Abstract
Metastatic events to the bone occur frequently in numerous cancer types such as breast, prostate, lung, and renal carcinomas, melanoma, neuroblastoma, and multiple myeloma. Accumulating evidence suggests that the inflammatory cytokine interleukin (IL)-6 is frequently upregulated and is implicated in the ability of cancer cells to metastasize to bone. IL-6 is able to activate various cell signaling cascades that include the STAT (signal transducer and activator of transcription) pathway, the PI3K (phosphatidylinositol-3 kinase) pathway, and the MAPK (mitogen-activated protein kinase) pathway. Activation of these pathways may explain the ability of IL-6 to mediate various aspects of normal and pathogenic bone remodeling, inflammation, cell survival, proliferation, and pro-tumorigenic effects. This review article will discuss the role of IL-6: 1) in bone metabolism, 2) in cancer metastasis to bone, 3) in cancer prognosis, and 4) as potential therapies for metastatic bone cancer.
Introduction
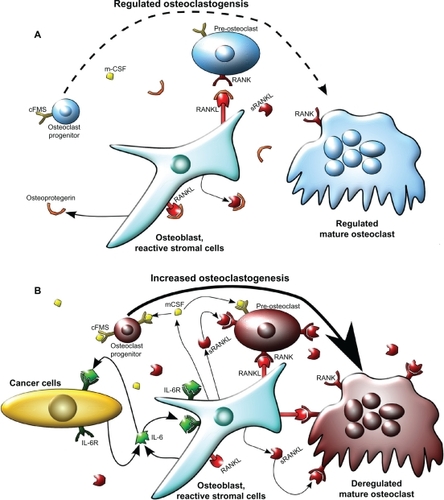
Bone homeostasis is maintained by a variety of cell types that control remodeling of the bone matrix. Two important cell types that mediate bone homeostasis are osteoblasts and osteoclasts. Osteoblasts contribute to the bone matrix by production of type I collagen, deposition of hydroxyapatite crystals into the collagen matrix, and regulation of osteoclast activity.Citation1,Citation2 Osteoblasts are of mesenchymal origin and differentiate from pre-osteoblasts. This process occurs via bone morphogenic proteins (BMPs) that induce runt-related transcription factor 2 (Runx2), leading to increased alkaline phosphatase activity.Citation1 Conversely, osteoclasts resorb bone matrixCitation3 and differentiate from the hematopoietic cell lineage upon stimulation in a differentiation process called osteoclastogenesis. Osteoclastogenesis is mediated by cytokines such as receptor activator of nuclear factor (NF)-κB ligand (RANKL) and macrophage-colony stimulating factor (m-CSF) ().Citation3,Citation4 RANKL, a membrane-bound ligand, and m-CSF a secreted factor, are predominantly produced by osteoblasts.Citation5 Osteoclastogenesis is regulated primarily via RANKL and osteoblast-produced osteoprotegrin (OPG) expression, a decoy receptor to RANKL that suppresses RANKL activity.Citation6 Osteoblasts that express RANKL have cell-to-cell contact with osteoclasts via ligand-receptor binding between RANKL and RANK (receptor activator of NF-κB) expressed on osteoclasts.Citation7 RANKL functions to promote osteoclast differentiation and activity through stimulation of various pathways including the phosphatidylinositol-3 kinase (PI3K) pathway and the mitogen activated protein kinase (MAPK) pathway. The MAPK pathway leads to the activation of c-fos, nuclear factor of activated T-cells-2 (NFAT2), and other transcription factors.Citation8,Citation9 Cleavage of RANKL from the cell membrane by proteinases such as matrix metalloproteinase-7 (MMP7) yields the soluble form of RANKL (sRANKL), which has a physiological function that is still disputed, although both anti- and pro-osteoclastogenic effects have been reported.Citation5,Citation10–Citation12
Figure 1 Model of osteoclastogenesis during bone homeostasis and tumor cell metastasis to bone. A) In normal bone, RANKL and m-CSF are produced primarily by osteoblasts. m-CSF binds to its receptor c-FMS, expressed on osteoclast progenitors, and RANKL binds to its receptor on pre-osteoclasts to promote osteoclastogenesis. Osteoprotegrin, also produced by osteoblasts, acts as a decoy receptor for RANKL and negatively regulates osteoclast differentiation. In this model, osteoblast and osteoclast activity are in homeostasis through careful regulation of osteoclastogenesis. B) When cancer cells metastasize to the bone, increased IL-6 may be produced by both the cancer cells and the osteoblasts, as an inflammatory response to the cancer cells. IL-6 then stimulates various types of stromal cells in the bone, which include bone marrow cells, osteoblasts, and fibroblasts in the area of the metastasis, to increase the expression of RANKL and m-CSF by osteoblasts. This IL-6-mediated increase in RANKL and m-CSF also occurs with injury and inflammation to the bone, but unlike in cancer metastasis, it is transient. RANKL and m-CSF then, in turn, activate the osteoclast differentiation cascade, where m-CSF strongly stimulates early stages of osteoclast differentiation, and RANKL stimulates late stages of osteoclast differentiation, as well as osteoclast activity. Once this occurs, osteoclast activity becomes dysregulated and reduces bone integrity.
Abbreviations: c-FMS, colony stimulating factor 1 receptor; IL-6, interleukin 6; IL-6R, IL-6 receptor; m-CSF, macrophage-colony stimulating factor; RANKL, receptor activator of nuclear factor κB ligand; sRANKL, soluble form of RANKL.
As osteoclasts differentiate in response to pro-osteoclastic factors, these cells create a segregated zone, a sealed area between the osteoclast and the bone matrix.Citation9 Osteoclasts then release hydrogen ions into the segregated zone, solubilizing the hydroxyapatite crystals and promoting acid-activated proteinases such as cathepsin K to degrade the collagen matrix.Citation9,Citation13 Osteoblasts generate new matrix to fill the vacant area. The rate at which osteoclasts differentiate and resorb bone is carefully regulated by osteoblast-produced RANKL and OPG. Other cells in the bone matrix such as osteocytes, terminally differentiated osteoblasts, are able to regulate the generation and resorption of bone matrix by affecting osteoblast and osteoclast activity.Citation14 When osteocytes are mechanically stimulated by shock to bone resulting in dynamic fluid movement, they promote alkaline phosphatase activity in osteoblasts by cell-to-cell contact through the RANK/RANKL complex, increasing bone mineralization and turnover.Citation15–Citation17 In this manner, damaged sections of the bone are removed and are replaced with new bone matrix by osteoblasts.
In normal bone, homeostasis is maintained and bone integrity is preserved by a continuous cycle of bone renewal. However, when cancer cells metastasize to the bone, the balanced and complex interplay of the cells is disrupted, leading to a pathologic condition that compromises bone integrity. One of the many characteristics that bone-homing cancer cells have in common is that most of them release copious levels of interleukin (IL)-6, which helps in facilitating bone invasion and growth of metastatic lesions.Citation18–Citation20 In this review article, the role of IL-6 in facilitating bone metastasis and approaches to measure serum IL-6 to predict progression of metastatic disease will be discussed. Additionally, new therapies targeting IL-6 and their potential efficacy in preventing bone metastasis will be reviewed.
