Abstract
T lymphocytes are believed to play an important role in the pathogenesis of chronic obstructive pulmonary disease (COPD). How T cells are recruited to the lungs and contribute to the inflammatory process is largely unknown. COPD is a heterogeneous disease, and discriminating disease phenotypes based on distinct molecular and cellular pathways may provide new approaches for individualized diagnosis and therapies. Bronchoalveolar lavage (BAL) and blood samples were obtained from 40 never-smokers, 40 smokers with normal lung function, and 38 COPD patients. T-cell chemokine receptor expression was analyzed with flow cytometry, and soluble BAL cytokines and chemokines were measured using a cytokine multiplex assay. Correlations with gender and clinical characteristics including lung imaging were investigated using multivariate modeling. Th1/Tc1- and Th2/Tc2-associated soluble analytes and T-cell chemokine receptors were analyzed as cumulative Th1/Tc1 and Th2/Tc2 immune responses. A higher expression of chemokine receptor CCR5 on CD8+ T cells in BAL and higher percentage of CXCR3+CD8+ T cells in blood was found in female smokers with COPD compared to those without COPD. CCR5 expression on CD4+ and CD8+ T cells was lower in BAL from male smokers with COPD compared to those without COPD. Among female smokers with COPD, Th1/Tc1 immune response was linked to BAL macrophage numbers and goblet cell density, and Th2/Tc2 response was associated with the measures of emphysema on high-resolution computed tomography. The highly gender-dependent T-cell profile in COPD indicates different links between cellular events and clinical manifestations in females compared to males. Our findings may reveal mechanisms of importance for the difference in clinical course in female COPD patients compared to males.
Introduction
Chronic obstructive pulmonary disease (COPD) is an inflammatory disease characterized by airflow limitation that is not fully reversible. In the industrialized world, the major risk factor for COPD is tobacco smoking. Historically considering a males’s disease, females have now surpassed males in COPD prevalence and hospitalizations.Citation1–Citation3 A large number of studies report the existence of gender differences in the manifestations of the disease, including a faster decline in lung function and worse symptoms among females, who also seem to be more susceptible to the toxic effects of cigarette smoke.Citation4,Citation5
The inflammation in COPD is characterized by increased number of macrophages, neutrophils, and T lymphocytes in the lung. The T lymphocytes are mainly CD8+ T-cytotoxic cells (Tc), but CD4+ T-helper (Th) cells are also increased in more severe disease. In the initial response to cigarette smoke, airway epithelial cells and macrophages induce airway inflammation, both through direct effects and through the recruitment of T cells from the periphery. CD8+ cells are able to destroy lung parenchyma through cytolytic activities while CD4+ cells recruit and activate other immune cells, including neutrophils, perpetuating the sustained inflammatory process. As T cells respond following antigen recognition, they differentiate in response to the inflammatory milieu to mount a response with a specific cytokine profile.Citation6–Citation9
A large body of evidence indicates a skewing toward a Th1/Tc1 response in COPD.Citation6 These cells typically produce cytokines such as interleukin-2 (IL-2), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α). However, there are studies that advocate that Th2/Tc2 cells, characterized by their expression of IL-4 and IL-13, can also be involved.Citation10–Citation12
T cells and other immune cells are directed to the site of inflammation by the binding of chemokines to cell-surface chemokine receptors. Th1/Tc1 cells can express CXCR3 and CCR5, which bind specifically to CXCL9–11 and CCL3–5, respectively. Th2/Tc2 cells can express CXCR4 binding specifically to CXCL12.Citation13 The resulting chemokine-orchestrated cell recruitment is vital for the protection against the cigarette smoke-induced events, which also contributes to disease development. Consequently, a substantial interest in developing receptor antagonists or chemokine blockers for new therapeutic solutions has evolved.Citation14
In this study, factors of plausible importance for the recruitment of T cells to the lung were studied. To do this, T cells and soluble analytes in bronchoalveolar lavage (BAL)Citation15 and blood from COPD patients, healthy smokers, and never-smokers were analyzed. It was hypothesized that patterns of inflammatory markers involved both in the response to cigarette smoke and in COPD in the lower airways may help reveal mechanistic gender differences and characterize disease phenotypes.
Materials and methods
Study subjects and patients
The subjects included in this study were part of the Karolinska COSMIC cohort (www.ClinicalTrials.gov/ct2/show/study/NCT2627872), consisting of 40 never-smokers, 40 smokers with normal lung function (smokers), and 38 COPD patients with GOLD stage I–II. Detailed characteristics of the subjects have been summarized in Citation16,Citation17 and also in the Supplementary materials. High-resolution computed tomography (HRCT) was performed by inspiratory and expiratory scans as described previously.Citation18 All participants provided written informed consent, and the study was approved by the Regional Ethics Committee in Stockholm on October 26, 2006 (ref: 2006/959-31/1).
