Abstract
The management of renal cell carcinoma (RCC) has undergone significant changes during the past 10 years, with the treatment of metastatic RCC undergoing the most radical changes. These developments reflect an enhanced understanding of this tumor’s underlying biology, which was then translated into the development of a new treatment paradigm. Current therapeutic approaches for the management of patients with metastatic RCC utilize knowledge of histology, molecular abnormalities, clinical prognostic factors, the natural history of this malignancy, and the treatment efficacy and toxicity of available agents. The treatment options available for patients with metastatic RCC have changed dramatically over the past 6 years. Interferon-α and interleukin-2 were the previous mainstays of therapy, but since December 2005, six new agents have been approved in the US for the treatment of advanced RCC. Three are multi-targeted tyrosine kinase inhibitors (TKI) including sunitinib, sorafenib, and pazopanib, two target the mammalian target of rapamycin (temsirolimus and everolimus), and one is a humanized monoclonal antibody (bevacizumab in combination with interferon-α). The current review focuses on the newest TKI available to treat patients with metastatic RCC, pazopanib. The development of this agent both preclinically and clinically is reviewed. The efficacy and safety data from the pivotal clinical trials are discussed, and the potential role of pazopanib in the treatment of patients with metastatic RCC in comparison to other treatment alternatives is critically appraised. This agent has a favorable overall risk benefit, and the available data demonstrate efficacy in patients with metastatic RCC who are either treatment-naïve or cytokine refractory. It therefore represents another alternative for treatment of metastatic RCC patients.
Keywords:
Introduction: management of metastatic renal cell carcinoma (RCC)
The management of RCC has undergone significant changes during the past 10 years. Surgical innovation has reduced morbidity and currently surgery utilizing less invasive approaches which preserve efficacy are emphasized. The therapy for metastatic RCC has seen the greatest change, reflecting an enhanced understanding of this tumor’s underlying biology, which was then translated into the development of a new treatment paradigm.
RCC accounts for 2% to 3% of all malignant tumors, and is the sixth leading cause of death in the US. An estimated 58,000 new renal tumors were diagnosed in 2010, with approximately 13,000 deaths reported.Citation1 It is most common in the seventh decade of life, and a male to female predominance of 1.6 to 1.0 is present. Worldwide, the incidence of RCC is over 200,000 new cases annually, with over 100,000 deaths per year.Citation2 Active and passive cigarette smoking is the major recognized risk factor for RCC, with a relative risk (RR) of approximately two- to three-fold.Citation3
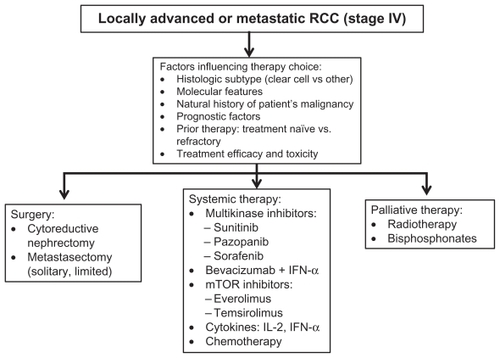
Current therapeutic approaches for management of patients with metastatic RCC utilize knowledge of histology, molecular abnormalities, clinical prognostic factors, knowledge of the natural history of RCC, and the treatment efficacy and toxicity of available agents (). Histology plays a significant role in treatment outcome and selection. Epithelial RCC includes various histologic subtypes, which have unique morphologic and genetic characteristics. Clear cell RCC is the most common epithelial subtype, accounting for 75%–85% of these tumors, and arises from the proximal convoluted tubule. Importantly, over 80% of sporadic clear cell RCC are associated with defects in the von Hippel- Lindau (VHL) gene.Citation4 Additional gene mutations and deletions have been recently identified,Citation5 however their importance and relevance are as yet unclear. The remaining subtypes of epithelial RCC are collectively referred to as non-clear cell carcinomas, with papillary RCC being the most common type (10%–15%). Understanding histologic subtypes and associated molecular alterations has provided the framework within which diseasespecific therapy has developed.
Figure 1 Flow diagram illustrating decision factors and therapeutic alternatives in patients with locally advanced and metastatic renal cell carcinoma.
Approximately 2%–3% of cases of RCC are familial and several autosomal dominant syndromes have been described, each with a distinct genetic basis and phenotype.Citation6 Most common is the VHL syndrome (1/36,000 births), characterized by the development of vascular tumors including clear cell RCC, hemangioblastomas of the central nervous system, and pheochromocytomas.Citation7 The tumor suppressor gene responsible for this syndrome is located on chromosome 3 (3p25-26).Citation8 Patients with the VHL syndrome inherit a defect on one allele of the VHL gene, and acquire a defect in the remaining allele. The majority of patients with sporadic (noninherited) clear cell RCC acquire defects of both alleles of the VHL gene with resulting dysfunction of the VHL protein. In sporadic clear cell RCC, both the maternal and paternal VHL alleles are inactivated by acquired mutations. The VHL protein functions as a tumor suppressor, and is responsible for ubiquination and proteasome degradation of hypoxia-inducible factor (HIF), a regulator of the hypoxic response.Citation9 Under hypoxia, or when VHL protein is nonfunctional, it does not bind and inactivate HIF-α, resulting in its accumulation. This in turn activates transcription of a variety of hypoxia-inducible genes, including vascular endothelial growth factor (VEGF), platelet-derived growth factor-β (PDGF-β), transforming growth factor-α, and erythropoietin. Clear cell cancers are highly vascular, secondary in part to stimulation of tumor associated angiogenesis. VHL protein plays a pivotal role in the control of neoangiogenesis, and loss of VHL gene function results in enhanced secretion of VEGF, PDGF, and creation of the vascular phenotype characteristic of clear cell RCC.
