Abstract
Long-term survival is quite uncommon in refractory small cell lung cancer (SCLC) patients, with less than 25% of patients with limited-stage disease and 1%–2% of patients with extensive-stage disease remaining alive at five years. Recent clinical studies have demonstrated the promising efficacy of amrubicin for patients with relapsed SCLC. This review presents the results of clinical studies showing the efficacy and safety of amrubicin for the treatment of relapsed SCLC. Amrubicin is a synthetic anthracycline agent with a similar structure to doxorubicin, in which the hydroxyl group at position 9 in amrubicin is replaced by an amino group to enhance efficacy. It is converted to an active metabolite, amrubicinol, which is 5–54 times more active than amrubicin. Amrubicin and amrubicinol are inhibitors of DNA topoisomerase II, exerting their cytotoxic effects by stabilizing a topoisomerase II-mediated cleavable complex. The toxicity of amrubicin is similar to that of doxorubicin, although amrubicin shows almost no cardiotoxicity. In the relevant trials, amrubicin was administered intravenously at a dose of 35–40 mg/m2 on days 1–3 every three weeks. The response rate was 34%–52% and median survival times were 8.1–12.0 months. Common hematologic toxicities included neutropenia, leucopenia, anemia, thrombocytopenia, and febrile neutropenia. Nonhematologic adverse events included Grade 3–4 anorexia, asthenia, hyponatremia, and nausea. The results of the studies which demonstrated the efficacy of monotherapy for relapsed SCLC involved mainly Japanese patients. Therefore, it is necessary to conduct more clinical studies in non-Japanese patients to confirm the efficacy of amrubicin.
Introduction
Small cell lung cancer (SCLC) accounts for approximately 15% of all lung cancer cases, with two-thirds of patients presenting with extensive disease (ED). Without treatment, tumor progression in patients with SCLC is rapid, with a poor prognosis. However, the disease shows a high response rate to chemotherapy and radiotherapy, except in a low percentage of patients. Treatment options for relapsed SCLC patients remain limited. A randomized trial demonstrated that single-agent topotecan was at least as efficacious as the three-drug combination of cyclophosphamide-doxorubicin-vincristine for the treatment of patients with sensitive relapsed cases.Citation1 Response rates and median survival times were 24% and 25.0 weeks for topotecan, and 18% and 24.7 weeks for cyclophosphamide-doxorubicin-vincristine, respectively. In previously untreated ED-SCLC, amrubicin yielded an extremely high response rate of 79% and a median survival time of 11 months, which was comparable with the results achieved with platinum.Citation2,Citation3 Recently, clinical studies have demonstrated the efficacy of amrubicin in patients with relapsed SCLC. This review presents the results of clinical studies showing the efficacy and safety of amrubicin for the treatment of relapsed SCLC.
Structure and characteristics
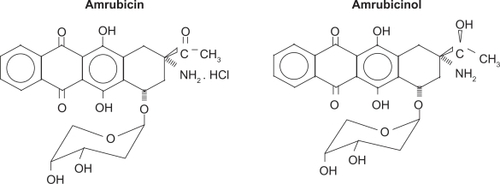
Amrubicin hydrochloride is a synthetic anthracycline agent, with a similar structure to doxorubicin, in which the hydroxyl group at position 9 group in amrubicin is replaced by an amino group to enhance efficacy (see ). Amrubicin is converted to an active metabolite, amrubicinol, which is 5–54 times more active than amrubicin, through reduction of its C-13 ketone group to a hydroxyl group by carbonyl reductase.Citation4 Other enzymes metabolizing amrubicin and amrubicinol are nicotinamide adenine dinucleotide phosphate (NADPH)-dependent P450 reductase and NAD[P]H-dependent quinone oxidoreductase. Doxorubicin, amrubicin, and amrubicinol are inhibitors of DNA topoisomerase II, exerting their cytotoxic effects by stabilizing a topoisomerase II-mediated cleavable complex.Citation5 In addition, they are more or less only one-tenth as potent as doxorubicin in producing DNA intercalation.