Frequency, consequences, and mechanisms of cancer cell metastases to bone
Various types of cancers metastasize to the bone, including breast, prostate, lung, thyroid, kidney, multiple myeloma, melanoma, and neuroblastoma.Citation21–Citation25 Usually the bone is only compromised at the site of metastasis, and not all types of bone metastases affect the bone in the same way. For example, breast cancer predominantly causes osteolytic lesions, resulting in an upregulation of osteoclast activity and subsequent decreased bone density and integrity that may lead to fractures.Citation22,Citation26 Conversely, prostate cancer results in primarily osteoblastic lesions that are caused by cytokine-induced upregulation of osteoblast activity and subsequent increased bone density.Citation26 This type of bone metastasis causes thickening of the bone, resulting in the possibility of nerve compression, vertebral fusion, and spinal cord compression depending on the location of the metastases. In contrast to what is found in normal bone where collagen fibers are highly organized and tightly packed, bone created by osteoblastic lesions contains disorganized and fragile collagen fibrils.Citation27 This leads to a high degree of bone brittleness, increase in potential fractures, and pain as the normal bone is replaced by abnormal bone created by the osteoblastic lesions. A subset of prostate cancers may also cause osteolytic lesions due to the expression of different cytokines that promote osteoclast activity rather than osteoblast activity.Citation28 Multiple myeloma causes only osteolytic lesions. Other cancers, including lung, kidney, and thyroid carcinomas, result in primarily osteolytic lesions, but osteoblastic lesions occur occasionally.Citation26,Citation29 Metastasis of the primary tumor to the bone occurs in about 60%–75% of patients with metastatic breast cancer, prostate cancer, neuroblastoma, or multiple myeloma.Citation21–Citation23,Citation30 Metastases to the bone from other cancers such as lung, kidney, and thyroid only occur in 30%–50% of patients with metastases.Citation24
The molecular mechanisms that determine when a cancer cell will metastasize to bone are not completely understood. Recent evidence shows that the CXC chemokine receptor 4/chemokine (C-X-C motif) ligand 12 CXCR4/CXCL12 axis may play a role in this metastatic process. Studies have demonstrated that cancer cells are attracted to the bone marrow due to the relatively high levels of CXCL12 expressed by osteoblasts, which acts as an attractant for the CXCR4 ligandpositive cancer cells.Citation31 Numerous studies have demonstrated that bone metastatic cancer cells from the breast, prostate, and myeloma overexpress the CXCR4 ligand, which promotes homing and metastasis to the bone and other organs.Citation32–Citation35 Inflammatory cytokines, such as IL-6, increase CXCR4 expression in breast cancer cells, specifically in a signal transducer and activator of transcription 3 (STAT3), and c-Jun-dependent manner.Citation36 Given these findings, therapeutics designed to block the CXCR4/CXCL12 axis are being evaluated in the prevention of bone metastases.Citation37
Once cancer cells colonize in the bone, they have to adapt to the challenges of cell survival and growth in a foreign tissue environment. The bone is a reservoir of a complex mixture of growth factorsCitation38 that are released as the bone is degraded by metastatic lesions. The mixture of these growth factors include transforming growth factor (TGF)-β, insulin like growth factor (IGF)-1, insulin-like growth factor (IGF)-2, platelet derived growth factor (PDGF), bone morphogenic proteins (BMP), fibroblast growth factors (FGF), and other factors that significantly improve tumor cell survival and growth.Citation39 These factors can promote the expression of prosurvival signals such as B-cell lymphoma 2 (Bcl-2) and AKT, which inhibit apoptosis in the cancer cells. In addition, these factors can also support further osteoclast differentiation and activity, leading to a vicious positive feedback loop (the vicious tumor–bone cycle) where additional growth factors are released, stimulating increased cancer cell growth and accelerated bone destruction. This accelerated bone destruction can lead to rapid loss of bone integrity in cancer patients causing fractures, pain, and loss of mobility.
IL-6, other cytokines, and growth factors in the bone microenvironment
IL-6 is a major pleiotropic, pro-inflammatory cytokine which plays a role in immune response, hematopoiesis, cell differentiation, wound repair, and bone remodeling.Citation40,Citation41 Inflammation in the bone caused by injury or disease increases expression of IL-6 by reactive stromal cells of the bone and infiltrating monocytes and macrophages, promoting bone remodeling evidenced by higher osteoclast activity.Citation42 The reactive stromal cells for bone metastases are generally the mesenchymal stem cells in the bone marrow as well as the fibroblasts, osteoblasts, and osteocytes in the region. IL-6 production is directly stimulated by prostaglandin E2 (PGE2) and TGF-β, while IL-1β and lipopolysaccharides indirectly stimulate IL-6 production via NF-κB activation ().Citation43–Citation48 IL-6 binds to its heterotrimeric receptor, consisting of two gp130 subunits and an IL-6 receptor subunit, on target cells and activates the STAT, MAPK, and PI3K pathways.Citation49–Citation52 IL-6 signaling through the Janus kinase (JAK)/STAT3 pathways lead to expression of RANKL from osteoblast/stromal cells, causing direct stimulation of osteoclast differentiation and activity and resulting in bone destruction ().Citation53,Citation54 Studies using IL-6 knockout mice have demonstrated that IL-6 is necessary for upregulating osteoclast activity and bone resorption in vivo. IL-6 knockout mice were shown to be protected from increased osteoclast activity and subsequent bone degradation when their bones were injected with the arthritis-inducing antigen heat-killed Mycobacterium tuberculosis.Citation55 IL-6 knockout bones that received antigen injections had less RANKL and IL-17 expression as well as reduced osteolysis and cartilage destruction near the site of injection compared with wild-type mice. IL-17 is a proinflammatory and pro-osteoclastogenic cytokine implicated in arthritis and tumorigenesis that is produced in CD4+ helper and tumor infiltrating T-cells when activated by IL-6.Citation56,Citation57 Additional mouse studies have demonstrated that inhibition of IL-6 activity, with an IL-6 receptor (IL-6R) antagonist that inhibits downstream receptor signaling, reduces bone resorption.Citation58 These results suggest that IL-6 plays a major role in the upregulation of additional pro-osteoclastic factors essential for osteoclast activity.
Figure 2 Factors that increase IL-6 production in response to various stimuli. Increased IL-6 production is associated with stimuli such as infection and inflammation. Infection, injury, and cancer can all stimulate inflammation that can lead to the increase of IL-6-modulating factors such as IL-1β, COX-2, PGE2, and TGF-β. Infection can also promote LPS secretion from bacteria, which increases NF-κB-dependent IL-6 levels. There are two main IL-6 production pathways: NF-κB-dependent and NF-κBindependent. NF-κB-independent pathways upregulate IL-6 secretion via TGF-β or PGE2, which is produced downstream of COX-2 activation. In the NF-κB-dependent pathway, LPS or IL-1β stimulate NF-κB activity that causes an increase in IL-6 production.
Abbreviations: COX-2, cyclooxygenase 2; IL-1β, interleukin 1β; IL-6, interleukin 6; LPS, lipopolysaccharide; NF-κB, nuclear factor κB; PGE2, prostaglandin E2; TGF-β, transforming growth factor β.