Table 1 Characteristics of the study subjects
Bronchoscopy with BAL, processing of BAL and blood cells, and cytospin preparations from bronchial brushings were performed as described previously.Citation19–Citation21 BAL characteristics, including BAL-cell differential counts, have been described elsewhere.Citation16
Flow cytometric analysis of T cells
BAL and blood cells were analyzed for their expression of the cell surface molecules CD3, CD4, CD8, CXCR3, CCR5, CXCR4, and CD69 using multicolor flow cytometry. Prior to monoclonal antibody staining, macrophage depletion from the BAL cells was performed as described previously.Citation22 BAL cells (1×106/100 μL cell wash) and 100 μL of blood were stained with monoclonal antibodies and analyzed with flow cytometry as described in the Supplementary materials. For statistical purposes, at least 50 detected events in the final gates for a specific analyte, as well as a clear separation between the positive and negative cell populations in the median fluorescence intensity (MFI) analysis were required for inclusion.
Multiplex analysis of inflammatory mediators
In order to investigate the recruitment of peripheral T cells to the lungs in smokers and COPD patients, combined analyses of selected chemokine receptors on T cells and chemokines they produce were performed, and the Th1/Th2 balance between chemokine receptors, cytokines and chemokines typically associated with the Th1/Tc1 and Th2/Tc2 profiles, respectively, were investigated. In addition to the Th1/Th2 panels, a set of variables directly associated with macrophage and epithelial processes in COPD was selected. Macrophage panel: IL-1β, CXCL9, and vascular endothelial growth factor (VEGF). These mediators can be produced and released by macrophages and have been postulated to play an important role in the pathophysiology of COPD. The epithelial cell panel is transforming growth factor-β2 (TGF-β2), TGF-β3, and IL-1β. IL-1β and TGF-β are important products of epithelial cells and are indicated to have important roles in the peripheral airways in COPD patients. TGF-β is increased in the airway epithelial cells in COPD patients and is suggested to be a mediator of peripheral airway fibrosis in COPD. As such, concentrated BAL supernatants were measured for the chemokines CXCL8, CXCL9, CXCL10, CXCL12, CCL3, CCL4, CCL5, the cytokines IL-1β, IL-4, IL-12 (p70), IL-13, IFN-γ, TNF-α, and the growth factors VEGF and TGF-β1, 2, and 3 using multiplex magnetic bead-based assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA) through LUMINEX® xMAP® technology. For further information, see Supplementary materials.
Statistical analyses
Univariate analyses were performed with GRAPHPAD PRISM® version 5.02 (GraphPad Software Inc., San Diego, CA, USA). Comparisons between two independent groups were performed by using Mann–Whitney U-test, and for more than two groups, one-way analysis of variance (ANOVA) test followed by Dunn’s multiple comparison test for nonparametric data (P<0.05) was performed.
Multivariate data analyses were performed on all T-cell variables and soluble analytes using SIMCA® version 14.0 (Umetrics AB, Umeå, Sweden) both by unsupervised (principal component analysis [PCA]) and supervised (orthogonal projections to latent structures [OPLS])Citation23 approaches (for more details, see Supplementary materials). Model performance is reported in terms of cumulative correlation coefficients (R2), predictive performance based on seven-fold cross validation (Q2), and cross-validated ANOVA (CV-ANOVA) P-values for the OPLS models. Variable selection for model optimization was performed iteratively on the basis of the scaled loadings of the predictive component of the OPLS models (P[corr]).Citation23
Pathway-based correlation analyses were performed on clinical data (for information on included clinical data, see www.ClinicalTrials.gov/ct2/show/study/NCT2627872) and immune cell pathways using partial least squares regression. Soluble analytes and T-cell chemokine receptors were structured into four groups; variables primarily related to Th1/Tc1 cell, Th2/Tc2 cell, macrophage, and epithelial cell processes, respectively ().
Table 2 Directly associated variables among the soluble analytes and T-cell chemokine receptors, structured in four groups of pathways
Results
T-cell chemokine receptor expression
Univariate analysis revealed higher median CXCR3 percentages among CD8+ T cells than among CD4+ T cells in BAL in all study groups. The percentage of CXCR3+CD4+ T cells in BAL was significantly lower in smokers compared to never-smokers (P<0.01) (). No differences in the proportions of CXCR3+CD8+ T cells were seen between the study groups ().
Figure 1 Results of univariate analysis.
Abbreviations: BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; MFI, median fluorescence intensity.