Preclinical studies
Antitumor activity and toxicologic aspects were first reported for amrubicin by Morisada et al.Citation6 They evaluated this agent in six murine systems and nine human tumor-nude mouse systems, and found that the antitumor activity of amrubicin was superior to adriamycin in human tumor xenografts, and almost equal against murine experimental tumors. They also evaluated toxicity in mice after a bolus intravenous injection. The acute toxic signs were body weight decrease, ataxia, hair loss, and myelosuppression, and these toxicities were qualitatively comparable with those induced by adriamycin. The maximum tolerated dose was estimated to be 25 mg/kg in four mouse strains, and the drug had anticancer activity against human lung cancer xenografts in vivo.Citation7
Cardiomyopathy is a burdensome toxicity with the anthracyclines. Suzuki et al evaluated the degree of cardiotoxicity of amrubicin compared with that of adriamycin in rabbits.Citation8 The drugs were intravenously administered three times a week for eight weeks. In this study, prolongation of the QTc interval and STT changes were observed in rabbits administered amrubicin and adriamycin. Morphologic studies showed that myocardial tissue damage in animals administered amrubicin was comparable with that seen in controls. Considering the results of the antitumor efficacy studies comparing amrubicin with adriamycin, they concluded that the cardiotoxicity of amrubicin was very slight.
Relapsed small cell lung cancer
Long-term survival is quite uncommon in refractory SCLC patients, with less than 25% of patients with limited-stage disease and 1%–2% of patients with extensive-stage disease remaining alive at five years. A Phase II study was conducted in patients with relapsed disease who had previously received one or two regimens, including at least one regimen of platinum-based chemotherapyCitation9 (). Sixty patients were enrolled in this multicenter trial. The disease progressed within 60 days after the final dose of previous chemotherapy in 16 and 44 refractory patients, respectively. The sensitive groups, in which complete response (CR) or partial response (PR) was observed with previous chemotherapy and the disease progressed or relapsed within 60 days of the final dose of previous chemotherapy, were eligible for the study, and were assessable for toxicity, response, and survival. Amrubicin was administered intravenously at a dose of 40 mg/m2 on days 1–3 every three weeks. The response rate was 52% (95% confidence interval [CI] 38%–65%). There were no differences in the response rate, ie, 50% (95% CI 25%–75%) for refractory disease and 52% [95% CI 37%–68%] for sensitive disease. The median survival times were 10.3 months in the refractory group and 11.6 months in the sensitive group, respectively (P = 0.0974, log rank test). Common adverse events were hematologic toxicities, including Grade 3–4 neutropenia (83%), leucopenia (70%), anemia (33%), thrombocytopenia (20%), and febrile neutropenia (5%). Nonhematologic adverse events included Grade 3–4 anorexia (15%), asthenia (15%), hyponatremia (8%), and nausea (5%).
Table 1 Phase II trial of amrubicin for treatment of refractory or relapsed small cell lung cancer (Thoracic Oncology Research Group Study 0301)Citation1
Another Phase II study of amrubicin in patients with previously treated SCLC was conducted by Kaira et alCitation10 (). Twenty-nine patients with relapsed SCLC who had previously received platinum-based chemotherapy were enrolled in the trial, including 10 patients with sensitive relapse and 19 patients with refractory relapse. Amrubicin was administered intravenously at a dose of 35 mg/m2 on days 1–3 every three weeks. The response rate was 44.8% (95% CI 26%–64%), being 60% for sensitive cases and 37% for refractory cases. No significant difference in the response rate was observed between sensitive cases and relapsed cases (P = 0.233, log rank test). The median progression-free survival and median survival times were 4.0 months (sensitive relapse, 4.0 months; refractory relapse, 4.0 months) and 12.0 months (sensitive relapse, 12.0 months; refractory relapse, 11.0 months), respectively. There was no difference in median progression-free survival and median survival time between sensitive relapse and refractory relapse. Grade 3 or 4 neutropenia and febrile neutropenia were observed in 42% and 3% of patients, respectively. Nonhematologic toxicity higher than Grade 3 was not observed. The results of this study show the efficacy of monotherapy for relapsed SCLC. However, this study involved only Japanese patients, so it would be necessary to conduct clinical studies in non-Japanese patients to confirm efficacy.