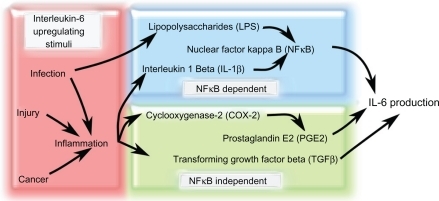
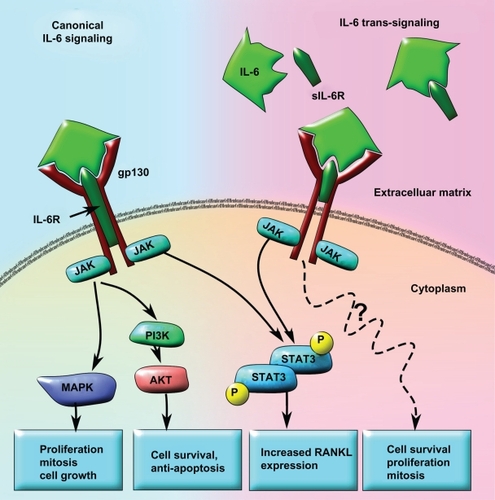
Figure 3 Model of canonical IL-6 signaling versus IL-6 trans-signaling in tumor progression and metastases. In the canonical IL-6 signaling pathway, the IL-6 receptor subunit is membrane bound and forms a heterotrimer with two gp130 subunits. When IL-6 binds to the receptor, STAT3 is activated in a JAK-dependent manner that leads to increased RANKL expression. IL-6 may also activate AKT via increased JAK-dependent PI3K activity and result in cell survival and anti-apoptosis signaling. Concomitantly, increased MAPK activity downstream of JAK activation can lead to upregulated cell growth, proliferation, and mitosis. In the IL-6 trans-signaling pathway, IL-6 first binds to the truncated sIL6R. The IL-6/sIL6R complex then binds to the membrane-bound gp130 dimer to form an IL-6 trans-signaling complex. Due to the fact that the sIL-6R lacks a membrane signaling domain, there appears to be significant differences in the intracellular signaling pathways. While IL-6 trans-signaling also leads to phosphorylation and activation of STAT3, increased cell survival, proliferation, and mitosis occurs in an AKT-and MAPK-independent manner. The exact mechanisms for IL-6 trans-signaling leading to increased cell survival, proliferation, and mitosis are not yet known.
Abbreviations: IL-6, interleukin 6; JAK, Janus kinase; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; RANKL, receptor activator of nuclear factor κB ligand; sIL6R, soluble IL-6 receptor; STAT3, signal transducer and activator of transcription 3.
Deregulation of IL-6 expression is implicated in disorders of bone homeostasis such as osteoporosis and osteopetrosis. Sex hormones such as 17-β-estradiol and testosterone have been shown to regulate IL-6 levels in the bone microenvironment. 17-β-estradiol is known for its bone-preserving effects, which is supported by the fact that post-menopausal women experience a decrease in bone mineralization and density that may lead to osteoporosis.Citation59 A recent study shows that 17-β-estradiol reduces both IL-6 and IL-8 production by monocytes and multiple myeloma cells through a mechanism that is not yet fully understood.Citation60,Citation61 The chemokine IL-8 is also a pro-inflammatory molecule, which like IL-6, can increase inflammation in the bone and cause excessive bone resorption by upregulating the transcription factor NF-κB.Citation62,Citation63 In turn, increased NF-κB activity stimulates IL-6 expression and secretion into the extracellular matrix.Citation64 Studies have shown that the binding of 17-β-estradiol to the estrogen receptor inhibits NF-κB transcriptional activity by preventing inhibitor of NF-κB alpha (IκBα) degradation, leading to decreased IL-6 expression.Citation60,Citation65 IκBα is normally constitutively expressed and bound to NF-κB, thus preventing the translocation of the transcription factor into the nucleus and initiation of the transcription of NF-κB-related genes.Citation66 17-β-estradiol has also been shown to suppress IL-6 activity by inhibiting STAT3 through upregulation of protein inhibitor of activated STAT3 (PIAS3).Citation67 In addition, testosterone decreases IL-6 expression by inhibiting NF-κB activity in osteoblasts via the hypothalamic-pituitary-adrenal axis, normally a potent stimulator of IL-6 production. Both of these result in testosterone-mediated bone-preserving effects.Citation68–Citation70 Therapies that involve suppression of testosterone and 17-β-estradiol are effective against androgen-dependent prostate and breast cancer respectively; however, bone density decreases significantly with these therapies leading to an increased chance of developing osteoporosis.Citation71
IL-6 production by cancer cells and stromal cells in the bone microenvironment facilitates invasion and metastasis
IL-6 produced by cancer cells initiates a variety of downstream signaling cascades that can lead to bone destruction (). Many cancer cell types that metastasize to the bone endogenously produce and secrete high levels of IL-6. On the other hand, other cancer cell types stimulate the surrounding stromal cells to release copious amounts of this cytokine. Some cancer cell types such as IL-6-dependent multiple myeloma cells do not express IL-6 and rely on the bone microenvironment’s reactive stromal cells to produce IL-6 in response to the presence of the tumor cells.Citation72 This stroma-dependent increase of IL-6 in the extracellular matrix may be specific to the microenvironment of the metastasis. For example, injection of Walker (W256) mouse mammary cancer cells and MatLyLu (MLL) mouse prostate cancer-like cells into mice has been shown to differentially express IL-6 depending on the location.Citation73 Specifically, local injection of W256 and MLL cells into the bone causes upregulation of IL-6, macrophage colony stimulating factor (m-CSF), RANKL, and Dickkopf-related protein 1 (DKK1) in the bone stromal cells. DKK1 is a member of the dickkopf family of factors that has been shown to be elevated in the bone marrow of patients with breast cancer bone metastases.Citation74 However, when these cells metastasized to nonosseous organs, there was little to no expression of IL-6, m-CSF, RANKL, or DKK1, indicating that some cancer cells stimulate surrounding cells to release pro-osteoclastic factors only in the bone microenvironment.Citation73,Citation75
It has been proposed that cancer cells induce an inflammatory response in osteoblasts which may lead to the stimulation of osteoclast differentiation and activity.Citation76,Citation77 The inflammatory response of osteoblasts in response to cancer cell-conditioned medium in vitro has been shown to cause an upregulation of PGE2, which induces IL-6 and activates osteoclasts via RANKL and PTHrP production.Citation18,Citation74,Citation75 This effect was seen in breast cancer cells, oral squamous carcinoma cell lines, and in neuroblastoma cells.Citation18,Citation75,Citation76 The induction of the inflammatory response to the cancer cellconditioned medium may be due to NFκB activation via an IL-6-independent mechanism within the osteoblasts.Citation77 Suppression of NFκB activity with methylseleninic acid reduced cytokine production by osteoblasts in response to cancer cell-conditioned medium, which may translate to reduced bone destruction in vivo.
IL-6 has been demonstrated to increase RANKL expression from osteoblasts and thus stimulate osteoclastogenesis. However inhibitors of RANKL fail to suppress IL-6-mediated osteoclastogenesis and bone resorption.Citation78,Citation79 This suggests that IL-6 has potential redundant pathways that upregulate bone destruction and could interfere with the efficacy of targeted therapies against RANKL such as denosumab, a humanized monoclonal antibody against RANKL.Citation80 RANKL-independent pathways could mediate IL-6 induced osteoclastogenesis. For instance, cancer induced inflammation leads to the stimulation of NF-κB activity, which initiates IL-6 production (). NF-κB activity is also able to stimulate cyclooxygenase (COX)-2 activity, which would result in the production of PGE2, stimulating more IL-6 release.Citation81 High levels of PGE2 have been shown to promote potent, pro-osteoclastic factors.Citation82 IL-6 may also be inducing other pro-osteoclastic factors that functions independently from RANKL such as IL-1β.Citation83 IL-1β has also been shown to increase NF-κB activityCitation84 that could result in a feedback loop that further increases IL-6.