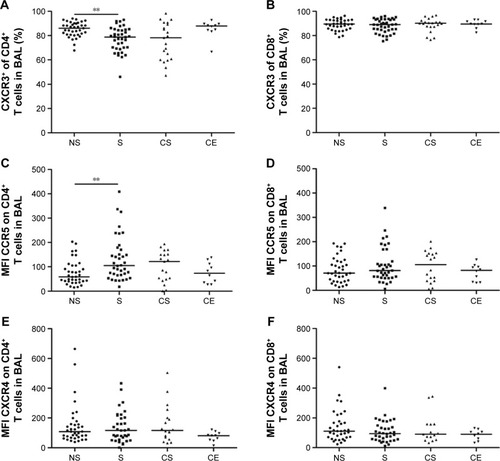
The expression of CCR5 on CD4+ T cells in BAL was significantly higher in smokers compared to never-smokers (P<0.01) (). No significant differences between the groups were observed in the CD8+ T-cell population (). In blood, the only significant difference was a decreased expression of CCR5 on CD8+ T cells from smokers compared to never-smokers (P<0.01) (data not shown).
No differences between the study groups were seen with regard to the expression of the Th2/Tc2 cell marker CXCR4 on CD4+ and CD8+ BAL T cells ().
Inflammatory mediators in BAL supernatant
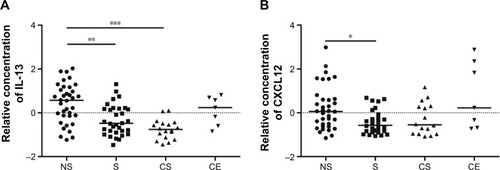
The relative concentration of the Th2/Tc2-associated cytokine IL-13 was lower in smokers and COPD smokers compared to never-smokers (P<0.01 and P<0.001, respectively), as were the Th2/Tc2-associated chemokine CXCL12 in smokers compared to never-smokers (P<0.05) (). There were no significant differences between the study groups in IL-4 levels, neither in the Th1/Tc1-associated TNF-α, IFN-γ, CCL3, -4, -5, or CXCL9, -10 (Figure S1A–H).
Figure 2 Relative concentrations of (A) IL-13 and (B) CXCL12 in BAL supernatant from healthy never-smokers (NS), smokers with normal lung function (S), smokers with COPD (CS), and ex-smokers with COPD (CE).
Abbreviations: BAL, bronchoalveolar lavage; IL, interleukin.
Compared to never-smokers, the relative concentration of the pro-inflammatory cytokine IL-1β was higher in BAL from smokers (P<0.001) and COPD smokers (P<0.05). Levels of IL-12 (p70), by contrast, were lower in both the groups of smokers compared to both never-smokers (P<0.001) and COPD ex-smokers (P<0.05) (Figure S2A and B).
The concentration of VEGF was significantly lower in smokers compared to never-smokers and COPD ex-smokers (P<0.001 for both) and in COPD smokers compared to never-smokers (P<0.001) and COPD ex-smokers (P<0.01). Of the three TGF-β isotypes measured, only TGF-β2 proved to be significantly different. The levels were lower in smokers compared to never-smokers (P<0.01) (Figure S2C and D).
Smokers versus COPD smokers
Multivariate modeling comparing T-cell subsets and soluble analytes of smokers with normal lung function versus smokers with COPD was performed in order to discriminate disease-related factors separate from the effects of smoking. Supervised modeling using OPLS-DA® resulted in a significant separation between smokers and COPD smokers, however, with a low predictive value (R2=0.46, Q2=0.29, P[CV-ANOVA] =0.006).
Gender differences
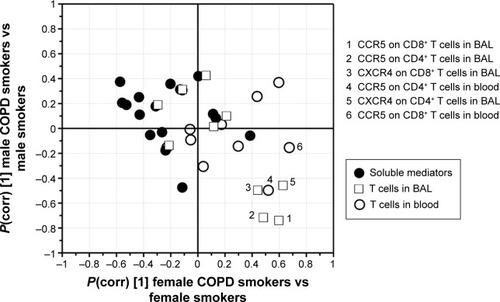
In order to assess gender-associated differences, females and males were analyzed separately. Comparison of all T-cell subsets and soluble analytes revealed a strong discrimination between female smokers and female COPD smokers (R2=0.50, Q2=0.41, P[CV-ANOVA] =8×10−4). Eleven variables were driving the separation between the two groups (). A less robust model was found for the corresponding male cohort, comparing male COPD smokers and male smokers (R2=0.32, Q2=0.25, P[CV-ANOVA] =0.02) (). Univariate analysis confirmed that three of the eleven variables were significantly higher in female COPD smokers compared to female smokers: the CCR5 expression on CD8+ T cells in both blood (P<0.01) and BAL (P<0.05), and the percentage of CXCR3+ among CD8+ T cells (P<0.05) ().
Figure 3 Multivariate modeling comparing T-cell subsets and soluble analytes of smokers with normal lung function versus COPD smokers.
