Abstract
Malignant ascites is frequently found with various solid tumors, and no established treatment options exist, apart from symptomatic paracentesis. Catumaxomab, a trifunctional bispecific monoclonal antibody, has two binding specificities directed to epithelial cell adhesion molecule (EpCAM) and the T cell antigen CD3. With its Fc-fragment, catumaxomab additionally binds accessory cells, including dendritic cells, macrophages, and natural killer cells. The trifunctional approach thus leads to a major histocompatibility complex-unrestricted but specific killing of epithelial tumor cells without need for preactivation or external costimulation. Because EpCAM is expressed in most solid tumors, but not in tissue of mesothelial origin, intraperitoneal treatment with catumaxomab is tumor-specific. Intraperitoneal treatment with catumaxomab resulted in a significant prolongation of puncture-free survival in patients with malignant ascites due to epithelial cancer. Catumaxomab has been approved in Europe for the intraperitoneal treatment of malignant ascites in patients with EpCAM-positive epithelial tumors where standard therapy is not available or no longer feasible.
Introduction
Peritonitis carcinomatosa indicates the presence of malignant cells in the peritoneal cavity, and is a well known complication of a number of malignant diseases. As a result, so-called malignant ascites develops. Malignant ascites is characterized by positive cytology of malignant cells. Impaired lymphatic drainage by occlusion of the lymphatic vessels and increased fluid production causes the accumulation of malignant ascites in the peritoneum.Citation1 Clinical manifestations include symptoms of abdominal pain, obstruction, fatigue, and abdominal swelling.
In their retrospective analysis of 209 patients with malignant ascites, Ayantunde et al found a median survival time of 5.7 months after diagnosis of ascites. Independent negative prognostic factors were type of cancer, liver metastasis, and low serum albumin, and, in contrast, patients with ovarian cancer had a favorable prognosis.Citation2
Treatment options are symptomatic, and the most common is paracentesis or surgical treatment, including peritoneovenous shunting. Systemic and local treatment options include systemic chemotherapy, intraperitoneal chemotherapy, or therapy with radioisotopes. Additionally, the vascular endothelial growth factor antibody bevacizumab is currently being tested in clinical studies to treat and prevent malignant ascites.Citation3
Catumaxomab
Mode of action
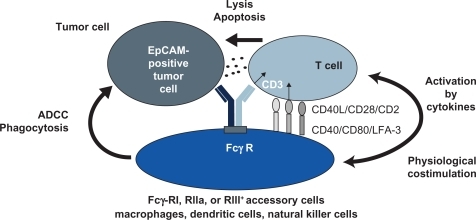
Catumaxomab (anti-EpCAM × anti-CD3) is a hybrid, hybridoma-derived, trifunctional, monoclonal bispecific antibody, combining two half-antibodies of mouse IgG2a and rat IgG2b that represent homologous immunoglobulin subclasses. Preclinical studies have shown the following three events to occur, demonstrating the trifunctional mode of action of the drug:
One antigen binding site, the mouse IgG2a, recognizes the tumor-specific antigenCitation4
The other antigen binding site (rat IgG2b) binds to CD3, part of the T cell receptor complexCitation5,Citation6
The Fc-fragment binds to FcγR Type I and III positive cells (eg, macrophages, dendritic cells, and natural killer cells) but not to inhibitory Type II FcγR (located on, eg, B cells).Citation7
The stimulation of immune cells is demonstrated by production of cytokines, including interleukin (IL)-1β, IL-2, IL-6, IL-12, and the dendritic cell cytokine 1. Activated accessory cells, including macrophages and dendritic cells, induce several costimulatory signals, eg, via CD40-CD40L to T cells to prevent T cell anergy. The simultaneous activation of T cells and accessory immune cells, and their mutual stimulation, leads to specific tumor cell killing by induction of apoptosis, release of cytokines, and perforin-mediated lysis, as well as antibody-dependent cellular cytotoxicity. Crucial in this process is the combination of the two potent immunoglobulin isotypes, mouse IgG2a and rat IgG2b, which, in contrast with other reported combinations, not only bind but also activate accessory cells. The trifunctional approach thus leads to major histocompatibility complex-unrestricted but specific killing of tumor cells without need for preactivation or external costimulationCitation5,Citation8 (see ).