Retrospective analysis of untreated metastatic RCC patients has identified clinical characteristics associated with differences in prognosis. An initial model was developed at Memorial Sloan-Kettering Cancer Center (MSKCC), and validated at the Cleveland Clinic.Citation10,Citation11 These risk criteria have now been utilized in a series of Phase III clinical trials. The five factors include low Karnofsky performance status (<80%), low serum hemoglobin, high corrected calcium, elevated lactate dehydrogenase, and short disease-free interval (<1 year). Prognostic groups were defined as favorable (no factors), intermediate (≤2 factors), and poor (≥3 factors), with median overall survival (OS) of 28.0, 13.6, and 4.6 months, respectively.Citation10 Recently, these criteria have been reexamined, and alternate models proposed.Citation12
Finally, an understanding of the natural history of RCC is critical in understanding the clinical course of metastatic RCC patients, and planning treatment. Issues such as management of synchronous metastatic disease, indolent disease patterns, long disease-free intervals in selected patients, patterns of metastatic disease, and the frequency of disease recurrence in sites such as the central nervous system and bone are important considerations. A complete discussion of these is beyond the scope of this review, and the reader is referred to recent reviews.Citation13 In this context, a new treatment paradigm utilizing molecularly targeted agents has been developed for patients with metastatic clear cell RCC.
Treatment options for patients with metastatic RCC have changed dramatically over the past 6 years, and a new paradigm has evolved. Interferon-α (INF-α) and interleukin- 2 (IL-2) were the previous mainstays of therapy,Citation14 but since December 2005, six new agents have been approved in the US for the treatment of advanced RCC. Three are multi- targeted tyrosine kinase inhibitors (TKIs) including sunitinib,Citation15 sorafenib,Citation16 and pazopanib,Citation17 two target the mammalian target of rapamycin (temsirolimusCitation18 and everolimusCitation19), and one is a humanized monoclonal antibody (bevacizumab in combination with INF-α) which targets VEGF.Citation20 Sunitinib has emerged as the standard-of-care for treatment-naïve RCC patients, with the recent approvals of the bevacizumab and IFN-α combination and pazopanib providing additional options for frontline therapy.
Pazopanib: mode of action, clinical pharmacology
Preclinical
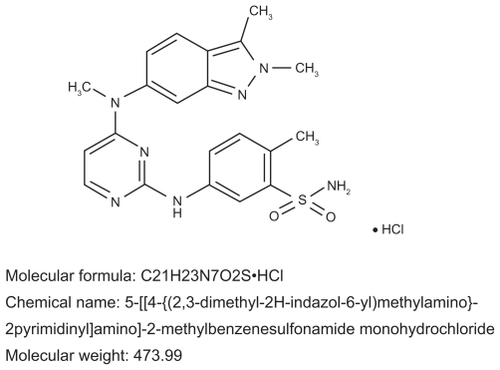
Pazopanib (GW786034; VotrientTM, GlaxoSmithKline, NC) is an indazolylpyrimidine 5-[[4-{(2, 3-dimethyl-2H-indazol- 6-yl)methylamino}-2-pyrimidinyl]amino]-2-methylbenzenesulfonamide that is orally bioavailable (). It was discovered during the screening of samples which included agents which inhibited the activities of VEGF receptor (VEGFR).Citation22 A monohydrochloride pyrimidine containing compound, pazopanib, which demonstrated both a favorable pharmokinetic profile and in vivo antitumor efficacy, was then selected for further development. When pazopanib was administered orally, optimal antitumor in vivo activity was noted. Since pazopanib demonstrated potency and selective inhibition of VEGFR, it was selected for further preclinical and clinical development.Citation22,Citation23
Figure 2 Molecular structure and chemical name of pazopanib.Citation21
Kumar et alCitation24 investigated the in vitro and in vivo pharmacodynamics of pazopanib. The inhibition of VEGF-induced phosphorylation of a wide variety of kinases in vitro was characterized. Kinases targeted for study included VEGFR1, VEGFR2 (from human, dog, mouse, and rat), VEGFR3, and a number of non-VEGFR kinases. Pazopanib inhibited the VEGFR kinase family, including VEGFR1, VEGFR2, and VEGFR3, and had similar activities against PDGFRα, PDGFRβ, and c-Kit (). Modest activity against fibroblast growth factor receptor 1, fibroblast growth factor receptor 3, and the c-fms receptor was also noted. It appeared from these data that pazopanib demonstrated modest selectivity in vitro.
Table 1 In vitro activity of pazopanib in purified human kinase and cell-based receptor assaysCitation24
Preclinical evaluation to assess the optimal pazopanib concentrations required for in vivo antitumor/antiangiogenic activity was also conducted.Citation24 In vivo inhibition of VEGFR2 phosphorylation in immunocomprised mice was assessed. Various transplantable tumor cell suspensions (HT29, A375P, PC3, Caki-2) were injected subcutaneously. In view of the poor solubility of pazopanib, sufficient parenteral drug levels were not achieved and a related compound, GW771806, with a kinase inhibitory spectrum similar to pazopanib was substituted. In vivo, Cmax, and drug exposure (area under curve [AUC]) did not appear to correlate with activity.
Pazopanib was further evaluated using oral administration. Dose-dependent growth inhibition of all tumor xenografts was reported, however, Caki-2, a RCC cell line, was the most sensitive to pazopanib (77% inhibition at 10 mg/kg dose, and complete inhibition at 100 mg/kg dose).
The effects of pazopanib on VEGF-induced VEGFR2 phosphorylation were then evaluated in vivo utilizing endothelial cells from mouse lungs and tumors. In the tumor xenograft studies, VEGFR2 phosphorylation of endothelial cells was not seen, perhaps reflecting low vessel density. In contrast, a single per os dose of 30 mg/kg of pazopanib inhibited lung endothelial VEGFR2 phosphorylation for over 8 hours. This dose corresponded to a plasma concentration >40 μmol/L. Below this, inhibition of VEGFR2 phosphorylation was minimal. These preclinical studies suggested pazopanib concentrations of ≥40 μmol/L would probably be required for optimal VEGFR2 inhibition, whereas the in vitro data suggested an IC50 of 0.02 μmol/L. The reasons for this difference may be related to the significant protein binding of pazopanib.