Notes: (A) OPLS-DA resulted in a significant separation between female smokers and female COPD patients (R2=0.50, Q2=0.41, P=8×10−4). Left panel shows the score plot with the predictive component along the x-axis and the y-axis indicating the numerical order of the subjects. The right panel shows the loadings plot, with primarily eleven variables driving a separation. (B) Among males, the analysis resulted in a significant separation between male smokers and male COPD patients (R2=0.32, Q2=0.25, P=0.016) with primarily eight variables driving a separation. Boxes above zero are markers upregulated and boxes below zero are markers downregulated due to COPD. Black dots: COPD smokers; white dots: smokers with normal lung function.
Abbreviations: BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; IFN, interferon; IL, interleukin; MFI, median fluorescence intensity; OPLS-DA, orthogonal projections to latent structures discriminate analysis; TGF, transforming growth factor.
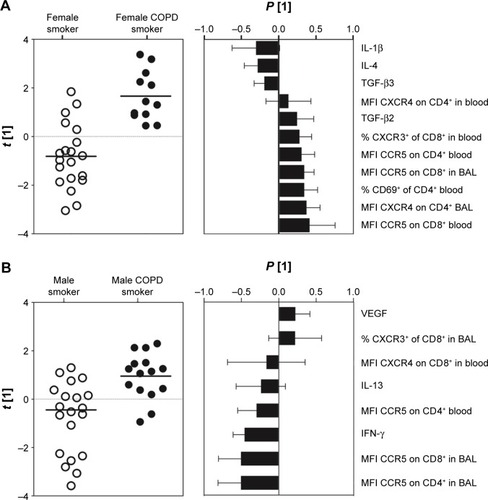
Figure 4 Results of univariate analysis on gender-associated factors separate in smokers with normal lung function and COPD smokers.
Abbreviations: BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; MFI, median fluorescence intensity.
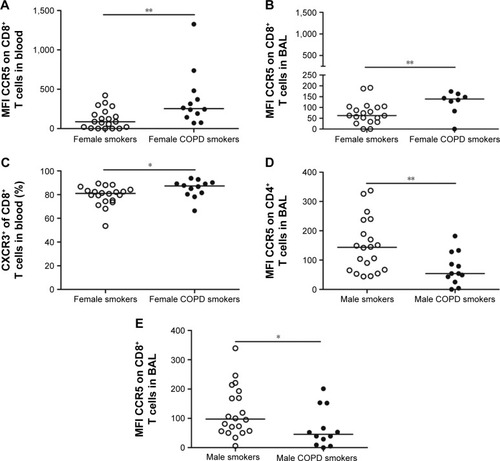
Among eight suggested variables driving the separation between male smokers and male COPD smokers, the univariate analysis revealed significantly lower MFI values of CCR5 on CD8+ and CD4+ T cells in BAL from the COPD smokers compared to smokers (P<0.05 and P<0.01, respectively) ().
Of the significantly different variables within each of the two OPLS® models described earlier, the MFI of CCR5 on CD8+ T cells in BAL was found significant in both the female and the male models. Notably, the alterations in abundances due to disease were found to be opposite, with increased levels in female COPD patients and decreased levels in male COPD patients, as highlighted by shared and unique structure analysis ().
Figure 5 SUS analysis comparing COPD-specific cytokine and T-cell profiles in the male (y-axis) versus female (x-axis) smoking populations.
Abbreviations: BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; OPLS, orthogonal projections to latent structures; SUS, Shared and Unique Structures.
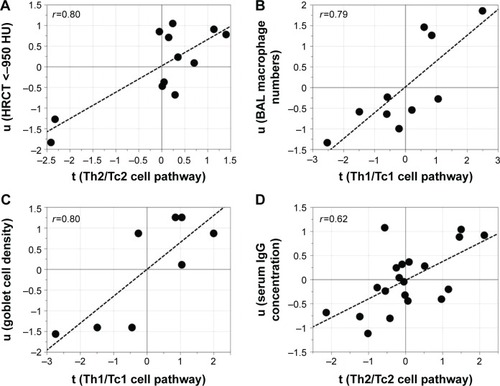
Subsequent multivariate correlation analysis on a pathway basis identified significant correlations between pathways and clinical parameters, especially among female COPD smokers, where the Th2/Tc2 pathway was associated with HRCT measures of emphysema, that is, an increase relative volume of lung tissue with attenuation <−950 Hounsfield units (HU),Citation24 (r=0.80, P=0.002). Furthermore, the Th1/Tc1 pathway was associated with macrophage numbers in BAL (r=0.79, P=0.006) and with bronchial epithelial goblet cell abundance in the airway epithelium sampled from bronchial brushings in female COPD smokers (r=0.80, P=0.02). Among male smokers, a correlation between the Th2/Tc2 pathway and levels of IgG in serum was found (r=0.62, P=0.003) (). No other correlations with clinical data were found.
