ATAC (3% improvement in OS) and the 76-month follow up of the monotherapy arms of BIG 1–98 (censored analysis, 19% improvement in OS). The NNT to avoid one early distant recurrence was significantly lower with letrozole versus anastrozole (100 vs 303, threefold difference), as was the NNT required to avoid one death (63 vs 161, 2.5-fold difference). The investigators speculated that the difference in the reduction of early DM events with letrozole versus anastrozole could account for the latter difference in deaths.Citation31 It also should be noted that the magnitude of the survival benefit seen with letrozole over tamoxifen, as expressed in terms of NNT, is comparable with that seen with other paradigm-changing adjuvant regimens (eg, the addition of taxanes to anthracycline-based chemotherapy regimens).Citation32
Binding and potency of anastrozole and letrozole
The potency of AIs relates to their ability to specifically suppress the enzyme aromatase, and in this regard, the third-generation AIs including letrozole and anastrozole demonstrate clear advantages over first-generation compounds such as aminoglutethimide. In an early, open-label, randomized, comparative trial of letrozole (n = 185, 2.5 mg letrozole; n = 192, 0.5 mg letrozole) with aminoglutethimide (n = 178) in women with advanced breast cancer, letrozole at 2.5 or 0.5 mg resulted in an objective response rate (ORR) of 19.5% and 16.7%, respectively, versus only 12.4% for aminoglutethimide, and there was a significantly better time to progression (RR 0.72; 95% CI: 0.57–0.92; P = 0.008), time to treatment failure (RR 0.70; 95% CI: 0.55–0.88; P = 0.003), and OS (RR 0.64; 95% CI: 0.49–0.85; P = 0.002) for letrozole 2.5 mg versus aminoglutethimide.Citation33 Another study of advanced breast cancer patients compared letrozole (n = 356) with anastrozole (n = 357) as second-line therapy.Citation34 In this study, median time to progression, the primary endpoint, was identical for the two drugs (5.7 months). However, ORR was significantly improved with letrozole relative to anastrozole (19.1% vs 12.3%, P = 0.013).Citation34 It is unclear whether these differences in clinical efficacy are directly correlated with the degree of aromatase inhibition and the corresponding suppression of plasma E2; however, it is notable that the potency of aminoglutethimide in this regard (90.6% suppression) is substantively lower than that of letrozole (nearly 99% suppression).Citation6,Citation35,Citation36
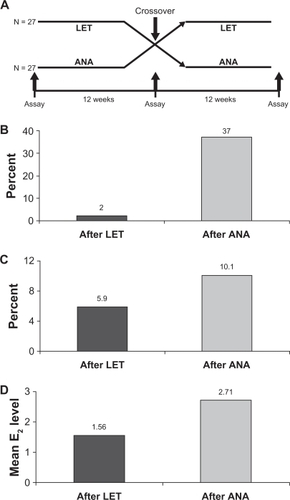
Although both anastrozole and letrozole are nonsteroidal AIs, there are important differences between these two compounds, which could, in part, underlie the clinical results that have been observed. Computer-assisted molecular modeling studies suggest that letrozole fits very tightly into the binding site of the aromatase enzyme and occupies the entire binding site, whereas anastrozole does not ().Citation4,Citation37 In a recent study, plasma samples from a neoadjuvant study using letrozole (16 weeks’ neoadjuvant treatment followed by surgery) were directly compared with samples from a similar neoadjuvant trial of anastrozole using a highly sensitive assay for E2 detection.Citation15 The authors found a significant difference in the degree of plasma E2 suppression for letrozole versus anastrozole (95.2% vs 92.8%, P = 0.018), and there were similar, significant differences observed for estrone (98.8% vs 96.3%, P = 0.003) and estrone sulfate (98.9% vs 95.3%, P = 0.003).Citation15 The suppression of plasma E2 by these two AIs also has been directly compared in the ALIQUOT (Anastrozole versus Letrozole, an Investigation of Quality Of Life and Tolerability) trial. This study compared outcomes following 3 months of adjuvant treatment with letrozole or anastrozole in a randomized crossover design in 54 PMW with HR+ breast cancer.Citation16 Patients were randomized to letrozole for 12 weeks followed by anastrozole for 12 weeks (n = 27), or the reverse sequence (n = 27), and plasma levels of E2 were assayed following each drug treatment (). Significantly fewer patients had E2 plasma values of 3 pmol/L or greater (), the percent residual E2 level was lower (), and the mean plasma E2 level after extrapolation was significantly lower following letrozole versus anastrozole treatment ().Citation16 These results were consistent regardless of which agent was given first in the study and regardless of prior tamoxifen use. Suppression of estrogen in the breast tumor tissue also has been found to be more pronounced following neoadjuvant letrozole compared with anastrozole. Mean percentages of suppression with letrozole and anastrozole were 97.6% and 89.0% for E2, 90.7% vs 83.4% for estrone, and 90.1% vs 72.9% for estrone sulfate, respectively.Citation15 Collectively, these findings provide evidence for a greater inhibition of aromatase and a greater corresponding suppression of plasma and tissue E2 levels with letrozole relative to anastrozole, and a possible explanation for their differences in the clinical setting. It is quite plausible that lower plasma E2 levels afforded by letrozole treatment provide for more complete eradication of micrometastatic tumor deposits, which could in turn result in fewer DM recurrences.Citation26