IL-6 and its soluble receptor as a prognostic factor for cancers that metastasize to bone
Predicting disease outcomes in cancer patients with metastasis to bone is difficult due to the inherent high level of tumor cell heterogeneity within a specific type of cancer. Current attempts at general prognostics are based mostly on tumor grading, staging, and invasive characteristics derived from histological and other types of physical analysis of biopsies.Citation85 Specific, factor-based categorization of cancer is limited to a handful of well characterized receptor and antigenic tests. For example, prostate specific antigen (PSA) has long been used as a prognostic factor to estimate progression of prostate cancer.Citation86 Immuno-assays are performed to detect receptors for estrogen (ER), progesterone (PR), and human epidermal growth factor receptor 2 (Her2/neu) to aid in directing treatment strategies for breast cancer.Citation87 Improving prediction accuracy by using more prognostic factors can hasten the detection of any changes in the progression of the disease.
Recently, interest in using serum IL-6 as a specific prognostic factor for prostate cancer and breast cancer has risen.Citation88–Citation90 Current research demonstrates that serum IL-6 levels are significantly increased in many cancer patients with invasive prostate cancer compared with benign prostatic hyperplasia (BPH).Citation91 It has been shown that higher levels of serum IL-6 in patients with castration-resistant prostate cancer correlates to shortened survival times.Citation92 Serum IL-6 is also elevated in prostate and breast cancer patients with distal metastases compared with patients without metastases,Citation92,Citation93 and higher serum IL-6 levels have been associated with lower patient survival rates in metastatic breast and prostate cancer.Citation94 The spread of breast cancer cells into the local lymphatic system is also significantly correlated with increased IL-6 levels.Citation93 Other studies have supported these findings and have shown that IL-6 correlates with the extent and size of prostate cancer bone metastases; specifically, the larger and more compromised the bone was, the higher the level of serum IL-6.Citation95,Citation96 Furthermore, significant elevation of IL-6 levels in the serum have been seen in prostate cancer patients who have experienced a relapse, where IL-6 levels positively correlate with cachexia.Citation90,Citation97 Additionally, IL-6 levels have been shown to correlate with measures of morbidity and poor patient health.Citation98 In one case study, a sharp increase in serum IL-6 was detected in terminally ill cancer patients who were experiencing extreme cachexia.Citation99
A comprehensive study involving patients with metastatic gastric cancer, which can also metastasize to the bone,Citation100,Citation101 demonstrated a significant correlation between serum IL-6 levels and the extent of gastric cancer progression.Citation102 Specifically, IL-6 levels correlated with tumor grade and the extent of invasion into the gastric organ as well as lymphatic and hepatic systems. Long-term survival rates were much higher with patients that had low levels of serum IL-6, and post-surgical probability of metastasis was higher in patients with high serum IL-6.Citation102 The use of serum IL-6 levels for prognosis in a clinical setting is limited by gaps in the current understanding of mechanisms by which IL-6 specifically mediates the progression of metastatic disease as well as a lack of large clinical trials to assess baseline and range of fluctuation of serum IL-6 levels.
In addition to serum IL-6 levels, the concentration of soluble receptor to IL-6 (sIL-6R) in the serum may also help predict the aggressiveness of cancer metastasis and the level of bone destruction. Even in the absence of cancer, high levels of serum concentration of sIL-6R can predict the rate and level of osteolysis in patients with hyperparathyroidism.Citation103 High levels of sIL-6R in the serum have also been associated with increased generalized inflammation, rheumatoid arthritis, inflammatory bowel disease, asthma, and inflammation-associated colorectal cancer.Citation104 sIL-6R enables a process called IL-6 trans-signaling, where cells that do not possess IL-6 receptor, or have low levels of it, can respond to IL-6 (). This occurs through an unclear mechanism by incorporating the sIL-6 receptor into the gp130 receptor dimer on the cells, forming a IL-6 receptor heterotrimer and enabling the cells to respond to IL-6.Citation105 Interest in IL-6 trans-signaling has increased in the past several years as new research show that sIL-6R is produced by various cancer cells, and the serum concentration is associated with decreased survival and increased aggressiveness of metastases in breast, prostate, and colorectal cancers.Citation95,Citation106,Citation107 Some data suggest that IL-6 trans-signaling causes various effects that promote cancer metastases including, increased detachment, proliferation, and migration through a pathway that is independent of STAT1, STAT3, or MAPK.Citation108 This suggests that IL-6 trans-signaling is distinct from the canonical IL-6 signaling pathway and could be due to the lack of the membrane signaling domain on the sIL-6 receptor subunit (). However, IL-6 trans-signaling does cause increased RANKL expression in synovial fibroblasts through a STAT3-dependent manner,Citation53 which suggests that trans-signaling may use some of the canonical IL-6 pathway to exert its effects. Although there is a convincing amount of evidence to suggest that higher serum sIL-6R levels may be associated with a worse cancer prognosis, little is known about the specifics of the IL-6 trans-signaling pathway, and more studies need to be done before assessing whether sIL-6R is a therapeutic target.
Serum IL-6 levels may predict response to cancer therapy
It is critical to determine throughout a patient’s treatment whether the current therapy plan should be maintained or whether new therapies need to be initiated. Changes in serum IL-6 levels in patients undergoing chemotherapies or targeted therapeutics may act as a biomarker that can predict whether a patient is responding or not. In one clinical study, combination therapy using docetaxel and zoledronic acid, a bisphosphonate that inhibits osteoclastic activity, was administered to prostate cancer patients with bone metastases.Citation109 Patients that responded to the therapy had a 35% decrease in overall serum IL-6 levels, while patients that did not respond had a 76% increase in serum IL-6 levels.Citation109 A confounding variable in this finding is that some of the increase in serum IL-6 may be due to a stress response to the chemotherapeutic agents themselves, and the high levels of IL-6 may actually confer drug resistance.Citation110 However, IL-6 has also been correlated to C-reactive protein (CRP) levels in the serum, and reduction in CRP levels alone may indicate positive biologic effects of chemotherapeutics indicated by a reduction in serum IL-6.Citation111,Citation112 Although there is a dearth of clinical studies using IL-6 as a predictive biomarker of therapeutic response, initial studies support the concept that changes in serum cytokine levels such as IL-6 are worthy of more investigation.
IL-6 promotes chemotherapy resistance
Chemotherapeutics traditionally have been and are currently, a mainstay in therapies against metastatic disease. However, resistance to chemotherapeutics is common, and the mechanisms mediating resistance have been difficult to determine. Recent experimental results suggest that chemotherapy resistance is mediated through a relatively heterogeneous set of mechanisms, including downregulation of apoptotic signals, increased drug clearing and deactivation from cancer cells, multidrug resistance gene mutations, and stimulation of cell survival pathways via gene amplification.Citation113–Citation115
A substantial amount of chemotherapy resistance research presently focuses on upstream mediators of cell survival. In the bone microenvironment, high concentrations of IL-6 have recently been shown to confer resistance to apoptosis in breast and prostate cancer cells as well as neuroblastoma cells.Citation18,Citation116,Citation117 Specifically, prostate cancer cell activity of NF-κB has been shown to cause high IL-6 production, which promotes docetaxel resistance in prostate tumors and associated bone metastases by upregulating the pro-survival AKT pathway in an IL-6-dependent manner ().Citation49 Additionally, resistance to paclitaxel is observed in breast cancer patients whose metastatic lesions show high levels of IL-6.Citation115 This high IL-6 production could itself be a function of the cancer cell’s response to chemotherapeutics. One particular study presented evidence that paclitaxel induced expression of IL-6 in cervical cancer cells via the c-Jun N-terminal kinase (JNK) signaling pathway.Citation110 More studies need to be conducted to assess the full role of IL-6 in conferring chemotherapeutic resistance, but these preliminary studies may support a rationale for using combination therapy of IL-6 inhibitors along with classical chemotherapeutic agents.