Figure 6 Associations between pathways and clinical parameters.
Abbreviations: BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; HRCT, high-resolution computed tomography; HU, Hounsfield units; IFN, interferon; IL, interleukin; PLS, partial least squares; TNF, tumor necrosis factor.
Discussion
This study extends previous findings on gender-dependent immune processes in COPD by demonstrating disparate profiles of T-cell recruitment to the lower airways of females and males. In addition, links between biological attributes and Th1/Tc1 or Th2/Tc2 immune patterns in respective gender are described.
An elevated expression of CCR5 on CD4+ T cells and reduced percentage of CXCR3+CD4+ T cells was found in BAL from smokers, but not from COPD smokers, compared to never-smokers. This finding suggests a CCR5-mediated recruitment of CD4+ T cells. A Th1/Tc1 response to smoking and/or that in COPD has been described previously, with infiltration of CCR5- and CXCR3-expressing T cells and higher levels of CCL5 and CXCL9–11 being observed in both the upper and the lower airways.Citation10,Citation25 Our findings on decreased levels of the Th2-associated IL-4 and CXCL12 in the lungs of current smokers further support the theory on a Th1 bias of smoke-induced inflammation and COPD.
There are several differences in the clinical presentation and prognosis between females and males with COPD. Extensive studies have shown that females have more severe symptoms compared to males, despite a shorter smoking history and a similar degree of obstruction and emphysema.Citation26 Several underlying molecular reasons for this could be proposed as previously reported while analyzing BAL-cell proteome from the same cohort.Citation17
Despite several disease-associated variables being significantly altered in the female and male models, respectively, expression of CCR5 on CD8+ T cells in BAL was the only alteration identified in both the models. Intriguingly, females and males showed opposing shifts in CCR5 abundance, due to COPD, indicating that the CCR5 receptor may play a crucial role in CD8+ T-cell recruitment to the lungs in a female-associated COPD phenotype. A functional role for CCR5 has been demonstrated at sites of inflammation. Among others, the receptor has been shown to accelerate recruitment of antigen-specific memory CD8+ T cells to the lung in response to respiratory viral infections.Citation15,Citation27 Fukada et alCitation28 observed an upregulation of CCR5 expression during antigen-specific CD8+ T-cell differentiation, coupled with an increased cytotoxic activity of these cells. The higher expression of CCR5 on CD8+ T cells in females may thus imply the presence of one or several specific antigens, autologous as well as foreign, that trigger T cells to a greater extent in the lungs of females. In fact, an autoimmune component in COPD has been reported.Citation29,Citation30 Moreover, autoimmune diseases are in general more common in females than males.Citation31
Intriguingly, previous studies show a relationship between gender-related hormones and lung function.Citation32 More respiratory symptoms and deteriorating lung function are reported for post-menopausal females, and a protective role for estrogen in the development of COPD has been proposed.Citation26 Among others, it has been shown to stimulate the expansion of CD4+CD25+ T regulatory cellsCitation33 important for controlling inflammation. Here, all female COPD smokers and half of the female smokers were post-menopausal.Citation17 However, the observed alterations related to COPD did not display any correlations with menopause (Figure S3), which is consistent with our findings from the BAL macrophage proteome of these subjects.Citation17
Pathway-based correlation analyses revealed a correlation between the Th2/Tc2 pathway and an HRCT-indicated emphysema among female COPD smokers. Our results implicate that the Th2/Tc2 network is associated with lung tissue destruction in females. Both Th1/Tc1 and Th2/Tc2 cytokines are probable initiators of respective downstream pathways generating emphysema.Citation34–Citation36 An involvement of IL-4 and IL-13 has primarily been suggested in patients exhibiting features of both COPD and asthma.Citation37 As the relative volume with attenuation <−950 HU was fairly small in the COPD patients in this study, it is possible that our findings indicate an effect of the Th2/Tc2 pathway on the early events of emphysema. However, whether this pathway could also be linked to protective mechanisms, for instance, through cross-inhibition of type 1 T cells, is yet to be explored.