Figure 2 Binding of nonsteroidal aromatase inhibitors anastrozole and letrozole. Computer-assisted molecular modeling displaying the active site fit and heme group binding in the aromatase enzyme. Whereas the distal portion of the letrozole molecule fits tightly and occupies the entire binding site, the same is not true for anastrozole.Citation4,Citation37 Reprinted with permission from Mouridsen HT, Bhatnagar AS. Letrozole in the treatment of breast cancer. Expert Opin Pharmacother. 2005;6:1389–1399.Citation4 Copyright © 2005, Ashley Publications Ltd.
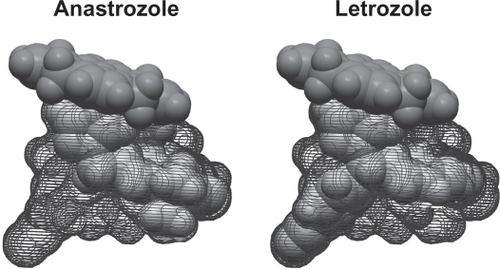
Figure 3 A) Study design of ALIQUOT (Anastrozole versus Letrozole, an Investigation of Quality Of Life and Tolerability), an open-label, crossover study comparing adjuvant therapy with letrozole and anastrozole. Postmenopausal women with hormone receptor-positive breast cancer (n = 27 per group) were randomized to 3 months’ treatment with letrozole (LET) followed by anastrozole (ANA), or the reverse sequence; plasma estrogen (E2) levels were assayed at the indicated time points. B) Percent of patients with an E2 value of 3 pmol/L or greater following treatment with LET or ANA; the difference was statistically significant. C) Mean residual E2 level (%) following treatment with LET or ANA. D) Mean E2 level in pmol/L after extrapolation following treatment with either LET or ANA; the difference was statistically significant.Citation16
FACE: trial design and methods
As outlined above, there is evidence from clinical and pharmacodynamic data to suggest a difference in potency between the nonsteroidal AIs anastrozole and letrozole. Nevertheless, the clinical data presented herein admittedly are from different trials (ATAC and BIG 1–98) that were conducted at different times and with different overall goals, and with substantive differences in study design, population, and follow-up reporting of results. Accordingly, only limited conclusions can be drawn from such indirect comparisons. More meaningful comparisons between agents ideally should come from head-to-head trails. The FACE trial was designed with the caveats of cross-trial comparisons in mind, and forthcoming results will begin to address the important issue of efficacy and safety differences between the third-generation AIs. FACE was designed as a head-to-head, Phase III, open-label, randomized, multicenter (up to 250 international sites) clinical trial comparing adjuvant treatment with anastrozole (1 mg daily) or letrozole (2.5 mg) in more than 4000 PMW with HR+ and N+ breast cancer.Citation38,Citation39 As of March 2008, accrual into the trial was completed, with 4172 patients enrolled. Consequently, safety and efficacy interim data are imminent. Patients who were N+ were specifically chosen for FACE, as these women are known to be at a higher overall relapse riskCitation19,Citation20 and are prone to earlier recurrence (within 2 years).Citation25 These women would therefore benefit the most from initial adjuvant therapy with an AI, as opposed to initial tamoxifen therapy. In addition, such a patient population could theoretically allow for a statistical difference in efficacy between these AIs to emerge in a shorter period of time. Treatment was to commence following standard chemotherapy or radiotherapy (if given), and patients were to receive treatment for up to 5 years, or until disease recurrence/relapse. Patients were to be stratified according to the number of involved nodes, as well as human epidermal growth factor receptor 2 status.Citation38,Citation39