IL-6 as a target for therapy
Currently, the only kinds of therapies that can treat bone metastases are supportive therapies using 1) bisphosphonates to reduce osteolytic burden, 2) radiotherapy and analgesics to alleviate pain, and 3) surgical intervention to reinforce weak bones.Citation24,Citation118,Citation119 The humanized monoclonal antibody to the IL-6 receptor, tocilizumab (Actemra®) was approved by the United States Food and Drug Administration (FDA) on January 11, 2010 and was previously approved in Japan and the European Medicines Agency (EMEA) in 2008 ().Citation120 Although tocilizumab is approved only for rheumatoid arthritis (RA) in the United States and Europe as well as Castleman’s disease in Japan, recent studies have shown that tocilizumab is also effective as an antitumor agent against U87MG glioma cells. Tocilizumab exerts an inhibitory effect on the JAK/STAT3 pathway by preventing IL-6 from binding to its receptor, thereby inhibiting IL-6 signaling.Citation121 Similar antitumor effects were seen with S6B45 multiple myeloma cells where a modified version of tocilizumab significantly inhibited the proliferation of these cells in vitro.Citation122 Tocilizumab has also been effective in blocking cartilage and bone destruction in IL-6-mediated autoimmune diseases such as synovitis and RA, where the mechanism of bone destruction is similar to that of bone metastases and high, local IL-6 levels were reported.Citation123 Thus, tocilizumab may be effective as part of a combination therapy with bisphosphonates to control cancer cell-mediated destruction of the bone. However, there is no public data that exists for the efficacy of tocilizumab in inhibiting the progression of bone metastases. Other inhibitors of IL-6 activity for the treatment of various autoimmune diseases such as lupus, RA, Crohn’s disease, and Castleman’s disease are being developed or are undergoing FDA approval.
Table 1 Targeted therapies for IL-6
Another anti-IL-6 drug that is being developed for bone metastatic prostate and renal carcinomas and multiple myeloma is (Centocor’s) CNTO-328 (Siltuximab).Citation124 This chimeric, monoclonal antibody to IL-6Citation120,Citation125 recently completed initial clinical trials for prostate cancer, kidney cancer, and renal cell carcinoma with mixed results. Some preliminary results from the completed trials indicate minimal side effects with the inhibitor; however, there was a general lack of correlation with IL-6 inhibition and reduction in tumor growth.Citation125,Citation126 The lack of tumor inhibition may be due to the nature of the trial that attempted to ascertain the safety profile of the drug, thereby leading to the use of a lower dose than may be effective. However, new clinical trials with dose escalation are planned. On the other hand, clinical trials on relapsed and refractory multiple myeloma is still ongoing. Preliminary results from a Phase 2 trial on these patients demonstrate positive results with manageable side effects and good safety profile.Citation127 This is supported by a study showing that siltuximab can inhibit prostate cancer cell growth in vitro and improve survival by reducing the level of cachexia in an animal model of prostate cancer.Citation128 In addition, siltuximab has been shown in mice to inhibit the conversion of androgen-dependent prostate cancer into a more aggressive, bone metastatic, and difficult to treat androgen-independent prostate cancer.Citation129 Treatment with siltuximab also decreased serum CRP levels, which correlated to improved outcome in treatment-resistant prostate cancer.Citation112 Other recent data indicate that STAT3 and MAPK activity is suppressed in patients taking siltuximab, which may inhibit IL-6 mediated drug resistance.Citation130 However, in a separate Phase 2 clinical trial involving castration-resistant prostate cancer where the disease had progressed beyond docetaxel therapy, siltuximab had a minimal clinical effect, despite positive biological IL-6 inhibition.Citation131 New clinical trials using a combination of siltuximab and chemotherapeutics such as docetaxel are underway.Citation131
The use of antibodies for therapeutically inhibiting cytokines such as IL-6 may soon be replaced by utilizing small protein, nonantibody-based inhibitors called avimers. Avimers may surpass monoclonal antibodies in efficacy and potency, while reducing cost. Because these proteins lack immunoglobulin domains, they are much less immunoreactive, and their smaller size (∼4 kDa) allows tighter interactions between the avimer and their target cytokine or receptor.Citation132,Citation133 In addition, due to their reduced immunoreactive nature, they should theoretically reduce occurrences of serious side effects such as acute allergic reactions, which currently are a common problem with antibody therapeutics. Because of the promising features of this type of biological therapeutic, many pharmaceutical companies are pursuing the development of drugs based on nonantibody protein compounds, but the majority of these compounds are still in preclinical or Phase 1 trials.
Avida recently developed an avimer against IL-6 called C326 or AMG-220.Citation134 Their studies show that this avimer has superior stability and drug longevity compared with antibody-based inhibitors,Citation73 resulting in an increase in both the half-life and the shelf-life of the drug. Avida published results demonstrating that their avimer against IL-6 has an IC50 in the picomolar range leading to much smaller doses, and as it can be produced in Escherichia coli, the cost is reduced.Citation133 AMG-220 is also being developed for Castleman’s disease, an autoimmune disorder that is characterized by high levels of serum IL-6 which is thought to cause the hyper-proliferation of B-cells, leading to high fevers, joint pain, weight loss, and anemia.Citation135 Currently, a Phase 1 trial for Crohn’s disease is also in progress and is recruiting volunteers with stable disease and generally good health.Citation133,Citation136,Citation137
Although not all IL-6 inhibitors currently being developed or on the market are designed for cancer, IL-6 inhibitors, in principle, should work similarly for all diseases where IL-6 is deregulated. Therefore, IL-6 inhibitors should effectively inhibit IL-6-dependent cancers by reducing metastases to the bone and bone destruction. Availability of IL-6 inhibitors for the treatment of various cancers and bone metastases should improve as new uses of the inhibitors are approved by the FDA.
Conclusion
Recent research and publications have demonstrated that IL-6 is one of the major factors upregulating and modulating cancer-mediated bone destruction. The information presented in this review illustrates the potential for IL-6 as a prognostic factor. In addition, fluctuations in serum IL-6 levels could help direct additional treatment strategies in the future, but clinical studies are needed to assess that potential. There is also evidence from in vitro, in vivo, and preliminary clinical trials to suggest that specific anti-IL-6 therapies may improve cancer survival rates and reduce metastatic burden in some types of cancers. However, additional studies and appropriate clinical trials need to be done to fully ascertain the effectiveness of anti-IL-6 therapies in cancer patients.
Disclosure
The authors declare that they have no conflict of interest.