Both goblet cell density and BAL macrophage numbers correlated with the Th1/Tc1 pathway in the female smokers with COPD. The interplay between macrophages and Th1 cytokines in COPD is well established. IFN-γ is the main cytokine responsible for the activation of macrophages, and the Th1/Tc1-associated chemokines are produced by macrophages.Citation38
The present study provides evidence for a Th1-dominated immune process, particularly through the CCR5 chemokine receptor, in the lower airways of smokers with normal lung function. We further propose a multivariate model with key factors important in the T-cell recruitment to the lung separating female smokers with normal lung function from female smokers with COPD. Associations between chemokine receptors and cytokine markers of the suggested Th2/Tc2 pathway and measures of emphysema were shown among female smokers with COPD, as well as associations between the suggested Th1/Tc1 pathway and both macrophage numbers in BAL and bronchial epithelial goblet cell density, respectively. Our findings add further support to the hypothesis that gender differences in clinical manifestations of COPD result from different cellular and biological events in the lung. An increased knowledge on the role of immune cell subtypes and inflammatory mediators in smoke-induced inflammation and COPD is central for future development of gender-specific caretaking and treatment.
Author contributions
HF was the principal investigator and was responsible for the integrity of the data and accuracy of data analysis. ÅMW and CMS initiated and conceived the project and provided funding and coordinated the research; HF, MY, MM, JW, ÅMW, and CMS contributed to study design; HF, MY, MM, RK, SN, BE, HM, JG, JW, ÅMW, and CMS contributed to data collection and analysis; HF, JW, ÅMW, and CMS contributed to drafting the manuscript. All the authors contributed to the interpretation of data and to the critical review of the manuscript, including approval of the final version.
Acknowledgments
The authors thank research nurses Gunnel de Forest, Heléne Blomqvist, and Margitha Dahl, as well as Louise Berg and Yvonne Sundström (Karolinska Institutet, Stockholm) for kindly providing the Bio-Rad Bio-Plex instrument and for their helpful recommendations. This study was supported by grants from the Swedish Heart-Lung Foundation, the King Oscar II Jubilee Foundation, the Mats Kleberg Foundation, King Gustaf V’s and Queen Victoria’s Freemasons’ Foundation, the Hesselmans Foundation, Sandoz A/S, Swedish Governmental Agency for Innovation Systems (VINNOVA), the Swedish Foundation for Strategic Research (SSF), European Union (EU) Fp6 Marie Curie International Reintegration Grant (IRF), the Foundation of the Finnish Anti-Tuberculosis Association, the Swedish Research Council (VR), the Stockholm County Council (ALF project), and Karolinska Institutet.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
Materials and methods
Study subjects and patients
To detect inflammatory processes related to COPD and not smoking, smokers with normal lung function and COPD patients were matched for smoking history in terms of pack-years. Patients treated with oral or inhaled corticosteroids, with a history of allergy or asthma, as stated in questionnaires, or with exacerbations during the last 3 months were not included. In vitro screenings for the presence of specific immunoglobulin E (IgE) antibodies (PHADIATOP®; Pharmacia, Uppsala, Sweden) were negative. Reversibility test was performed after inhalation of two doses of 0.25 mg terbutaline (BRICANYL® TURBUHALER®; AstraZeneca, Södertälje, Sweden). Serum IgG was analyzed according to routines at Karolinska University Laboratory, Department of Clinical Chemistry.
Flow cytometric analysis of T cells
Bronchoalveolar lavage (BAL) and blood cells were stained with antibodies against the surface molecules CD3 (Pacific Blue), CD4 (allophycocyanin-Cy7), CD8 (Amcyan), CXCR3 (allophycocyanin), CCR5 (phycoerythrin), CXCR4 (phycoerythrin-Cy5), and CD69 (fluorescein isothiocyanate) or with matched isotype controls (Becton Dickinson [BD], Mountain View, CA, USA) for 25 minutes in the dark (BAL cells at 4°C and blood at room temperature). The blood cells were thereafter incubated with fluorescence-activated cell sorting lysing solution (BD) for 8 minutes and both BAL, and blood cells were washed twice with cell wash (BD). T cells were acquired and analyzed using an 8-parameter flow cytometer FACSCanto (BD) and FACS Diva 6.1.2 software (BD). Because of difficulties in discriminating CCR5- and CXCR4-positive T cells from negative, the expression of these receptors was measured as the difference between median fluorescence intensity (MFI) and the fluorescence of the corresponding isotype control.
Multiplex analysis of inflammatory mediators
Frozen BAL supernatants were thawed at room temperature and concentrated using Amicon Ultra 15ML 3K centrifugal filters (Millipore Corporation, Bedford, MA, USA) prior to cytokine analysis. The assay was performed according to the manufacturer’s instructions, and bead MFI was detected with a Bio-Plex 200 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Multiplex data were analyzed using Bio-Plex Manager software, version 6.0 (Bio-Rad). All samples were run in duplicate. Data out of range or below the lowest limit of quantification (defined as 5× standard deviation of the background noise) or sample with high technical variance (defined as a coefficient of variance of replicates >50%) were excluded from all further analyses. Correction for batch effects was performed through mean-centering and scaling to univariate variance. Strong intra-group outliers, as identified by Dixon’s q-test (P<0.05), were excluded.