Clinical
The pharmacokinetics of pazopanib in human subjects were investigated in a Phase I clinical trialCitation25 in which 63 patients with refractory solid tumors received escalating doses of pazopanib (50 mg three times weekly, 50–2000 mg daily, and 300–400 mg twice daily). Plasma pazopanib was detected at all dose levels, however, its oral bioavailability and solubility are low, and therefore, absorption at doses above 800 mg once daily was limited. Geometric mean pazopanib t½ values ranged from 18.1 hours to 52.3 hours. In patients receiving 800 mg once daily the mean t½ was 30.9 hours. Mean Cmax and AUC0–24 on day 1 increased with increasing pazopanib dose levels. The highest values were seen in patients receiving 2000 mg daily. No evidence of drug accumulation was observed, and the steady-state exposure plateaued at 800 mg daily. These data suggested increasing pazopanib doses above 800 mg would not produce increased plasma drug levels. This schedule and dose were subsequently selected for Phase II and Phase III clinical trials.
Pazopanib absorption increases when administered with food, therefore, it is administered in the fasting state.Citation26 It is highly protein bound (>98.8%)Citation24 and is metabolized by cytochrome P450 (CYP) 3A4, and to a lesser degree by CYP1A2 and CYP2C8.Citation26 Potential interactions with CYP3A4 inhibitors or inducers are possible, but none have been reported. Pazopanib is excreted primarily via the fecal route. Studies estimate less than 4% is excreted in the urine, and therefore impaired renal function is not likely to alter systemic exposure. Citation26 Pazopanib metabolites are produced at low levels, and probably do not contribute to drug activity. Finally, age, race, and gender are reported to have minimal effects on the pharmacokinetics of pazopanib,Citation26 however, formal studies in Asians have not been reported.
Pazopanib: clinical trials
A series of clinical trials investigating the toxicity and efficacy of pazopanib have been reported. These include a Phase I trial in solid tumor patients, two large randomized trials in patients with advanced RCC, and a series of Phase I and II studies to assess pazopanib in various RCC patient subsets, or in combination with other targeted agents.
Phase I trial
The Phase I pazopanib clinical trial demonstrated that it was well tolerated and had antitumor activity.Citation25 Mild-to-moderate hypertension, diarrhea, hair depigmentation, and nausea were observed. Hypertension was the most frequent grade 3 adverse event. In 12 patients with metastatic RCC, a partial response was seen in two, stable disease in four, and progressive disease in four. These results demonstrated pazopanib was tolerated over a range of doses. The 800 mg once daily dose was recommended for future studies.
Phase II randomized discontinuation trial (RDT)
Based on this initial information, a Phase II RDTCitation27 was designed.Citation28 Metastatic RCC patients received 800 mg pazopanib orally on a daily schedule. Eligibility required metastatic or locally recurrent predominant clear cell RCC, treatment-naïve or cytokine/bevacizumab refractory patients, and measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST). An RDT design was employed initially, with all subjects receiving study drug. Patients with stable disease at 12 weeks were randomized to either continue pazopanib or a placebo. After approximately 60 patients had been entered, an interim analysis was performed. The overall response rate (ORR) in this group was 38%, and the Data Monitoring Committee recommended the trial be modified. The RTD approach was discontinued, and all patients received open-label drug until disease progression. The original sample size estimate as defined in the RTD study was utilized, namely, the progressive disease rate at 16 weeks post-randomization.
There were 225 patients entered into this trial, including the 55 patients from the RDT portion. The ORR was 35% (95% confidence interval [CI]: 28%–41%), when assessed by an independent review committee (IRC), and 34% (95% CI: 28%–40%) by investigator assessment. The ORR was similar in the treatment-naïve (34%, 95% CI: 26%–41%) and refractory patients (37%, 95% CI: 26%–49%). The median response duration was 68 weeks, and median progression free survival (PFS) 52 weeks (95% CI: 44–60 weeks).
Pazopanib was well tolerated, and the most common adverse events reported included diarrhea, fatigue, and hair depigmentation. Elevations of hepatic enzymes were seen, with increases of alanine aminotransferase (ALT) (54%) and/or aspartate aminotransferase (AST) (53%). Grade 3 or 4 elevations occurred in 7% and 10% of patients, respectively.
These Phase II results demonstrated significant clinical activity in patients with metastatic clear cell RCC. In view of previous reports documenting tumor regression and improved PFS in metastatic RCC patients receiving sunitinib,Citation15 sorafenib,Citation16 or bevacizumab,Citation20 this type of clinical result should have been expected. The RDT design was therefore not optimal, since it required discontinuation of an active agent in the setting of stable disease and/or an evolving clinical response.
Phase II randomized trial
Demonstration of pazopanib’s clinical benefit in metastatic RCC required a randomized, double-blind, placebo- controlled Phase III trial.Citation17 This pivotal study was designed and conducted at a time when standard therapy for metastatic RCC was in transition. This transition occurred at different times in different areas of the world. The study was placebo-controlled in order to definitively establish the activity of pazopanib, and provided placebo subjects the opportunity to crossover to pazopanib upon progression. When sunitinib or sorafenib were made available in various regions, enrolment ceased, unless access to the new agent was not possible. Untreated or cytokine refractory/intolerant patients with metastatic clear cell RCC were eligible. PFS based on IRC review was the primary endpoint. Secondary endpoints included ORR, OS, and toxicity. 435 patients (233 untreated, 202 previous cytokine therapy) were randomized (2:1 ratio) to either pazopanib 800 mg once daily (n = 290) or a placebo (n = 145). At the time of progression patients on the placebo arm were unblinded, and were then eligible for crossover to open-label pazopanib (extension trial, VEG107769). Blinding was discontinued for all subjects after the final PFS analysis, and placebo-treated subjects without progressive disease had the option to receive pazopanib (VEG107769). The trial design permitted detection of an 80% improvement in PFS and 50% improvement in OS.
