Abstract
Airway mucus hypersecretion is a frequent symptom associated with acute and chronic airway disease. Inhibition of mucus production or promotion of mucolysis not only relieved symptoms but also improved disease outcomes. There are numerous available mucoactive medicines for prescription, and how to select them properly for different diseases is important for clinical practice. So far, there is no one consensus or guideline reported. A group of Chinese pulmonary physicians worked together to complete this consensus based on literature review, summarized mechanism and usage of each classical mucoactive medicine. In general, antioxidant mucoactive medicines play an important role in chronic airway disease, including but not limited to airway mucus clearance, reduced acute exacerbation and improved pulmonary function.
Keywords:
Introduction
COPD is defined as persistent air flow limitation with continuous progression. But it is a treatable and preventable disease. A survey among 20,245 adults in 7 areas of the People’s Republic of China showed that the prevalence of COPD in the population aged older than 40 years was 8.2%.Citation1 Global disease burden report indicated that COPD would rank as the fourth disease in the People’s Republic of China in 2013.Citation2 Most importantly, acute exacerbation of COPD (AECOPD) accelerates pulmonary function decline, reduces quality of life and increases medical cost. Risk factors analysis suggested that patients who have acute exacerbations more than 3 times carried 4 times higher mortality and morbidity compared to those who do not have acute exacerbations.Citation3 Therefore, the prevention of acute exacerbation is an important strategy to reduce lung function decline, improve quality of life and eventually reduce COPD mortality.Citation4
COPD is a heterogeneous disease based on clinical presentation, genetic background, pathophysiology and therapeutic response. There is no doubt that precision medicine is required to treat COPD, considering its versatile and complicated profile. Cilium-beating dysfunction, mucus hypersecretion, bacteria colonization, airway inflammation and oxidative stress contribute to COPD pathogenesis, while identification of the gene susceptible to occupational exposure and smoke may reveal intrinsic factors. Thus, COPD prevention, diagnosis and treatments should be a long–term, comprehensive, persistent and individualized program. In the past 10 years, results from several randomized controlled trials (RCTs) have increased the understanding of the role of expectorant/antioxidant therapy in COPD. These results have also been cited in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, with contribution from Chinese scholars.Citation5,Citation6
Considering that there is no guideline or consensus on COPD expectorant/antioxidant therapy, the editorial office of the International Respiratory Journal brought together specialists in the field to draft this consensus in order to guide clinical use of expectorant/antioxidant medicine in COPD.
Methodology
The PubMed, Chinese Biology Abstract, Chinese Academic Journal database and WanFang database were used to identify relevant articles published from 2005 to 2016. The initial literature search identified 316 published articles, of which there were 80 potentially relevant references (41 from English and 39 from Chinese literatures). Finally, 76 references were eligible for this review after group discussion, and eventually 66 references were included in this review. Disagreements were resolved by consensus.
Importance of mucoactive therapy
Cough and phlegm are the main clinical presentations, as well as key criteria, for COPD phenotype classification. Airway mucus hypersecretion is one of the insulting factors for airflow limitation, lung function decline and COPD acute exacerbation.Citation7 Several studies have shown that persistent cough with sputum is correlated with the decline of forced expiratory volume in one second (FEV1), hospitalization and mortality,Citation8,Citation9 while mucoactive therapy could relieve small airway obstruction, reduce bacteria colonization and acute exacerbation, and improve health-related quality of life. A study conducted by Vestbo et alCitation10 showed that chronic airway mucus hypersecretion is correlated with FEV1 decline, especially in male patients, with additional 22.8 mL decrease each year. Khurana et alCitation9 showed evidence that sputum neutrophil and eosinophil counts, eotaxin-1, monocyte chemoattractant protein (MCP)-1, tumor necrosis factor (TNF)-α and interleukin (IL)-6 levels in sputum supernatant in persistent-expectoration COPD patients was higher than the levels in patients who do not have cough and sputum secretion. It indicated that mucoactive therapy may relieve airway inflammation and expectoration symptoms.Citation11 Other studies showed that COPD patients with and without long-term cough and sputum had acute exacerbation at the rate of 2.2/patient-year and 0.97/patient-year; 1.8/patient-year and 0.66/patient-years for moderate exacerbation; 0.43/patient-year and 0.22/patient-year for hospitalization, respectively. The proportions of acute exacerbation were 55% and 22% individually.Citation12 These results strongly indicated the necessity of mucoactive therapy in COPD.