References
- JensenEDGopalakrishnanRWestendorfJJRegulation of gene expression in osteoblastsBiofactors2010361253220087883
- OrimoHThe mechanism of mineralization and the role of alkaline phosphatase in health and diseaseJ Nippon Med Sch201077141220154452
- TeitelbaumSLBone resorption by osteoclastsScience200028954841504150810968780
- DucyPSchinkeTKarsentyGThe osteoblast: a sophisticated fibroblast under central surveillanceScience200028954841501150410968779
- NakashimaTKobayashiYYamasakiSProtein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokinesBiochem Biophys Res Commun2000275376877510973797
- KearnsAEKhoslaSKostenuikPJReceptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and diseaseEndocr Rev200829215519218057140
- MartinTJNgKWMechanisms by which cells of the osteoblast lineage control osteoclast formation and activityJ Cell Biochem19945633573667876329
- TakayanagiHKimSKogaTInduction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclastsDevelopmental Cell20023688990112479813
- BoyleWJSimonetWSLaceyDLOsteoclast differentiation and activationNature2003423693733734212748652
- HikitaAYanaIWakeyamaHNegative regulation of osteoclastogenesis by ectodomain shedding of receptor activator of NF-κB ligandJ Biol Chem200628148368463685517018528
- LynchCCHikosakaAAcuffHBMMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKLCancer Cell20057548549615894268
- SasanoTSuzukiOKanzakiHIs RANKL shedding involved in immune cell-mediated osteoclastogenesis?Interface Oral Health Science 2009SpringerJapan2010403405
- VaesGCellular biology and biochemical mechanism of bone resorption. A review of recent developments on the formation, activation, and mode of action of osteoclastsClin Orthop Relat Res19882312392713286076
- TetiAZalloneADo osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisitedBone2009441111618977320
- HeinoTJHentunenTAVaananenHKOsteocytes inhibit osteoclastic bone resorption through transforming growth factor-beta: enhancement by estrogenJ Cell Biochem200285118519711891862
- TaylorAFSaundersMMShingleDLCimbalaJMZhouZDonahueHJMechanically stimulated osteocytes regulate osteoblastic activity via gap junctionsAm J Physiol Cell Physiol20072921C545C55216885390
- ChanMLuXHuoBA trabecular bone explant model of osteocyte–osteoblast co-culture for bone mechanobiologyCell Mol Bioeng20092340541520827376
- AraTSongLShimadaHInterleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cellsCancer Res200969132933719118018
- PauleBClercDRudantCEnhanced expression of interleukin-6 in bone and serum of metastatic renal cell carcinomaHum Pathol19982944214249563797
- ThomasRJGuiseTAYinJJBreast cancer cells interact with osteoblasts to support osteoclast formationEndocrinology1999140104451445810499498
- ColemanREClinical features of metastatic bone disease and risk of skeletal morbidityClin Cancer Res200612206243s6249s17062708
- ColemanRERubensRDThe clinical course of bone metastases from breast cancerBr J Cancer198755161663814476
- DuBoisSGKalikaYLukensJNMetastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survivalJ Pediatr Hematol/Oncol1999213181189
- RicciardiSde MarinisFTreatment of bone metastases in lung cancer: the actual role of zoledronic acidRev Recent Clin Trials20094320521120028333
- YangMJiangPAnZGenetically fluorescent melanoma bone and organ metastasis modelsClin Cancer Res19995113549355910589771
- GuiseTAMohammadKSClinesGBasic mechanisms responsible for osteolytic and osteoblastic bone metastasesClin Cancer Res20061220 Pt 26213s6216s17062703
- EricAGBKristiannMDKennethJPCharlesCCThomasJRLaurieKMSkeletal metastasis of prostate adenocarcinoma in rats: morphometric analysis and role of parathyroid hormone-related proteinProstate199939318719710334108
- KellerETBrownJProstate cancer bone metastases promote both osteolytic and osteoblastic activityJ Cell Biochem200491471872914991763
- RoodmanGDMechanisms of bone metastasisN Engl J Med2004350161655166415084698
- ColemanREMetastatic bone disease: clinical features, pathophysiology and treatment strategiesCancer Treat Rev200127316517611417967
- TaichmanRSCooperCKellerETPientaKJTaichmanNSMcCauleyLKUse of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone20026218321837
- ChinniSRYamamotoHDongZSabbotaABonfilRDCherMLCXCL12/CXCR4 transactivates HER2 in lipid rafts of prostate cancer cells and promotes growth of metastatic deposits in boneMol Cancer Res20086344645718337451
- HintonCVAvrahamSAvrahamHKRole of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to the brainClin Exp Metastasis20102729710518814042
- LukerKELukerGDFunctions of CXCL12 and CXCR4 in breast cancerCancer Lett20062381304116046252
- OoiLLDunstanCRCXCL12/CXCR4 axis in tissue targeting and bone destruction in cancer and multiple myelomaJ Bone Miner Res20092471147114919419311
- HelbigGChristophersonKW2ndBhat-NakshatriPNF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4J Biol Chem200327824216312163812690099
- HirbeACMorganEAWeilbaecherKNThe CXCR4/SDF-1 chemokine axis: a potential therapeutic target for bone metastases?Curr Pharm Des201016111284129020166978
- HauschkaPVMavrakosAEIafratiMDDolemanSEKlagsbrunMGrowth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-sepharoseJ Biol Chem19862612712665126743745206
- KhanSNBostromMPLaneJMBone growth factorsOrthop Clin North Am200031337538810882464
- NauglerWEKarinMThe wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancerTrends Mol Med200814310911918261959
- NakaTNishimotoNKishimotoTThe paradigm of IL-6: from basic science to medicineArthritis Res20024Suppl 3S233S24212110143
- AthanasouNAPathology of bone injuryDiagn Histopathol2009159437443
- TosatoGJonesKDInterleukin-1 induces interleukin-6 production in peripheral blood monocytesBlood1990756130513102310829
- HoltIDavieMWBraidmanIPMarshallMJProstaglandin E2 stimulates the production of interleukin-6 by neonatal mouse parietal bonesBone Miner199425147578061551
- EickelbergOPanskyAMussmannRTransforming growth factor-beta1 induces interleukin-6 expression via activating protein-1 consisting of JunD homodimers in primary human lung fibroblastsJ Biol Chem199927418129331293810212284
- ZhangYBroserMRomWActivation of the interleukin 6 gene by Mycobacterium tuberculosis or lipopolysaccharide is mediated by nuclear factors NF IL 6 and NF-kappa BProc Natl Acad Sci U S A199592836327724610
- LibermannTABaltimoreDActivation of interleukin-6 gene expression through the NF-kappa B transcription factorMol Cell Biol1990105232723342183031
- FitzgeraldDCMeadeKGMcEvoyANTumour necrosis factor-alpha (TNF-alpha) increases nuclear factor kappaB (NFkappaB) activity in and interleukin-8 (IL-8) release from bovine mammary epithelial cellsVet Immunol Immunopathol20071161–2596817276517
- Domingo-DomenechJOlivaCRoviraAInterleukin 6, a nuclear factor-kappaB target, predicts resistance to docetaxel in hormone-independent prostate cancer and nuclear factor-kappaB inhibition by PS-1145 enhances docetaxel antitumor activityClin Cancer Res200612185578558617000695
- HeinrichPCBehrmannIMuller-NewenGSchaperFGraeveLInterleukin-6-type cytokine signalling through the gp130/Jak/STAT pathwayBiochem J1998334Pt 22973149716487
- MurakamiMHibiMNakagawaNIL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinaseScience19932605115180818108511589
- WegielBBjartellACuligZPerssonJLInterleukin-6 activates PI3K/Akt pathway and regulates cyclin A1 to promote prostate cancer cell survivalInt J Cancer200812271521152918027847
- HashizumeMHayakawaNMiharaMIL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17Rheumatology (Oxford)200847111635164018786965
- O’BrienCAGubrijILinSCSaylorsRLManolagasSCSTAT3 activation in stromal/osteoblastic cells is required for induction of the receptor activator of NF-kappaB ligand and stimulation of osteoclastogenesis by gp130-utilizing cytokines or interleukin-1 but not 1,25-dihydroxyvitamin D3 or parathyroid hormoneJ Biol Chem199927427193011930810383440