Statistical analyses
Unsupervised analysis by principal component analysis (PCA) was used for quality control and outlier identification, and Orthogonal Projections to Latent Structures Discriminate Analysis (OPLS-DA) for separation between groups. In contrast to PCA, OPLS is a supervised modeling approach designed to separate structured noise unrelated (orthogonal) to the predictive variance of interest (eg, between COPD patients and smokers), thereby increasing the ability to identify biomarkers driving the observed group separation.
Prior to analysis, data were log transformed, scaled to unit variance, and mean-centered to avoid weighting based on variable abundance in the analyses.
The results of the OPLS analysis are displayed in a scores plot displaying the separation of groups (predictive component) along the y-axis and within-group variation between individuals (orthogonal components) in the x-axis, as well as the corresponding loadings plot or variables’ importance plot, showing the relative importance of variables for separating the groups.
Figure S1 The relative concentration of (A) TNF-α, (B) IFN-γ, (C) IL-4, (D) CCL3, (E) CCL4, (F) CCL5, (G) CXCL9, and (H) CXCL10 in BAL from healthy never-smokers (NS), smokers with normal lung function (S), smokers with COPD (CS), and ex-smokers with COPD (CE).
Notes: Data have been group mean-centered for the removal of batch effects. Horizontal lines represent the median values.
Abbreviations: BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
Figure S2 The relative concentration of (A) IL-1β, (B) IL-12 (p70), (C) VEGF, and (D) TGF-β2 in BAL from healthy never-smokers (NS), smokers with normal lung function (S), smokers with COPD (CS), and ex-smokers with COPD (CE). Horizontal lines represent the median values. *P<0.05; **P<0.01; ***P<0.001.
Abbreviations: BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; MFI, median fluorescence intensity; IL, interleukin; TGF, transforming growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
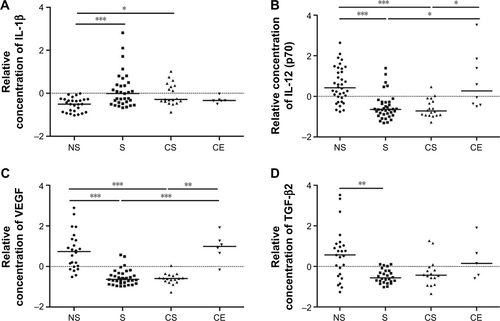
Figure S3 Comparison of the predictive components of the model for all female smokers versus female COPD smokers (x-axis) with the model for female postmenopausal smokers versus female postmenopausal COPD smokers (y-axis).
Note: P(corr) [1] is the scaled loadings of predictive component for the respective OPLS model.
Abbreviations: COPD, chronic obstructive pulmonary disease; OPLS, orthogonal projections to latent structures.
![Figure S3 Comparison of the predictive components of the model for all female smokers versus female COPD smokers (x-axis) with the model for female postmenopausal smokers versus female postmenopausal COPD smokers (y-axis).Note: P(corr) [1] is the scaled loadings of predictive component for the respective OPLS model.Abbreviations: COPD, chronic obstructive pulmonary disease; OPLS, orthogonal projections to latent structures.](/cms/asset/228894aa-01e9-42bf-b45d-e80c2056ab39/dcop_a_113625_sf0003_b.jpg)
References
- The National Board of Health and Welfare (Socialstyrelsen)Hälsooch sjukvårdsrapport 2009 Socialstyrelsen; Stockholm, Sweden. Available from: http://www.socialstyrelsen.se/publikationer2009/2009-126-72Accessed October 6, 2016
- ManninoDMHomaDMAkinbamiLJFordESReddSCChronic obstructive pulmonary disease surveillance – United States, 1971–2000MMWR Surveill Summ2002516116
- AkinbamiLJLiuXChronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998–2009NCHS Data Brief20116318
- LanghammerAJohnsenRGulsvikAHolmenTLBjermerLSex differences in lung vulnerability to tobacco smokingEur Respir J20032161017102312797498
- Ben-Zaken CohenSParePDManSFSinDDThe growing burden of chronic obstructive pulmonary disease and lung cancer in women: examining sex differences in cigarette smoke metabolismAm J Respir Crit Care Med2007176211312017413125
- BrusselleGGJoosGFBrackeKRNew insights into the immunology of chronic obstructive pulmonary diseaseLancet201137897951015102621907865
- HoggJCTimensWThe pathology of chronic obstructive pulmonary diseaseAnnu Rev Pathol2009443545918954287
- EnelowRIMohammedAZStolerMHStructural and functional consequences of alveolar cell recognition by CD8(+) T lymphocytes in experimental lung diseaseJ Clin Invest19981029165316619802879
- FinkelsteinRFraserRSGhezzoHCosioMGAlveolar inflammation and its relation to emphysema in smokersAm J Respir Crit Care Med19951525 Pt 1166616727582312