EpCAM is a Type I transmembrane glycoprotein that mediates Ca2+-independent homophilic cell adhesions.Citation9 In humans, EpCAM is expressed only in epithelium and neoplasms derived from epithelia.Citation10 EpCAM is also strongly expressed in carcinomas of various origins, including colon, rectum, gastric, ovarian, esophagus, lung, pancreas, breast, head, and neck.Citation11–Citation13 Because of the wide expression on tumor cells, EpCAM is considered a tumor-associated antigen, and innovative immunotherapeutic approaches targeting EpCAM are of special interest. EpCAM expression is a negative prognostic marker for overall survival in patients suffering from either nodal-positive or node-negative breast cancer. Schmidt et al demonstrated that EpCAM overexpression was independently associated with poor survival in node-negative patients. This effect was particularly strong in the subgroup of triple-negative breast cancer, making EpCAM an attractive therapeutic target in this patient population.Citation14,Citation15
EpCAM has a direct impact on cell cycle and proliferation, and the ability to upregulate the proto-oncogene, c-myc, and cyclin A/E rapidly. Furthermore, EpCAM weakens cadherin-mediated cell adhesion, and thereby modulates proliferation, differentiation, and tissue maintenance.Citation16 Blocking of EpCAM leads to a decrease in proliferation and metabolism in human carcinoma cells.Citation17 Recently, Maetzel et al demonstrated how EpCAM affects nuclear function by shedding of its ectodomain, EpEX, and nuclear translocation of its intracellular domain, EpICD, dependent on the presence of tumor necrosis factor-alpha-converting enzyme (TACE, ADAM17) and presenilin-2.Citation18 Furthermore, Lindhofer et al showed elimination of putative EpCAM-positive cancer stem cells (CD 133+/EpCAM+) in patients with malignant ascites treated with catumaxomab.Citation19 Recently Hirschhaeuser et al demonstrated a strong, dose-dependent effect of catumaxomab on multicellular tumor spheroids of human EpCAM-positive FaDu tumor cells when cocultured with human peripheral blood monocytes in terms of volume reduction and infiltration of immune cells.Citation20
Intraperitoneal administration
The first reported clinical pilot study treated eight patients with malignant ascites by intraperitoneal application of catumaxomab or ertumaxomab binding EpCAM or human epidermal growth receptor 2/neu antigen on tumor cells, respectively. Treatment consisted of four to six applications within nine to 23 days, using a total amount of 145–940 μg of the antibody. Seven of eight patients required no further paracentesis during follow-up or until death, with a mean paracentesis-free interval of 38 weeks. Complete elimination of tumor cells in ascites was seen at total doses of 40–140 μg. Clinical response with disappearance of ascites accumulation was correlated with elimination of tumor cells (P = 0.0014).Citation21
In a Phase I/II study reported by Burges, patients with malignant ascites due to ovarian cancer were treated with escalating intraperitoneal doses of catumaxomab. The maximum tolerated dose was defined as 10, 20, 50, 200, and 200 μg on days 0, 3, 6, 9, and 13. The dose-limiting toxicities were large bowel obstruction Common Toxicity Criteria Grade 3 and gamma glutamyl transferase elevation Grade 4. All patients had treatment-emergent adverse events, with fever, nausea, vomiting, abdominal pain, lymphopenia, and general pain being the most common events. In terms of efficacy, 22 of 23 patients did not require any further paracentesis during a follow-up period of up to 37 days. The authors concluded that a dose regimen of 10, 20, 50, and 150 μg would be the recommended treatment schedule for further investigation.Citation22
This led to a pivotal Phase II/III study in patients with symptomatic malignant ascites secondary to epithelial cancers requiring symptomatic therapeutic paracentesis. The study compared paracentesis with intraperitoneal catumaxomab versus paracentesis alone in a two-arm, open-label, randomized trial. The primary endpoint was puncture-free survival, defined as the time to first need for therapeutic puncture or death after treatment. Secondary endpoints were time to next paracentesis, ascites signs defined by the patient, ascites signs defined by the investigator, and overall survival. The investigators were to follow an algorithm when a paracentesis was indicated (ascites >1L as assessed by a computed tomography scan and signs and symptoms of ascites assessed by the investigator using physical examination and a patient questionnaire) to ensure a comparable decision on when to perform paracentesis by the different investigators in the different treatment arms. Patient in the paracentesis-only treatment group were allowed to cross over to catumaxomab treatment if they still fulfilled the inclusion and exclusion criteria, and had had at least two paracenteses after day 0 of the study. The percentage and outcome of the crossover patients were not reported, although they might influence the secondary endpoint of overall survival. Treatment consisted, as recommended, of four constant-rate intraperitoneal infusions at doses of 10, 20, 50, and 150 μg of catumaxomab on days 0, 3, 7, and 10. The antibody was administered via intraperitoneal catheter in an inpatient setting, and the control group was treated with a paracentesis. Toxicity was as expected, with predominantly cytokine-release-like symptoms, including pyrexia (in 60.5% of patients), nausea, and vomiting. Ileus was reported in 6.4% of the patients treated with catumaxomab. There were no treatment-related deaths. In total, 258 patients were randomized, of whom 170 received catumaxomab and paracentesis and 88 received paracentesis alone. One hundred and twenty-nine patients had ovarian cancer, and 129 patients suffered from nonovarian cancer, mostly gastric cancer (n = 66). The primary endpoint of puncture-free survival was significantly prolonged in catumaxomab patients in both strata (ovarian: 52 versus 11 days, and nonovarian cancer: 37 versus 14 days, P < 0.0001, respectively) as well as in the pooled analysis (46 versus 11 days). The secondary endpoint of median overall survival was not prolonged in the pooled analysis (72 days for catumaxomab versus 68 days for paracentesis only, P = 0.0846) as well as in the stratified groups. Subgroup analysis showed a significant (P = 0.0313) survival benefit for catumaxomab in the gastric cancer patients (71 versus 44 days). The authors concluded that the treatment regimen demonstrated a clinically relevant benefit in patients with malignant ascites from epithelial cancer.Citation23
Summary
Catumaxomab, given intraperitoneally in ascending, repetitive doses, prolongs puncture-free survival in patients with malignant ascites. Side effects are explained by the mode of action of the drug and are usually reversible. Common side effects with intraperitoneal treatment include cytokine release-related symptoms, like fever, chills, nausea, and vomiting.