- WongPKQuinnJMSimsNAvan NieuwenhuijzeACampbellIKWicksIPInterleukin-6 modulates production of T lymphocyte-derived cytokines in antigen-induced arthritis and drives inflammation-induced osteoclastogenesisArthritis Rheum200654115816816385511
- McGeachyMJBak-JensenKSChenYTGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathologyNat Immunol20078121390139717994024
- WangLYiTKortylewskiMPardollDMZengDYuHIL-17 can promote tumor growth through an IL-6-Stat3 signaling pathwayJ Exp Med200920671457146419564351
- MoongaBSAdebanjoOAWangHJDifferential effects of interleukin-6 receptor activation on intracellular signaling and bone resorption by isolated rat osteoclastsJ Endocrinol2002173339540512065229
- GambaccianiMVaccaFPostmenopausal osteoporosis and hormone replacement therapyMinerva Med200495650752015785435
- KandaNWatanabeS17beta-estradiol, progesterone, and dihydrotestosterone suppress the growth of human melanoma by inhibiting interleukin-8 productionJ Invest Dermatol2001117227428311511305
- KramerPRKramerSFGuanG17 beta-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophagesArthritis Rheum20045061967197515188374
- BendreMSMontagueDCPeeryTAkelNSGaddyDSuvaLJInterleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone diseaseBone2003331283712919697
- MannaSKRameshGTInterleukin-8 induces nuclear transcription factor-kappaB through a TRAF6-dependent pathwayJ Biol Chem200528087010702115591054
- NovotnyNMMarkelTACrisostomoPRMeldrumDRDifferential IL-6 and VEGF secretion in adult and neonatal mesenchymal stem cells: role of NFkBCytokine200843221521918621544
- LesmeisterMJJorgensonRLYoungSLMisfeldtML17Beta-estradiol suppresses TLR3-induced cytokine and chemokine production in endometrial epithelial cellsReprod Biol Endocrinol200537416384532
- Bhat-NakshatriPNewtonTRGouletRJrNakshatriHNF-kappaB activation and interleukin 6 production in fibroblasts by estrogen receptor-negative breast cancer cell-derived interleukin 1alphaProc Natl Acad Sci U S A19989512697169769618523
- WangLHYangXYMihalicKXiaoWLiDFarrarWLActivation of estrogen receptor blocks interleukin-6-inducible cell growth of human multiple myeloma involving molecular cross-talk between estrogen receptor and STAT3 mediated by co-regulator PIAS3J Biol Chem200127634318393184411429412
- ColettaRDReynoldsMAMartelli-JuniorHGranerEAlmeidaOPSaukJJTestosterone stimulates proliferation and inhibits interleukin-6 production of normal and hereditary gingival fibromatosis fibroblastsOral Microbiol Immunol200217318619212030972
- TuckSPFrancisRMTestosterone, bone and osteoporosisFront Horm Res20093712313219011293
- PapadopoulosADWardlawSLTestosterone suppresses the response of the hypothalamic-pituitary-adrenal axis to interleukin-6Neuroimmunomodulation200081394410859487
- HershmanDNarayananRPrevention and management of osteoporosis in women with breast cancer and men with prostate cancerCurr Oncol Rep20046427728415161581
- BarilleSColletteMBatailleRAmiotMMyeloma cells upregulate interleukin-6 secretion in osteoblastic cells through cell-to-cell contact but downregulate osteocalcinBlood1995868315131597579410
- BlouinSBasleMFChappardDInteractions between microenvironment and cancer cells in two animal models of bone metastasisBr J Cancer200898480981518253114
- Voorzanger-RousselotNGoehrigDJourneFIncreased Dickkopf-1 expression in breast cancer bone metastasesBr J Cancer200797796497017876334
- DeyamaYTeiKYoshimuraYOral squamous cell carcinomas stimulate osteoclast differentiationOncol Rep200820366366818695921
- KinderMChislockEBussardKMShumanLMastroAMMetastatic breast cancer induces an osteoblast inflammatory responseExp Cell Res2008314117318317976581
- ChenYCSosnoskiDMGandhiUHNovingerLJPrabhuKSMastroAMSelenium modifies the osteoblast inflammatory stress response to bone metastatic breast cancerCarcinogenesis200930111941194819759193
- MizutaniKSudSPientaKJProstate cancer promotes CD11b positive cells to differentiate into osteoclastsJ Cell Biochem2009106456356919170075
- KudoOSabokbarAPocockAItonagaIFujikawaYAthanasouNAInterleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanismBone20033211712584029
- SchwarzEMRitchlinCTClinical development of anti-RANKL therapyArthritis Res Ther20079Suppl 1S717634146
- LeeKMKangBSLeeHLSpinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivityEur J Neurosci200419123375338115217394
- KajiHSugimotoTKanataniMFukaseMKumegawaMChiharaKProstaglandin E2 stimulates osteoclast-like cell formation and boneresorbing activity via osteoblasts: role of cAMP-dependent protein kinaseJ Bone Miner Res199611162718770698
- KuriharaNBertoliniDSudaTAkiyamaYRoodmanGDIL-6 stimulates osteoclast-like multinucleated cell formation in long term human marrow cultures by inducing IL-1 releaseJ Immunol199014411422642302341718
- RenardPZacharyMDBougeletCEffects of antioxidant enzyme modulations on interleukin-1-induced nuclear factor kappa B activationBiochem Pharmacol19975321491609037247
- SoerjomataramILouwmanMWRibotJGRoukemaJACoeberghJWAn overview of prognostic factors for long-term survivors of breast cancerBreast Cancer Res Treat2008107330933017377838
- LiljaHUlmertDVickersAJProstate-specific antigen and prostate cancer: prediction, detection and monitoringNat Rev Cancer20088426827818337732
- HarrisLFritscheHMennelRAmerican Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancerJ Clin Oncol200725335287531217954709
- CuligZHobischARole of IL-6 in regulating the androgen receptorAndrogen Action in Prostate Cancer2009451463
- KnüpferHPreißRSignificance of interleukin-6 (IL-6) in breast cancer (review)Breast Cancer Res Treat2007102212913516927176
- KurodaKNakashimaJKanaoKInterleukin 6 is associated with cachexia in patients with prostate cancerUrology200769111311717270630
- TumminelloFMBadalamentiGIncorvaiaLFulfaroFD’AmicoCLetoGSerum interleukin-6 in patients with metastatic bone disease: correlation with cystatin CMed Oncol2008261101518461289
- GeorgeDJHalabiSShepardTFThe prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: results from cancer and leukemia group B 9480Clin Cancer Res20051151815182015756004
- AhmedOIAdelAMDiabDRGobranNSPrognostic value of serum level of interleukin-6 and interleukin-8 in metastatic breast cancer patientsEgypt J Immunol2006132616818689272
- RobertoSSaraJInaBCirculating interleukin-6 predicts survival in patients with metastatic breast cancerInt J Cancer2003103564264612494472
- ShariatSFAndrewsBKattanMWKimJWheelerTMSlawinKMPlasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasisUrology20015861008101511744478
- ShariatSFKattanMWTraxelEAssociation of pre- and postoperative plasma levels of transforming growth factor beta(1) and interleukin 6 and its soluble receptor with prostate cancer progressionClin Cancer Res20041061992199915041717
- StrassmannGFongMKenneyJSJacobCOEvidence for the involvement of interleukin 6 in experimental cancer cachexiaJ Clin Invest1992895168116841569207
- TwillieDAEisenbergerMACarducciMAHseihWSKimWYSimonsJWInterleukin-6: a candidate mediator of human prostate cancer morbidityUrology19954535425497879350
- IwaseSMurakamiTSaitoYNakagawaKSteep elevation of blood interleukin-6 (IL-6) associated only with late stages of cachexia in cancer patientsEur Cytokine Netw200415431231615627639
- KangSHKimJIMoonHSOvert bone marrow metastasis from early gastric cancerEndoscopy200840Suppl 2E34E3518278722
- SudoHTakagiYKatayanagiSBone metastasis of gastric cancerGan To Kagaku Ryoho20063381058106016912521
- AshizawaTOkadaRSuzukiYClinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factorGastric Cancer20058212413115864720
- NakchbandiIAMitnickMALangRGundbergCKinderBInsognaKCirculating levels of interleukin-6 soluble receptor predict rates of bone loss in patients with primary hyperparathyroidismJ Clin Endocrinol Metab200287114946495112414855
- ChalarisAGarbersCRabeBRose-JohnSSchellerJThe soluble interleukin 6 receptor: generation and role in inflammation and cancerEur J Cell Biol. Epub 2010 Dec 7.