Importance of antioxidant therapy
Oxidative stress is one of the key contributors to COPD pathogenesis, due to the imbalance of oxidant and antioxidant systems, which leads to the accumulation of reactive oxygen species (ROS), resulting in organ tissue injury. Multiple cells are involved in the pathogenesis of COPD, including neutrophils, eosinophils, macrophages, lymphocytes, as well as the airway epithelial cells etc. The activation of these cells results in persistent and chronic inflammation, as well as the imbalance of oxidant/antioxidant status. Smoking and air pollution are two major risk factors for COPD. Smoking could increase ROS production. When antioxidants cannot metabolize ROS, the cell membrane, proteins, glycosides and DNA of the airway epithelium are damaged due to chronic inflammation.Citation13 Moreover, the endogenous source of oxidative stress is the inflammatory cells such as macrophages, neutrophils and eosinophils. These cells release large amounts of ROS after smoking exposure. Although antioxidants could scavenge free radicals, quantities of ROS would be accumulated when the antioxidants are exhausted.Citation14 The compound 8-isoprostane, a biomarker of oxidation, increased in expiration condensation fluid obtained from COPD patients and smokers;Citation15,Citation16 moreover, this also correlated positively with the degree of emphysema and modified Medical Research Council (mMRC) dyspnea score, while being negatively correlated with partial pressure of arterial oxygen PaO2, diffusing capacity of the lungs for carbon monoxide (DLCO), 6 min walk test and maximum exercise work load.Citation17,Citation18
Mucoactive and antioxidant drugs
There are many mucoactive medicines, such as mucolytics, mucokinetic agents, mucoregulators and expectorants. Not all mucoactive medicines have both expectorant and antioxidant properties. Herein, we summarized 4 of them that have been frequently used in clinical practice with evidence of literature support, such as N-acetylcysteine (NAC), carbocysteine, erdosteine and ambroxol. They also are routinely prescribed drugs for COPD ().
Table 1 Summary of mucoactive medicines
N-acetylcysteine
NAC has been used in clinical practice since the 1960s. It contains one free thiol and it breaks down the disulfide bond, depolymerizes the oligomer accumulation of mucin and then reduces sputum viscosity.Citation19 The overall functions of NAC are described as follows.Citation20
Mucolytic activity
NAC breaks down the disulfide bond in mucin glycopeptides to reduce sputum viscosity and makes expectoration easy. NAC can also lyse sputum DNA, increase airway surface liquid thickness and promote airway clearance. It also inhibits mucus secretion and cell hyperplasia, as well as increasing MUC5AC expression.Citation21 In addition, it increases beating of cilia, stimulates gastric–lung vagus reflexion to improve expectoration.Citation22
Antioxidant property
NAC has direct and indirect antioxidant properties (). The direct function includes the binding of the thiol group to free radicals, hydrogen peroxide and hypochlorite to clear ROS.Citation23 It also binds to glutathione peroxidase to reduce production of lipid peroxide. The indirect functions include synthesis of glutathione and maintenance of adequate levels of glutathione to prevent cell damage (). Oral intake of 600 mg/d NAC for 5 d can significantly increase bronchoalveolar lavage fluid (BALF) glutathione levels, indicating that NAC plays an important role in indirect antioxidation.
Figure 1 Mechanism of NAC pharmacology in COPD patients.
Notes: NAC can directly break disulfide bonds in mucus to decrease mucus viscosity, thus improving ciliary beating and mucus clearance. NAC clears ROS through –SH binding, possessing antioxidant properties, as well as having the indirect function of facilitating GSH accumulation. The decrease of ROS and increase of GSH reduce airway inflammation and airway mucus production. All these contribute to improved lung function and reduced acute exacerbation.
Abbreviations: –SH, thiol; GSH, glutathione; NAC, N-acetylcysteine; ROS, reactive oxygen species.
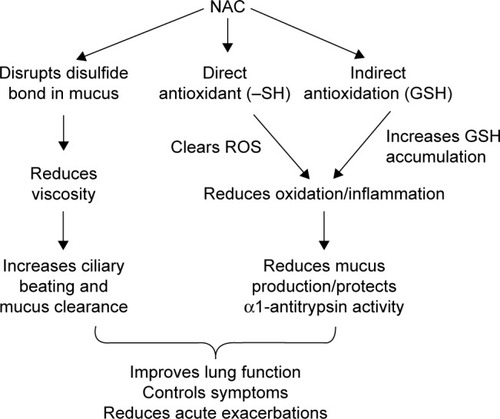
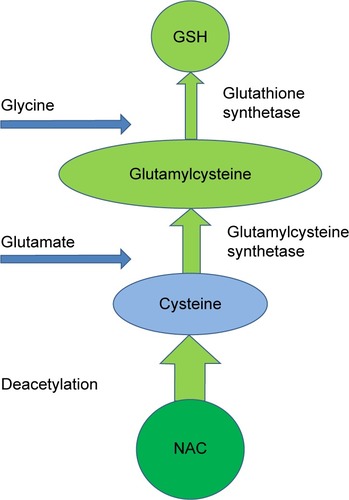
Figure 2 The synthesis of GSH from NAC and its metabolites.