The study results have been updated on several occasions.Citation17,Citation29,Citation30 The two arms were well balanced, and 95% of patients were either favorable or intermediate risk (MSKCC criteria).Citation10 All patients had clear cell (90%) or predominantly clear cell histology (10%). Prior nephrectomy had been performed in approximately 90% of patients. The intent-to-treat population included 233 untreated and 202 cytokine refractory patients. The study demonstrated improvement in median PFS for patients receiving pazopanib compared with the placebo group (9.2 months vs 4.2 months, hazard ratio [HR] for progression: 0.46, P < 0.0000001). This difference was more pronounced in treatment-naïve patients (11.1 months vs 2.8 months, HR: 0.40, P < 0.0000001) than in the cytokine refractory group (7.4 months vs 4.2 months, HR: 0.54, P < 0.001). A prespecified analysis of trial subgroups demonstrated that improvement of PFS was independent of age, performance status, gender, and MSKCC risk group. The data for the various MSKCC risk groups are not yet available.
ORR was higher in all patients receiving pazopanib compared with the control group (30% vs 3%). In treatment-naïve subjects, the ORR was 32% vs 4% for the placebo group. The median response duration was 59 weeks.
Selected efficacy data reported in various first-line Phase II/III trials of VEGF/VEGFR inhibitors in metastatic RCC patients (excluding the temsirolimus trial) are summarized in (PFS), and (OS). The ORR in treatment- naïve patients varies between 5.2% and 47% depending upon the trial, agent utilized, and type of analysis (independent vs investigator). The most active agent appears to be sunitinib, with an ORR of 37% (47% investigator assessment).Citation15,Citation34 The ORR observed with pazopanib appears similar (32% vs 37%). Responses appear to be durable with all agents, with median response durations between 11.0 months and 14.0 months.
Table 2 Progression free survival in frontline metastatic renal cell cancer randomized trials
Table 3 Overall survival in randomized trials: frontline metastatic renal cell cancer patients
An interim survival analysis in the pazopanib Phase III trial initially reported a median OS of 21.1 months for pazopanib vs 18.7 months for the placebo patient group (HR: 0.73, one-sided P = 0.02).Citation17 Final OS data are available, and revealed a median OS of 22.9 months for the pazopanib vs 20.5 months in the placebo cohort (HR: 0.91, 95% CI: 0.71– 1.16, stratified log rank P = 0.224).Citation29 A high rate of secondary therapy in placebo patients compared with those randomized to pazopanib was reported (66% vs 30%), with 54% of the placebo group ultimately receiving pazopanib.Citation29 In an inverse probability censoring weighted analysis which adjusts for the activity of pazopanib vs placebo, pazopanib therapy was associated with a 50% reduction in the risk of death.
Direct comparisons between the various trial results are not possible in view of the different trial designs and patient populations treated. Since the trials were conducted using similar endpoints and evaluation methods, the PFS data from these studies is illustrated in . The effect of pazopanib on PFS appears comparable to that of the other anti-angiogenic agents in either treatment-naïve or cytokine pretreated subjects.
Figure 3 Comparison of progression free survival data from recent phase II and II randomized clinical trials utilizing a variety of targeted agents in treatment-naïve or cytokine refractory patients with metastatic renal cell carcinoma.
Notes: apatient number; bhazard ratio (95% confidence interval).
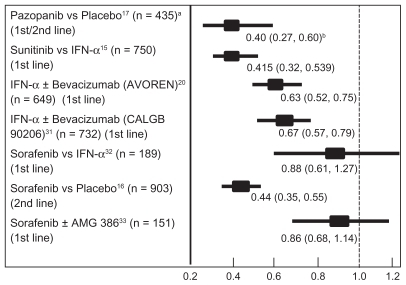
The pazopanib data have been comparedCitation37 to the Phase III trial results with sunitinib,Citation15,Citation34 sorafenib,Citation16,Citation38 and bevacizumab plus IFN-α.Citation20,Citation35 The adjusted indirect comparison methodology was utilized. Patient characteristics were reported as similar across the various trials. This approach suggested that pazopanib is superior to IFN-α with a PFS HR (HR: 0.50, 95% CI: 0.31–0.81). In treatment-naïve patients, the indirect PFS HR suggested pazopanib was not statistically different from sunitinib (HR: 0.93, 95% CI: 0.56–1.56) or bevacizumab plus IFN-α (HR: 0.79, 95% CI: 0.48–1.32). In cytokine refractory patients, the indirect PFS HR suggests that pazopanib is not different from sorafenib (HR: 0.98, 95% CI: 0.61–1.58). Further information from comparative randomized trials is now required to validate such comparisons. Currently, significant improvement in PFS is a surrogate endpoint accepted as demonstrating clinical benefit of therapy in metastatic RCC patients, and appears to correlate with OS.Citation39 The ultimate effect on survival however, remains a critical determinant of effectiveness. Comparisons of OS data were not reported by this group.
Phase II pazopanib trials: refractory patients
Recently, several groups have examined the efficacy of pazopanib in RCC patients who have progressed on other targeted agents including TKIs, bevacizumab, and mammalian target of rapamycin inbitors.Citation40,Citation41 A Phase II trial in 44 patients who had progressed or were intolerant of first-line sunitinib or bevacizumab was reported by Reeves et al.Citation40 These results are summarized in and suggest pazopanib has activity in this subset of patients. A report from MD Anderson HospitalCitation41 retrospectively reviewed 88 consecutive refractory RCC patients who had received one or more targeted therapies which included sunitinib (78%), sorafenib (40%), temsirolimus (20%), everolimus (51%), or bevacizumab (26%). This was a heavily pretreated group, with 26% having also received prior chemotherapy, and 16% prior cytokines. In patients failing one prior agent, an ORR of 42% was found, in contrast to 18% in patients failing more than one targeted treatment. In both reports, the toxicity of pazopanib was similar to that noted previously. Seven percent and 10% of patients discontinued pazopanib secondary to adverse drug events, respectively. These two reports suggest pazopanib has clinical activity not only in the cytokine refractory patient, but also in patients failing targeted agents.