The primary endpoint of FACE is DFS at 5 years for letrozole and anastrozole, with the important additional secondary endpoints of OS, TTDR, time to CLBC, and safety. The safety results also will be particularly important to determine any potential differences between these AIs with respect to bone health and cardiovascular adverse events. The trial is sufficiently powered to detect a difference of 3.5% between the two treatment arms at 5 years (ie, 5 years’ DFS of 76.5% for anastrozole vs 80.0% for letrozole); this corresponds to an HR of 0.83 in favor of letrozole. Interim efficacy analyses for DFS also are planned when approximately a third of the maximum number of events have occurred, and again when about two-thirds of the maximum events have occurred (about 320 and 639 events, respectively). Finally, a number of correlative science studies will be undertaken in FACE. These include an assessment of E2 suppression during treatment with anastrozole or letrozole, and the use of exploratory proteomics and genomics studies to identify differential protein, blood metabolite, and/or gene expression profiles, if any, that are associated with differential DFS and OS with anastrozole or letrozole therapy. As with BIG 1–98, the data will be provided by an independent academic organization, the Michelangelo Foundation, and will be reviewed in a confidential manner by an Independent Data Monitoring Committee (IDMC) chaired by Dr H Rugo; the IDMC will make recommendations to the Trial Steering Committee, co-chaired by Professor I Smith and Dr J O’Shaughnessy. The Steering Committee has developed a publication plan, and will determine when abstracts and/or manuscripts originating from the trial will be developed and presented. At annual intervals, and at the time of the interim analyses, the validated database will be transferred to the Michelangelo Foundation, which will work closely with the IDMC to monitor the study. Only after the final analysis has been completed will the database be transferred to the study sponsor, Novartis.
Conclusions
Evidence from the pivotal trials of anastrozole and letrozole, ATAC and BIG 1–98, has revealed important efficacy differences between these two drugs (), particularly in the prevention of early DM events, which are by far the most prevalent and the most deadly types of breast cancer recurrence events.Citation22,Citation23,Citation25,Citation27,Citation28,Citation30 Differences in the binding of letrozole and anastrozole to the aromatase enzyme (), and their corresponding inhibitory activity, may in part underlie the more complete suppression of E2 levels with letrozole versus anastrozole that has been observed in directly comparative clinical studies such as ALIQUOT (). It is plausible that the more complete suppression of E2 in plasma and tissues by letrozole in turn causes a greater eradication of distant micrometastatic breast cancer deposits, which can develop later into fulminant DM recurrences. This especially may be relevant for patients with N+ disease, as these patients present a greater risk for early recurrence (). Although letrozole effectively suppresses recurrence in all relevant patient subgroups, its benefit is especially pronounced in the high-risk subgroup of N+ patients.Citation9,Citation10 In addition, while not reported in the 76-month follow-up of the monotherapy arms, subgroup analysis in the PCA population at 60.5 months shows a significant DFS and OS benefit in the N+ subgroup, despite the crossover of about 25% of patients from tamoxifen to letrozole.Citation12
There is precedent for an association between a lower degree of aromatase inhibition (ie, in the case of aminoglutethimide) and poorer outcomes compared with letrozole in advanced cancer patients.Citation33 Despite these observations, however, comparisons with anastrozole remain indirect, and only directly comparative head-to-head trials will be useful in resolving the question of potential efficacy differences between letrozole and anastrozole. The development and design of the FACE trial undoubtedly will provide a conclusive resolution to this question, and demonstrate unequivocally which AI is more appropriately used upfront in the high-risk population of N+ breast cancer patients. It is hoped that other similarly designed trials will be implemented to enable further comparisons among the third-generation AIs in different patient populations and treatment settings. In this regard, the National Cancer Institute of Canada MA. 27 trial is investigating upfront anastrozole versus exemestane in the adjuvant setting in PMW with HR+ breast cancer.Citation40 Analysis of the trial is forthcoming; to date, 6830 patients have been accrued. Until the results of this trial and the FACE trial are reported, however, clinicians need to rely on the available clinical evidence, which strongly favors the use of upfront letrozole for PMW with HR+ early breast cancer.