Table 2 Phase II trial of amrubicin for treatment of relapsed small cell lung cancerCitation10
A randomized Phase II trial of amrubicin versus topotecan as second-line treatment for sensitive ED-SCLC was therefore conductedCitation11 (). Seventy-six patients who had previously received platinum-based firstline chemotherapy were enrolled. All were sensitive cases, in which CR or PR had been observed with previous chemotherapy and the disease had then progressed or relapsed within at least 90 days of the final dose. Patients were randomized at a 2:1 ratio to receive either amrubicin or topotecan. Amrubicin was administered intravenously at a dose of 40 mg/m2 on days 1–3 every three weeks. Topotecan was administered intravenously at a dose of 1.5 mg/m2 on days 1–5 every three weeks. The response rate for amrubicin was 34% (95% CI 22%–48%), and for topotecan was 4% (95% CI 1%–19%). There was a trend towards a longer progression-free survival time in the amrubicin group (4.6 months, 95% CI 64–187) than in the topotecan group (3.5 months, 95% CI 75–177). This study showed that amrubicin is also active in non-Japanese patients and is well tolerated, with myelotoxicity being the main dose-limiting toxicity. Inoue et al conducted a randomized Phase II trial comparing amrubicin with topotecan in not only sensitive relapsed but also refractory casesCitation12 (). Sensitive cases were defined as CR or PR being achieved with the previous chemotherapy, after which the disease progressed or relapsed at least 90 days after the final dose. Fifty-nine patients were randomized in a 1:1 ratio to receive either amrubicin or topotecan. Amrubicin was administered intravenously at a dose of 40 mg/m2 on days 1–3 every three weeks. Topotecan was administered intravenously at a dose of 1.0 mg/m2 on days 1–5 every three weeks. The response rate for amrubicin was 38% (95% CI 60%–92%) and for topotecan was 13% (95% CI 4%–31%). In sensitive relapsed cases, the response rate for amrubicin was 53% (95% CI 28%–77%), and for topotecan was 21% (95% CI 6%–46%). In refractory relapsed cases, the response rate for amrubicin was 17% (95% CI 2%–48%), and for topotecan was 0% (95% CI 0%–28%). The median progression-free survival time with amrubicin was 3.5 months and with topotecan was 2.2 months. The median overall survival time with amrubicin was 8.1 months and with topotecan was 8.4 months. There was no difference in the frequency of hematologic toxicity more than Grade 3 between amrubicin and topotecan. These studies show that amrubicin monotherapy is an encouraging regimen for second-line treatment of SCLC.
Table 3 Randomized Phase II trial of amrubicin versus topotecan for treatment of sensitive relapsed small cell lung cancerCitation11
Table 4 Randomized Phase II trial comparing amrubicin with topotecan in patients with sensitive and refractory relapsed small cell lung cancerCitation12
Conclusion
Clinical investigation of the novel anticancer agent, amrubicin, has increased quickly, and there are high expectations for this agent in trials to improve the outcome for relapsed SCLC patients. Amrubicin is an active agent for the treatment of relapsed SCLC, but because it is strongly myelotoxic, particular care should be taken with its use.
Disclosure
The authors declare no potential conflicts of interest.
References
- von PawelJSchillerJHShepherdFATopotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancerJ Clin Oncol19991765866710080612
- YanaTNegoroSTakadaMPhase II study of amrubicin in previously untreated patients with extensive-disease small cell lung cancer: West Japan Thoracic Oncology Group (WJOG) studyInvest New Drugs20072525325817039404
- NodaKNishiwakiYKawaharaMIrinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancerN Engl J Med2002346859111784874
- YamaokaTHanadaMIchiiSCytotoxicity of amrubicin, a novel 9-aminoanthracycline, and its active metabolite amrubicinol on human tumor cellsJpn J Cancer Res199889106710739849587
- HanadaMMizunoSFukushimaAA new antitumor agent amrubicin induces cell growth inhibition by stabilizing topoisomerase II-DNA complexJpn J Cancer Res199889122912389914793
- MorisadaSYanagiYNoguchiTAntitumor activities of novel 9-aminoanthracycline (SM-5887) against mouse experimental tumors and human tumor xenograftsJpn J Cancer Res19898069762496061
- MorisadaSYanagiYKashiwazakiYToxicological aspects of a novel 9-aminoanthracycline, SM-5887Jpn J Cancer Res19898077822496062
- SuzukiTMinamideSIwasakiTCardiotoxicity of a new anthracycline derivative (SM-5887) following intravenous administration to rabbits: Comparative study with doxorubicinInvest New Drugs1997152192259387044
- OnodaSMasudaNSetoTPhase II trial of amrubicin for treatment of refractory or relapsed small-cell lung cancerJ Clin Oncol2006245448545317135647
- KairaKSunagaYTomizawaYA Phase II study of amrubicin, a synthetic 9-aminoanthracycline, in patients with previously treated lung cancerLung Cancer200934513456
- JotteRMConklingPRReynoldsCA randomized Phase 2 trial of amrubicin (AMR) vs topotecan as second-line treatment in extensive-disease small cell lung cancer (SCLC) sensitive to platinum-based first-line chemotherapyJ Clin Oncol20082615S8040
- InoueASugawaraSYamazakiKRandomized phase II trial comparing amrubicin with topotecan in patients with previously treated small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0402J Clin Oncol2008265401540618854562