Table 4 Phase II trial pazopanib in patients refractory to sunitinib or bevacizumabCitation40
Phase I pazopanib combination trials
Several Phase I trials are in progress investigating administration of pazopanib with other targeted agents, including bevacizumab (NCT 00992121)Citation42 and everolimus (NCT 01184326).Citation43 A recent reportCitation44 summarized the results of a phase I dose escalation trial combining temsirolimus and pazopanib to define the combination’s dose limiting toxicity (DLT). Solid tumor patients were eligible. The starting dose level included temsirolimus administered at a dose of 15 mg intravenously weekly, and pazopanib 400 mg per os daily. DLT was encountered at dose level 1, and a second dose level utilizing dose reductions of temsirolimus to 10 mg intravenously weekly and pazopanib to 200 mg daily was opened. DLT was again encountered. This trial enrolled only eight patients, but the authors suggest further development of this combination is not recommended secondary to grade 3 fatigue and electrolyte disturbances which limited therapy at lower than optimal dose levels. The possibility pazopanib will resemble sunitinib and sorafenib when combined with other targeted agents and produce unacceptable toxicity is suggested by these preliminary observations, and the results of the other combination trials are needed to fully assess this issue.
Strategies to increase pazopanib efficacy
One approach to enhance the efficacy of current RCC therapy involves development of biomarkers for patient selection. Clinical response to pazopanib therapy varies between patients, and biomarkers possibly predictive of treatment outcome have recently been identified. Xu and colleaguesCitation45 evaluated 27 single nucleotide polymorphisms within 13 genes in 397 patients with RCC receiving pazopanib. The association with PFS and ORR was analyzed, with a recent update of the results examining correlations with OSCitation46 (). Polymorphisms in IL-8, HIF1A, NR1l2, and VEGFA showed nominally significant association (P ≤ 0.05) with PFS when compared with the wild-type genotypes. Similarly, polymorphisms in IL-8, fibroblast growth factor receptor 2, VEGFRA, FLT4, and NR1l2 in 241 patients were associated with OS. The data suggest germline variants in angiogenesis- and drug exposure-related genes may predict pazopanib efficacy in metastatic RCC patients, and may also be useful in predicting treatment failure in certain patients. Validation of these results is now required, but these approaches appear to provide an alternative strategy to enhance efficacy.
Table 5 Genotypes for single nucleotide polymorphisms associated with pazopanib efficacyCitation45,Citation46
Finally, the relationship of drug exposure and efficacy of various TKIs in metastatic RCC has been suggested by several investigators.Citation47 Suttle et alCitation48 have investigated this relationship between pazopanib plasma concentrations at 4 and 12 weeks in patients treated in the phase II RTD pazopanib trial. A Cox regression analysis was utilized. Pharmacokinetic data were available from 205 patients. The median PFS in patients with a plasma pazopanib concentration ≥20.6 μg/mL (n = 143) at week 4 was 49.4 weeks vs 20.3 weeks for those with lower values (n = 62, P = 0.0041). Additionally, pazopanib concentrations at week 4 above 20.6 μg/mL were associated with a significantly higher ORR (64/143 vs 11/62 or 45% vs 18%, P = 0.000017). These data suggest pazopanib concentrations >20.6 μg/mL may be associated with improved efficacy. Prospective studies to optimize pazopanib exposure in non-responding patients who have levels ≤20.6 μg/mL would be of interest.
Comparative Phase II pazopanib trials
Several Phase III trials comparing pazopanib and sunitinib are in progress, and will provide important information on the comparative efficacy and tolerability of these two TKIs. VEGF pathway inhibitors produce a constellation of common side effects including fatigue, diarrhea, hypertension, and nausea. Potential differences between adverse event profiles may reflect the mechanisms of action, types of targets inhibited, potency, VEGF pathway inhibition selectivity, and pharmacokinetic differences. In the case of pazopanib, a Phase III trial directly comparing pazopanib and sunitinib is underway. This international blinded controlled trial (COMPARZ trial) randomizes untreated patients with metastatic clear cell RCC to either to pazopanib 800 mg daily or sunitinib 50 mg/day for 4 weeks on and 2 weeks off therapy.Citation49 The study is adequately powered (n = 876) for noninferiority, and will provide information on the efficacy and, importantly, the tolerability of pazopanib compared with the most frequently used agent in metastatic RCC patients.
A second Phase III trial is underway to examine patient preferences with regard to initial therapy for metastatic RCC (PISCES trial).Citation50 The design involves a randomized double blind, crossover trial in which 160 patients will receive either pazopanib or sunitinib at standard doses for two 10-week periods separated by a 2 week wash out phase. The trial will assess the tolerability and safety of these two TKIs and utilize patient reported outcomes to investigate differences.
Pazopanib safety and tolerability
Patients in the Phase II pazopanib RTD trial,Citation28 tolerated therapy without difficulty. The most common adverse events reported included diarrhea, fatigue, and hair depigmentation. Laboratory abnormalities reported included elevated ALT (54%) and AST (53%), with ≥grade 3 elevations noted in 7% and 9% of patients, respectively. In contrast, only mild hematologic toxicity (≤grade 2) was encountered, with neutropenia and thrombopenia reported in 27% and 26% of patients, respectively.
This toxicity profile was confirmed in the Phase III pivotal trial comparing pazopanib to a placebo.Citation17 In this study, pazopanib was also well tolerated. summarizes the most common adverse events (all grades) experienced by patients in both treatment arms. These included diarrhea (52%), hypertension (40%), and hair color changes (38%). Grade 3 and 4 adverse events were uncommon. Hand-foot syndrome, arterial thrombotic events, hypothyroidism, proteinuria, and stomatitis were noted in 3%–9% of patients. The most common laboratory abnormalities observed were grade 2 or less hepatic enzyme abnormalities and hyperglycemia. In a subsequent report,Citation30 the frequency and severity of adverse events did not change despite a 30% increase in cumulative pazopanib exposure.