Acknowledgements
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. We thank Maria Soushko, PhD, of Phase Five Communications Inc., for medical editorial assistance with this manuscript.
Disclosure
The author discloses no conflicts of interest.
References
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trialsLancet20053651687171715894097
- FisherBCostantinoJPWickerhamDLTamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 StudyJ Natl Cancer Inst199890137113889747868
- BraithwaiteRSChlebowskiRTLauJGeorgeSHessRColNFMeta-analysis of vascular and neoplastic events associated with tamoxifenJ Gen Intern Med20031893794714687281
- MouridsenHTBhatnagarASLetrozole in the treatment of breast cancerExpert Opin Pharmacother200561389139916013988
- RieberAGTheriaultRLAromatase inhibitors in postmenopausal breast cancer patientsJ Natl Compr Canc Netw2005330931416002003
- GeislerJLonningPEAromatase inhibition: translation into a successful therapeutic approachClin Cancer Res2005112809282115837728
- Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ GroupForbesJFCuzickJBuzdarAHowellATobiasJSBaumMEffect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trialLancet Oncol20089455318083636
- HowellACuzickJBaumMATAC Trialists’ GroupResults of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancerLancet2005365606215639680
- Breast International Group (BIG) 1–98 Collaborative GroupThürlimannBKeshaviahACoatesASA comparison of letrozole and tamoxifen in postmenopausal women with early breast cancerN Engl J Med20053532747275716382061
- CoatesASKeshaviahAThürlimannBFive years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98J Clin Oncol20072548649217200148
- BIG 1–98 Collaborative GroupMouridsenHGiobbie-HurderAGoldhirschALetrozole therapy alone or in sequence with tamoxifen in women with breast cancerN Engl J Med200936176677619692688
- ThurlimannBBIG 1-98 Collaborative and International Breast Cancer Study GroupsLetrozole versus tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive breast cancer39th St. Gallen Symposium2009 May 7–9St. Gallen, Switzerland Abstr 0161.
- CoombesRCKilburnLSSnowdonCFIntergroup Exemestane StudySurvival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trialLancet200736955957017307102
- GossPEIngleJNMartinoSRandomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17J Natl Cancer Inst2005971262127116145047
- GeislerJHelleHEkseDLetrozole is superior to anastrozole suppressing breast cancer tissue and plasma estrogen levelsClin Cancer Res2008146330633518829517
- DixonJMRenshawLYoungOLetrozole suppresses plasma estradiol and estrone sulphate more completely than anastrozole in postmenopausal women with breast cancerJ Clin Oncol2008261671167618375896
- BaumMBuzdarAUCuzickJfor the ATAC Trialists GroupAnastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomized trialLancet200235921312139 Erratum in: Lancet.2002360152012090977
- Arimidex [package insert]. Wilmington, DE: AstraZeneca; Rev. 12/08.
- McArthurHLOlivottoIGelmonKARisk of early relapse in post-menopausal women with early stage, estrogen receptor positive (ER+) breast cancer on tamoxifenBreast Cancer Res Treat200594Suppl 1S124 Abstr 3001.
- HortobagyiGNKauS-WBuzdarAUWhat is the prognosis of patients with operable breast cancer (BC) five years after diagnosis?Proc Am Soc Clin Oncol20042323 Abstr 585.
- BuzdarAUGuastallaJPNabholtzJMCuzickJATAC Trialists’ GroupImpact of chemotherapy regimens prior to endocrine therapy: Results from the ATAC (Anastrozole and Tamoxifen, Alone or in Combination) trialCancer200610747248016804925
- LameratoLHavstadSGandhiSJonesDChlebowskiRBreast cancer recurrence and related mortality in US pts with early breast cancerJ Clin Oncol20052316S62s Abstr 738.