- Rose-JohnSNeurathMFIL-6 trans-signaling: the heat is onImmunity20042012414738759
- AtreyaRNeurathMFSignaling molecules: the pathogenic role of the IL-6/STAT-3 trans signaling pathway in intestinal inflammation and in colonic cancerCurr Drug Targets20089536937418473764
- KnupferHPreissRLack of knowledge: breast cancer and the soluble interleukin-6 receptorBreast Care (Basel)20105317718021049067
- SanterFRMalinowskaKCuligZCavarrettaITInterleukin-6 trans-signalling differentially regulates proliferation, migration, adhesion and maspin expression in human prostate cancer cellsEndocr Relat Cancer200917124125319966016
- Woods IgnatoskiKMFriedmanJEscara-WilkeJChange in markers of bone metabolism with chemotherapy for advanced prostate cancer: interleukin-6 response is a potential early indicator of response to therapyJ Interferon Cytokine Res2008292105112
- WangTHChanYHChenCWPaclitaxel (Taxol) upregulates expression of functional interleukin-6 in human ovarian cancer cells through multiple signaling pathwaysOncogene200625354857486616547493
- StarkJRLiHKraftPCirculating prediagnostic interleukin-6 and C-reactive protein and prostate cancer incidence and mortalityInt J Cancer2009124112683268919189403
- PinskiJKGoldmanBDorffTSWOG S0354: a phase II trial of CNTO328, a monoclonal antibody against interleukin-6 (IL-6), in chemotherapy pretreated patients (pts) with castration-resistant prostate cancer (CRPC)J Clin Oncol20092715S5143
- LuqmaniYAMechanisms of drug resistance in cancer chemotherapyMed Princ Pract200514Suppl 1354816103712
- WilsonTRJohnstonPGLongleyDBAnti-apoptotic mechanisms of drug resistance in cancerCurr Cancer Drug Targets20099330731919442051
- RinconMBroadwaterGHarrisLInterleukin-6, multidrug resistance protein-1 expression and response to paclitaxel in women with metastatic breast cancer: results of cancer and leukemia group B trial 159806Breast Cancer Res Treat2006100330130816773437
- ChungTDYuJJKongTASpiottoMTLinJMInterleukin-6 activates phosphatidylinositol-3 kinase, which inhibits apoptosis in human prostate cancer cell linesProstate20004211710579793
- Garcia-TunonIRicoteMRuizAFraileBPaniaguaRRoyuelaMIL-6, its receptors and its relationship with bcl-2 and bax proteins in infiltrating and in situ human breast carcinomaHistopathology2005471828915982327
- PetrutBTrinkausMSimmonsCClemonsMA primer of bone metastases management in breast cancer patientsCurr Oncol200815Suppl 1S50S5718231649
- ColemanREManagement of bone metastasesOncologist20005646347011110597
- MeltonLCoombsAActemra poised to launch IL-6 inhibitorsNat Biotechnol200826995795918779787
- KudoMJonoHShinrikiSAntitumor effect of humanized anti-interleukin-6 receptor antibody (tocilizumab) on glioma cell proliferationJ Neurosurg2009111221922519326989
- Yoshio-HoshinoNAdachiYAokiCPereboevACurielDTNishimotoNEstablishment of a new interleukin-6 (IL-6) receptor inhibitor applicable to the gene therapy for IL-6-dependent tumorCancer Res200767387187517283116
- FonsecaJESMCanhãoHChoyEInterleukin-6 as a key player in systemic inflammation and joint destructionAutoimmun Rev20098753854219189867
- LiJHuXFXingPXCNTO-328 (Centocor)Curr Opin Investig Drugs200566639645
- PuchalskiTPrabhakarUJiaoQBernsBDavisHMPharmacokinetic and pharmacodynamic modeling of an anti-interleukin-6 chimeric monoclonal antibody (siltuximab) in patients with metastatic renal cell carcinomaClin Cancer Res20101651652166120179212
- RossiJFNegrierSJamesNDA Phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancerBr J Cancer201010381154116220808314
- VoorheesPMMangesRFSomloGA phase II multicenter study of CNTO 328, an anti-IL-6 monoclonal antibody, in patients (pts) with relapsed or refractory multiple myeloma (MM)J Clin Oncol (Meeting Abstracts)20092715S8527
- ZakiMHNemethJATrikhaMCNTO 328, a monoclonal antibody to IL-6, inhibits human tumor-induced cachexia in nude miceInt J Cancer2004111459259515239138
- WallnerLDaiJEscara-WilkeJInhibition of interleukin-6 with CNTO328, an anti-interleukin-6 monoclonal antibody, inhibits conversion of androgen-dependent prostate cancer to an androgen-independent phenotype in orchiectomized miceCancer Res20066663087309516540658
- KarkeraJSteinerHLiWThe anti-interleukin-6 antibody siltuximab down-regulates genes implicated in tumorigenesis in prostate cancer patients from a Phase I studyProstate Epub 2011 Feb 14.
- DorffTBGoldmanBPinskiJKClinical and correlative results of SWOG S0354: a Phase II trial of CNTO328 (Siltuximab), a monoclonal antibody against interleukin-6, in chemotherapy-pretreated patients with castration-resistant prostate cancerClin Cancer Res201016113028303420484019
- SheridanCPharma consolidates its grip on post-antibody landscapeNat Biotechnol200725436536617420728
- SilvermanJLiuQBakkerAMultivalent avimer proteins evolved by exon shuffling of a family of human receptor domainsNat Biotechnol200523121556156116299519
- BraddockM11th annual inflammatory and immune diseases drug discovery and development summitExpert Opin Investig Drugs200716909917
- BrandtSJBodineDMDunbarCENienhuisAWDysregulated interleukin 6 expression produces a syndrome resembling Castleman’s disease in miceJ Clin Invest19908625925992384605
- WurchTLowePCaussanelVBesCBeckACorvaiaNDevelopment of novel protein scaffolds as alternatives to whole antibodies for imaging and therapy: status on discovery research and clinical validationCurr Pharm Biotechnol2008950250919075688
- GebauerMSkerraAEngineered protein scaffolds as next-generation antibody therapeuticsCurr Opin Chem Biol200913324525519501012