Table 6 Phase II trial of pazopanib versus placebo in metastatic renal cell carcinoma – selected adverse events and clinical chemistry abnormalitiesCitation17,Citation29,Citation30
Severe hepatic toxicity (grade 3 or worse) has been reported in 4%–12% of patients receiving pazopanib.Citation28 Results of a meta-analysis investigating hepatic toxicity related to pazopanib are now available.Citation51 The studies analyzed included trials in which the pazopanib starting dose was 800 mg daily. Summary incidence rates, RR, and 95% CI were calculated using a fixed-effects or random-effects model. Eight trials involving 1155 patients with various solid tumors were included in the analysis. The incidence of ALT elevation was 41.7% (95% CI: 33.9–49.9) with 8.2% (95% CI: 5.9–11.3) characterized as high grade (≥grade 3). A significant increase in high grade ALT elevation in RCC patients (10.9% vs 5.7%, P = 0.012) was noted. The incidence of AST elevation was 39.3% (95% CI: 30.2–49.2) with 6.4% (95% CI: 4.6–8.8) being high grade. In this case, no differences between RCC and non-RCC patients (7.4% vs 4.8%, P = 0.22) were found. When compared to controls, an increased risk of high grade ALT elevation (RR: 7.95, 95% CI: 2.22–28.55, P = 0.001) and high grade AST elevation (RR: 9.01, 95% CI: 1.71–47.50, P = 0.01) were noted. The authors conclude pazopanib administration may be associated with a risk of ≥grade 3 hepatotoxicity, and the frequency may be dependent upon tumor type.
The most common adverse events associated with pazopanib, sunitinib, and bevacizumab plus IFNα are summarized in . Grade 3 hepatic toxicity may be more frequent with pazopanib than with other TKIs such as sunitinib. In contrast, neutropenia and thrombocytopenia appear less frequent. Such comparisons of toxicity must be investigated prospectively, however, the adverse event profiles of these three treatments have been indirectly compared.Citation37 Adverse events were common with all agents (92%–99%). The rates of serious adverse events were also similar among these treatments (27%–34%). The frequency of ≥grade 3 adverse events was lower with pazopanib (44%, 95% CI: 40%–48%) compared to sunitinib (67%, 95% CI: 62%–71%) or bevacizumab plus IFN-α (60%, 95% CI: 55%–66%). This type of indirect comparison method suggests there may be differences between these agents, even including class effects such as hypertension. Safety comparisons must be interpreted based on the length of exposure to drug as well as other trial variables.
Table 7 Selected adverse events (all grades and ≥grade 3): sunitinib, bevacizumab + IFN-α, and pazopanib pivotal trials
The health-related quality of life (HRQOL) data from the Phase III pazopanib vs placebo trial are also of interest when considering patient tolerability.Citation52 HRQOL outcome was assessed using European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ-C30) and EQ-5D index and visual analog scale. The time to ≥20% decline from baseline HRQOL was estimated for all patients, by prior therapy, and stratified by response (RECIST). The authors noted placebo treated patients were more likely to experience ≥20% HRQOL decline (QLQ-C30) (HR: 0.77, 95% CI: 0.57–1.03, P = 0.0817). Patients with RECIST responses experienced significantly less HRQOL deterioration compared to progressive disease patients. These results supporting the tolerability profile of pazopanib and suggest patients who had tumor response also experienced better HRQOL compared to those without response.
Conclusion: role of pazopanib in RCC therapy
In the Phase III randomized double-blind placebo-controlled trial, pazopanib treatment produced a highly significant PFS improvement for either treatment-naïve or cytokine pretreated patients. The efficacy and safety results from this study were similar to those from the Phase II nonrandomized open label RTD trial in a similar population. Certain adverse reactions are common to the anti-VEGF agent class, however, their incidence and severity varies widely. The potential difference in the safety profiles of these agents provides treatment options for patients with advanced RCC. Pazopanib represents a treatment option with comparable efficacy, and potential important differences in tolerability vs the current standard of care. As such, it appears to represent a valuable addition to the treatment of advanced RCC. The following observations on efficacy and toxicity are relevant when considering the role of pazopanib in patients with metastatic clear cell RCC:
Efficacy of pazopanib
A large and very significant improvement in PFS was demonstrated with pazopanib compared to a placebo in patients with advanced metastatic RCC. The concordance of the IRC and the investigator assessments demonstrate the robust nature of the findings.
Subgroup analyses demonstrate PFS improvement was consistent across multiple clinically relevant subgroups. Importantly, these improvements were seen in prespecified groups, including both treatment-naïve and cytokine refractory patients.
The median PFS reported in the Phase II RTD study (10.4 months) was similar to that reported in the pivotal study (all patients: 9.2 months, treatment-naïve: 11.1 months), and resembles those of the current standard metastatic RCC therapy sunitinib (11 months in first-line).
The survival data suggest a favorable trend associated with pazopanib treatment, in spite of significant crossover by subjects in the placebo arm.
The median OS of placebo patients in the pivotal study was substantially longer than reported for a historical group of metastatic RCC patients receiving cytokines.Citation53 This may reflect the confounding effects of secondary therapy.
The Phase II results in TKI refractory patients with pazopanib resemble those reported with other TKIs, and are consistent with a lack of cross resistance.
Safety of pazopanib
The most common side effects of pazopanib include diarrhea, hypertension, hair color changes, nausea, fatigue, anorexia, and vomiting. Most adverse events are grade 1 or 2, and do not require discontinuation of therapy.
The most common serious adverse events associated with pazopanib include diarrhea, dyspnea, pleural effusion, abdominal pain, and vomiting. More serious liver function abnormalities, arterial/thrombotic events, and hemorrhagic events have also been reported.
The most common laboratory abnormalities produced include grade 1 or 2 ALT, AST, and bilirubin elevations, hyperglycemia, and electrolyte abnormalities. The most common serious laboratory abnormalities (≥grade 3) were hepatic enzyme elevations. Cytopenias occur in pazopanib treated patients, but ≥grade 3 hematologic toxicity is seldom seen.