- DoughtyJCMansellJMonypennyIDistant recurrence and subsequent mortality in breast cancer patients treated at 5 UK centres39th St. Gallen Symposium2009 May 7–9St. Gallen, Switzerland Abstr 0188.
- VakaetLBuyseMComparative results of two adjuvant trials comparing 5 years of an aromatase inhibitor (AI) with 5 years of tamoxifen (TAM) in postmenopausal women with early breast cancer (EBC)Breast Cancer Res Treat2006100Suppl 1S108S109 Abstr 2084.
- MauriacLKeshaviahADebledMBIG 1-98 Collaborative GroupInternational Breast Cancer Study Group. Predictors of early relapse in postmenopausal women with hormone receptor-positive breast cancer in the BIG 1-98 trialAnn Oncol20071885986717301074
- GligorovJAzriaDPivotXPenault-LlorcaFSpielmannMNamerMClinical impact of upfront adjuvant AI therapy on the early risk of recurrenceEur J Cancer20075Suppl209 Abstr 2089.
- HoughtonJon behalf of the ATAC Trialists’ GroupInitial adjuvant therapy with anastrozole (S) reduced rates of early breast cancer recurrent and adverse events compared with tamoxifen (T) – data reported on behalf of the ATAC (‘Arimidex’, TAM, Alone or in Combination) Trialists’ groupAnn Oncol200617Suppl 9ix93 Abstr 243PD.
- HowellAForbesJCuzickJInitial adjuvant therapy with anastrozole – early and late event data from the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial in the hormone-responsive population39th St. Gallen Symposium2009 May 7–9St. Gallen, Switzerland Abstr 0130.
- RugoHRourkeMDranitsarisGKauraSLetrozole or anastrozole for the prevention of early recurrences in postmenopausal women with early stage breast cancer: Using number needed to treat (NNT) to compare benefitEur J Cancer Suppl20086118 Abstr 236.
- MansellJMonypennyIJSkeneAIPatterns and predictors of early recurrence in postmenopausal women with estrogen receptor-positive early breast cancerBreast Cancer Res Treat2009117919819112615
- RugoHSKauraSDranitsarisGThe number needed to treat to save a life: a comparison between aromatase inhibitors39th St. Gallen Symposium2009 May 7–9St. Gallen, Switzerland Poster 136.
- DhillonNKauraSDranitsarisGRugoHNumber needed to treat (NNT) to compare benefits of letrozole (LET) with adjuvant chemotherapy (CT) in patients (pts) with node-positive (N+) breast cancer (BC)J Clin Oncol20092715s Abstr 581.
- GershanovichMChaudriHACamposDLetrozole, a new oral aromatase inhibitor: Randomised trial comparing 2.5 mg daily, 0.5 mg daily and aminoglutethimide in postmenopausal women with advanced breast cancerAnn Oncol199896396459681078
- RoseCVtorayaOPluzanskaAAn open randomized trial of second-line endocrine therapy in advanced breast cancer. Comparison of the aromatase inhibitors letrozole and anastrozoleEur J Cancer2003392318232714556923
- DowsettMJonesAJohnstonSRJacobsSTrunetPSmithIEIn vivo measurement of aromatase inhibition by letrozole (CGS 20267) in postmenopausal patients with breast cancerClin Cancer Res19951151115159815951
- MacNeillFAJonesALJacobsSLønningPEPowlesTJDowsettMThe influence of aminoglutethimide and its analogue rogletimide on peripheral aromatisation in breast cancerBr J Cancer1992666926971419608
- FuretPBatzlCBhatnagarAFrancotteERihsGLangMAromatase inhibitors: synthesis, biological activity, and binding mode of azole-type compoundsJ Med Chem199336139314008496907
- MonnierARefining the postmenopausal breast cancer treatment paradigm: the FACE trialExpert Rev Anticancer Ther200661355135917069521
- de BoerRSrBurrisHAMonnierAon behalf of the H2H trial steering committeeThe Head to Head trial: Letrozole vs anastrozole as adjuvant treatment of postmenopausal patients with node positive breast cancerJ Clin Oncol20062418s582s Abstr 10672
- NCIC Clinical Trials GroupExemestane or anastrozole in treating postmenopausal women who have undergone surgery for primary breast cancer http://clinicaltrials.gov/ct2/show/NCT00066573. Accessed 2010 Jun 7.