- FreemanCMCurtisJLChensueSWCC chemokine receptor 5 and CXC chemokine receptor 6 expression by lung CD8+ cells correlates with chronic obstructive pulmonary disease severityAm J Pathol2007171376777617640964
- BarceloBPonsJFusterAIntracellular cytokine profile of T lymphocytes in patients with chronic obstructive pulmonary diseaseClin Exp Immunol2006145347447916907916
- BarczykAPierzchalaWKonOMCosioBAdcockIMBarnesPJCytokine production by bronchoalveolar lavage T lymphocytes in chronic obstructive pulmonary diseaseJ Allergy Clin Immunol200611761484149216751017
- SyrbeUSivekeJHamannATh1/Th2 subsets: distinct differences in homing and chemokine receptor expression?Springer Semin Immunopathol199921326328510666773
- OnufferJJHorukRChemokines, chemokine receptors and small-molecule antagonists: recent developmentsTrends Pharmacol Sci2002231045946712368070
- PalmerLASaleGEBalogunJIChemokine receptor CCR5 mediates alloimmune responses in graft-versus-host diseaseBiol Blood Marrow Transplant201016331131920025985
- ForsslundHMikkoMKarimiRDistribution of T-cell subsets in BAL fluid of patients with mild to moderate COPD depends on current smoking status and not airway obstructionChest2014145471172224264182
- KohlerMSandbergAKjellqvistSGender differences in the bronchoalveolar lavage cell proteome of patients with chronic obstructive pulmonary diseaseJ Allergy Clin Immunol2013131374375123146379
- KarimiRTornlingGForsslundHLung density on high resolution computer tomography (HRCT) reflects degree of inflammation in smokersRespir Res2014152324564813
- LofdahlJMCederlundKNathellLEklundASkoldCMBronchoalveolar lavage in COPD: fluid recovery correlates with the degree of emphysemaEur Respir J200525227528115684291
- KarimiRTornlingGGrunewaldJEklundASkoldCMCell recovery in bronchoalveolar lavage fluid in smokers is dependent on cumulative smoking historyPLoS One201273e3423222479573
- BancroftJDGambleMTheory and Practice of Histological Techniques6th edEdinburghChurchill Livingstone2008
- WongDMVaresioLDepletion of macrophages from heterogeneous cell populations by the use of carbonyl ironMethods Enzymol19841083073136527653
- WheelockAMWheelockCETrials and tribulations of ‘omics data analysis: assessing quality of SIMCA-based multivariate models using examples from pulmonary medicineMol Biosyst20139112589259623999822
- GevenoisPADe VuystPde MaertelaerVComparison of computed density and microscopic morphometry in pulmonary emphysemaAm J Respir Crit Care Med199615411871928680679
- SaettaMMarianiMPanina-BordignonPIncreased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2002165101404140912016104
- OharJFromerLDonohueJFReconsidering sex-based stereotypes of COPDPrim Care Respir J201120437037821922124
- KohlmeierJEMillerSCSmithJThe chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infectionsImmunity200829110111318617426
- FukadaKSobaoYTomiyamaHOkaSTakiguchiMFunctional expression of the chemokine receptor CCR5 on virus epitope-specific memory and effector CD8+ T cellsJ Immunol200216852225223211859109
- PackardTALiQZCosgroveGPBowlerRPCambierJCCOPD is associated with production of autoantibodies to a broad spectrum of self-antigens, correlative with disease phenotypeImmunol Res2013551–3485722941590
- DaffaNITighePJCorneJMFaircloughLCToddINatural and disease-specific autoantibodies in chronic obstructive pulmonary diseaseClin Exp Immunol2015180115516325469980
- SelmiCBrunettaERaimondoMGMeroniPLThe X chromosome and the sex ratio of autoimmunityAutoimmun Rev2012116–7A531A53722155196
- TamAMorrishDWadsworthSDorscheidDManSFSinDDThe role of female hormones on lung function in chronic lung diseasesBMC Womens Health2011112421639909
- TaiPWangJJinHInduction of regulatory T cells by physiological level estrogenJ Cell Physiol2008214245646417654501
- GrumelliSCorryDBSongLZAn immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysemaPLoS Med200411e815526056
- ShanMChengHFSongLZLung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysemaSci Transl Med2009144ra10
- WangZZhengTZhuZInterferon gamma induction of pulmonary emphysema in the adult murine lungJ Exp Med2000192111587160011104801
- ZhengTZhuZWangZInducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysemaJ Clin Invest200010691081109311067861
- BarnesPJThe cytokine network in asthma and chronic obstructive pulmonary diseaseJ Clin Invest2008118113546355618982161