Grade 3 or greater elevations of hepatic enzymes occur in from 8% to 13% of patients treated with pazopanib. The time course of this development is well characterized, and recommendations for monitoring liver functions are available. Importantly, these liver enzyme elevations will generally normalize with adequate follow-up. In patients who continue to receive pazopanib despite transaminase elevations, adaptation has been noted.
Fatal liver toxicity related to pazopanib is rare (0.05%– 0.1%), and has also been reported with other TKIs such as sunitinib and sorafenib.
Adverse events previously described with other TKIs, such as cardiac/cerebral ischemia, hemorrhage, and bowel perforation, are also observed with pazopanib. However, no evidence of left ventricular dysfunction has been reported secondary to pazopanib administration.
Patients receiving pazopanib may exhibit differences in tolerability when compared to individuals treated with the other approved anti-VEGF TKIs. This includes a possible lower incidence of mucositis, hand-foot syndrome, fatigue, and hematologic toxicity ().
In summary, the overall risk benef it of pazopanib appears favorable. The available data demonstrate pazopanib has efficacy in patients with metastatic RCC who are either treatment-naïve or cytokine refractory. The significant improvement of PFS compared to a placebo noted in the pivotal Phase III trial is an acceptable surrogate of clinical benefit. The final analysis of OS failed to demonstrate significant improvement, however, the influence of crossover and the confounding effects of secondary therapy may be responsible factors. The final median OS for pazopanib patients in the combined population of treatment-naive and cytokine refractory individuals was 22.4 months, for the placebo subjects was 20.1 months. The OS of this latter group is different from historical data for metastatic RCC patients who have received either a placebo or cytokine therapy. This may be a reflection of the crossover study design.
These benefits should be examined in the context of possible pazopanib associated toxicity. Pazopanib’s safety profile is well documented, and the majority of adverse events are grade 1 or 2. The frequency of grade 3 and 4 events is generally low. The majority of associated adverse events are easily managed. It is important to note that the adverse events reported with pazopanib have also been reported with the other agents approved for metastatic RCC. The incidence and severity of various adverse events varies between agents, with these differences potentially impacting drug utilization. The relatively low incidence of severe myelosuppression, hand-foot syndrome, stomatitis, and fatigue compared with the safety profile of other agents of this class such as sunitinib in metastatic RCC position pazopanib as a potential therapeutic option. The COMPARZ clinical trial which compares the safety and efficacy of pazopanib and sunitinib will provide the comparative data required to determine whether pazopanib has similar efficacy and an improved toxicity profile. Generally efficacy guides therapeutic decisions when toxicity is equivalent.
Disclosure
Consultant: GlaxoSmithKline, Pfizer, Genentech, Novartis, Argos, Agenus. Speaking: Pfizer, Genentech, Novartis.
References
- JemalASiegelRXuJWardECancer statistics, 2010CA Cancer J Clin.201060527730020610543
- FerlayJShinHRBrayFFormanDMathersCParkinDMInternational Agency for Research on CancerGLOBOCAN 2008Cancer incidence and mortality worldwide: CancerBase102008 Available from: http://globocan.iarc.frAccessed June 21, 2011
- TsivianMMoreiraDMCasoJRMouravievVPolascikTJCigarette smoking is associated with advanced renal cell carcinomaJ Clin Oncol.201129152027203121502558
- YoungACCravenRACohenDAnalysis of VHL gene alterations and their relationship to clinical parameters in sporadic conventional renal cell carcinomaClin Cancer Res.200915247582759219996202
- VarelaITarpeyPRaineKExome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinomaNature.2011469733153954221248752
- GeorgeDJKaelinWGJrThe von Hippel-Lindau protein, vascular endothelial growth factor, and kidney cancerN Engl J Med.2003349541942112890838
- ButmanJALinehanWMLonserRRNeurologic manifestations of von Hippel-Lindau diseaseJAMA.2008300111334134218799446
- LatifFToryKGnarraJIdentification of the von Hippel–Lindau disease tumor suppressor geneScience.19932605112131713208493574
- KaelinWGJrThe von Hippel-Lindau tumor suppressor protein and clear cell renal carcinomaClin Cancer Res.2007132 Pt 2680s684s17255293
- MotzerRJBacikJMurphyBARussoPMazumdarMInterferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinomaJ Clin Oncol.200220128929611773181
- MekhailTMAbou-JawdeRMBoumerhiGValidation and extension of the memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinomaJ Clin Oncol.200523483284115681528
- HengDYXieWReganMMPrognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter studyJ Clin Oncol.200927345794579919826129
- RiniBICampbellSCEscudierBRenal cell carcinomaLancet.200937396691119113219269025
- AtkinsMBReganMMcDermottDUpdate on the role of interleukin 2 and other cytokines in the treatment of patients with stage IV renal carcinomaClin Cancer Res.20041018 Pt 26342S6346S15448028
- MotzerRJHutsonTETomczakPSunitinib versus interferon alfa in metastatic renal-cell carcinomaN Engl J Med.2007356211512417215529
- EscudierBEisenTStadlerWMSorafenib in advanced clear-cell renal-cell carcinomaN Engl J Med.2007356212513417215530
- SternbergCNDavisIDMardiakJPazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trialJ Clin Oncol.20102861061106820100962
- HudesGCarducciMTomczakPTemsirolimus, interferon alfa, or both for advanced renal-cell carcinomaN Engl J Med.2007356222271228117538086
- MotzerRJEscudierBOudardSEfficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trialLancet.2008372963744945618653228
- EscudierBPluzanskaAKoralewskiPBevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trialLancet.200737096052103211118156031