Disclosure
The author has received lecture fees from and previously consulted for Fresenius Biotech.
References
- TamsmaJThe pathogenesis of malignant ascitesCancer Treat Res200713410911817633049
- AyantundeAAParsonsSLPattern and prognostic factors in patients with malignant ascites: A retrospective studyAnn Oncol200718594594917298959
- El-ShamiKElsaidAEl-KermYOpen-label safety and efficacy pilot trial of intraperitoneal bevacizumab as palliative treatment in refractory malignant ascitesJ Clin Oncol200725Suppl 189043
- SchmittMSchmittAReinhardtPOpsonization with a trifunctional bispecific (alphaCD3 x alphaEpCAM) antibody results in efficient lysis in vitro and in vivo of EpCAM positive tumor cells by cytotoxic T lymphocytesInt J Oncol200425484184815375531
- RufPGiresOJagerMFellingerKAtzJLindhoferHCharacterisation of the new EpCAM-specific antibody HO-3: Implications for trifunctional antibody immunotherapy of cancerBr J Cancer200797331532117622246
- ZeidlerRReisbachGWollenbergBSimultaneous activation of T cells and accessory cells by a new class of intact bispecific antibody results in efficient tumor cell killingJ Immunol199916331246125210415020
- ZeidlerRMysliwietzJCsanadyMThe Fc-region of a new class of intact bispecific antibody mediates activation of accessory cells and NK cells and induces direct phagocytosis of tumour cellsBr J Cancer200083226126610901380
- RiesenbergRBuchnerAPohlaHLindhoferHLysis of prostate carcinoma cells by trifunctional bispecific antibodies (alpha EpCAM x alpha CD3)J Histochem Cytochem200149791191711410615
- LitvinovSVVeldersMPBakkerHAFleurenGJWarnaarSOEp-CAM: A human epithelial antigen is a homophilic cell-cell adhesion moleculeJ Cell Biol199412524374468163559
- BalzarMWinterMJde BoerCJLitvinovSVThe biology of the 17–1A antigen (Ep-CAM)J Mol Med1999771069971210606205
- OstaWAChenYMikhitarianKEpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapyCancer Res200464165818582415313925
- WentPVaseiMBubendorfLFrequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancersBr J Cancer200694112813516404366
- WentPTLugliAMeierSFrequent EpCam protein expression in human carcinomasHum Pathol200435112212814745734
- SchmidtMHasencleverDSchaefferMPrognostic effect of epithelial cell adhesion molecule overexpression in untreated node-negative breast cancerClin Cancer Res200814185849585518794096
- SchmidtMPetryIBBohmDEp-CAM RNA expression predicts metastasis-free survival in three cohorts of untreated node-negative breast cancerBreast Cancer Res Treat2010330 [Epub ahead of print].
- LitvinovSVBalzarMWinterMJEpithelial cell adhesion molecule (Ep-CAM) modulates cell-cell interactions mediated by classic cadherinsJ Cell Biol19971395133713489382878
- MunzMKieuCMackBSchmittBZeidlerRGiresOThe carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferationOncogene200423345748575815195135
- MaetzelDDenzelSMackBNuclear signalling by tumour-associated antigen EpCAMNat Cell Biol200911216217119136966
- LindhoferHSchoberthAPelsterDHessJHeroldJJagerMElimination of cancer stem cells (CD133+/EpCAM+) from malignant ascites by the trifunctional antibody catumaxomab: Results from a pivotal phase II/III studyJ Clin Oncol200927Suppl 15301419364954
- HirschhaeuserFWalentaSMueller-KlieserWEfficacy of catumaxomab in tumor spheroid killing is mediated by its trifunctional mode of actionCancer Immunol Immunother201059111675168420652245
- HeissMMStrohleinMAJagerMImmunotherapy of malignant ascites with trifunctional antibodiesInt J Cancer2005117343544315906359
- BurgesAWimbergerPKumperCEffective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: A phase I/II studyClin Cancer Res200713133899390517606723
- HeissMMMurawaPKoralewskiPThe trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trialInt J Cancer201012792209222120473913