- LiuDQChenTKMcGuireMAKordASAnalytical control of genotoxic impurities in the pazopanib hydrochloride manufacturing processJ Pharm Biomed Anal.200950214415019427156
- HarrisPACheungMHunterRN3rdDiscovery and evaluation of 2-anilino-5-aryloxazoles as a novel class of VEGFR-2 kinase inhibitorsJ Med Chem.20054851610161915743202
- HarrisPABoloorACheungMDiscovery of 5-[[4-[(2,3- dimethyl-2H-indazol-6-yl)methylamino]-2 pyrimidinyl]amino] 2 methylbenzenesulfonamide (pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitorJ Med Chem.200851154632464018620382
- KumarRKnickVBRudolphSKPharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activityMol Cancer Ther.2007672012202117620431
- HurwitzHIDowlatiASainiSPhase I trial of pazopanib (GW786034), an oral multikinaseangiogenesis inhibitor, in patients with advanced cancer: results of safety, pharmacokinetics, and clinical activityClin Cancer Res.200915124220422719509175
- Food and Drug AdministrationVotrientTM. Full prescribing information102009 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022465lbl.pdfAccessed July 23, 2011
- RatainMJEisenTStadlerWMPhase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinomaJ Clin Oncol.200624162505251216636341
- HutsonTEDavisIDMachielsJHEfficacy and safety of pazopanib in patients with metastatic renal cell carcinomaJ Clin Oncol.201028347548020008644
- SternbergCNHawkinsRESzczylikCRandomized, double- blind phase III study of pazopanib in patients with advanced/metastatic renal cell carcinoma (MRCC): final overall survival (OS) resultsAnn Oncol201021Suppl 8 abstract LBA22
- SternbergCNHawkinsRESzczylikCA randomized, double-blind phase III study (VEG105192) of pazopanib (paz) versus placebo (pbo) in patients with advanced/metastatic renal cell carcinoma (mRCC): updated safety resultsJ Clin Oncol201129Suppl 7 abstract 313
- RiniBIHalabiSRosenbergJEPhase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206J Clin Oncol.201028132137214320368558
- EscudierBSzczylikCHutsonTERandomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinomaJ Clin Oncol.20092781280128719171708
- RiniBISzczylikCTannirNMAMG 386 in combination with sorafenib in patients (pts) with metastatic renal cell cancer (mRCC): a randomized, double-blind, placebo-controlled, phase II studyJ Clin Oncol201129Suppl 7 abstract 309
- MotzerRJHutsonTETomczakPOverall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinomaJ Clin Oncol.200927223584359019487381
- EscudierBBellmuntJNegrierSPhase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survivalJ Clin Oncol.201028132144215020368553
- RiniBIHalabiSRosenbergJEBevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206J Clin Oncol.200826335422542818936475
- McCannLAmitOPanditeLAmadoRGAn indirect comparison analysis of pazopanib versus other agents in metastatic renal cell carcinoma (mRCC)J Clin Oncol201028Suppl abstract e15128
- EscudierBEisenTStadlerWMSorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trialJ Clin Oncol.200927203312331819451442
- HengDYXieWBjarnasonGAProgression-free survival as a predictor of overall survival in metastatic renal cell carcinoma treated with contemporary targeted therapyCancer.2011117122637264221656741
- ReevesJASpigelDRDanielDBFriedmanEKBurrisHAHainsworthJDPazopanib in patients with metastatic renal cell carcinoma previously treated with sunitinib or bevacizumab: a Sarah Cannon Research Institute phase II trialJ Clin Oncol201129Suppl abstract 4659
- MatranaMRAtkinsonBJCornPGJonaschETannirNMMetastatic renal cell carcinoma treated with pazopanib after progression on other targeted agents: a single-institution experienceJ Clin Oncol201129Suppl 7 abstract 351
- ClinicalTrials.govAn open-label pharmacodynamic study of bevacivumab and pazopanib in renal cell carcinoma Oct 82009 Available from: http://clinicaltrials.gov/ct2/show/NCT00992121Accessed June 22, 2011
- ClinicalTrials.govPazopanib and everolimus in patients with advanced solid tumors and previously treated kidney cancer Aug 172010 Available from: http://clinicaltrials.gov/ct2/show/NCT01184326Accessed June 22, 2011
- SemradTJEddingsCDutiaMPChristensenSLauDLaraPPhase I study of temsirolimus (Tem) and pazopanib (Paz) in solid tumors with emphasis on renal cell carcinoma (RCC)J Clin Oncol201129Suppl abstract e15113
- XuCBingNXBallHAPazopanib efficacy in renal cell carcinoma: evidence for predictive genetic markers in angiogenesis-related and exposure-related genesJ Clin Oncol.201129182557256421576632
- XuCBallHABingNAssociation of genetic markers in angiogenesis- or exposure-related genes with overall survival in pazopanib (P) treated patients (Pts) with advanced renal cell carcinomaJ Clin Oncol201129Suppl 7 abstract 303
- HoukBEBelloCLPolandBRosenLSDemetriGDMotzerRJRelationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysisCancer Chemother Pharmacol.201066235737119967539
- SuttleBBallHAMolimardMRelationship between exposure to pazopanib (P) and efficacy in patients (pts) with advanced renal cell carcinoma (mRCC)J Clin Oncol201028Suppl abstract 3048
- ClinicalTrials.govPazopanib versus sunitinib in the treatment of locally advanced and/or metastatic renal cell carcinoma (COMPARZ) July 222008 Available from: http://clinicaltrials.gov/ct2/show/NCT00720941Accessed June 22, 2011
- ClinicalTrials.govPatient preference study of pazopanib versus sunitinib in advanced or metastatic kidney cancer (PISCES) February 42010 Available from: http://clinicaltrials.gov/ct2/show/NCT01064310Accessed June 22, 2011
- KapadiaSHapaniSWuSRisk of high-grade liver toxicity with pazopanib in patients with cancer: a meta-analysisJ Clin Oncol201129Suppl abstract 4595
- PickardACellaDDuhMSHealth-related quality of life in patients with advanced renal cell carcinoma receiving pazopanib or placebo in a randomized phase III trialJ Clin Oncol201129Suppl abstract 9096
- CoppinCPorzsoltFAwaAKumpfJColdmanAWiltTImmunotherapy for advanced renal cell cancerCochrane Database Syst Rev20051CD00142515674877