Abbreviations: GSH, glutathione; NAC, N-acetylcysteine.
Inhibition of lung inflammation
Oral intake of NAC can decrease the H2O2 level in expiration air and reduce NF-κB-mediated lung inflammation.Citation24 Signal transduction in redox-sensitive cells is also inhibited by NAC to reduce endothelial injury, improve imbalance of oxidant-antioxidant and further prevent airway injury.Citation25 The thiol group in NAC reduces the activity of elastase, decreases plasma myeloperoxidase (MPO) and the capacity of elastic protease, as well as decreasing the production of lactoferrin and eosinophil cationic protein in BALF, neutrophil chemoattractant activityCitation26 and neutrophil chemoattractant release in sputum of COPD patients.Citation27 It also attenuates the lung injury induced by oxidative stress, lung inflammation and airway remodeling.
Decreases microbial pathogenicity
NAC could reduce the adhesion of Haemophilus influenzae and Streptococcus pneumonia to epithelia of oropharynx, inhibit bacteria colonization and growth, improve the anti-infective ability, and decrease the frequency of acute exacerbation.Citation28 NAC also could inhibit virus replication and reduce virus titer through decreasing cell cytosol H2O2 and restore cell sulfhydryl levels.Citation29 By inhibiting expression of adhesion molecules after respiratory syncytial virus infection, NAC could adjust cytosol H2O2 level to restore glutathione for epithelium protection.Citation30
Carbocysteine
Mucoregulator
Carbocysteine is the thiol derivative of L-cysteine with free radical-scavenging and anti-inflammatory properties.Citation31 Carbocysteine stimulates the production of low-viscosity sialomucin and decreases the production of high-viscosity mucin to improve sputum clearance. It also binds to the disulfide bond through the carboxymethyl group to improve sputum elasticity and viscosity to increase ciliary clearance.Citation32
Anti-inflammatory and antioxidant properties
Carbocysteine is a strong scavenger of hypochlorite and free radicals. It can significantly inhibit IL-8 production from peripheral neutrophils.Citation33 It also inhibits conversion of xanthine dehydrogenase to xanthine oxidase to exert anti-inflammatory effects.Citation34,Citation35
Anti-infective activity
Carbocysteine reduces bacterial colonization by decreasing the expression of adhesion molecule-1, especially for Streptococcus pneumoniae.Citation36
Erdosteine
Erdosteine is an antioxidant and mucoactive medicine containing thiol group. It has the following activities.
Viscosity regulation
Three free thiol metabolites are produced after oral intake, and these thiol metabolites break the disulfide bond to change sputum viscosity and promote airway clearance while retaining the antitussive effects.
Anti-inflammatory and antioxidant activity
Erdosteine scavenges free radicals and protects α1-antitrypsin activityCitation37 to exert its anti-inflammation and antioxidant properties.
Anti-infective activity
Erdostetine metabolites significantly increase secretory immunoglobulin A (sIgA)/albumin and lactoferrin/albumin ratios, as well as improving antibiotic penetration in airway mucosa, thus reducing bacterial adhesion.Citation38,Citation39
Ambroxol
Similar to NAC, ambroxol regulates mucus secretion and exerts anti-inflammatory effect at large doses. The main mechanisms are described as follows.
Viscosity regulation
Ambroxol stimulates serous secretion and increases airway surface liquid depth, in addition to thinning of the thick mucus and sputum. It also promotes surfactant production, increases ciliary beating and promotes expectoration.Citation40,Citation41
Anti-inflammatory and antioxidant effects
Ambroxol has high affinity to lung tissue; it has anti-inflammatory and antioxidant properties. Through promotion of surfactant production, the surface tension of alveoli could be reduced, thereby preventing alveolar trap and decreasing alveolar and airway pressure. By free radical clearance and inhibition of leukotriene and histamine production, ambroxol effectively attenuates inflammation from macrophages and neutrophils. It also activates the cytosolic glutathione system to promote glutathione production to clear hyperoxidates, leading to reduced airway responsiveness and reactivity.Citation42
Antibacterial effects
Combination of antibiotics and ambroxol could increase antibiotic concentration in the lung tissue and improve bacterial clearance and lung infection, while reducing antibiotic use.Citation43
Clinical studies of mucoactive/antioxidant drugs on COPD
Mucoactive/antioxidant drugs could decrease acute exacerbation
Several RCT studies have shown that mucoactive/antioxidant medicines could significantly decrease acute exacerbation in COPD. Pela et alCitation44 reported, for the first time, on the effects of NAC in stable COPD patients and found that NAC reduced acute exacerbation in 169 moderate-to-severe COPD patients. The patients enrolled in that study were assigned randomly to control standardized treatment group and standardized treatment with NAC group with a dose of 600 mg/d. After 6 months, the overall exacerbation in the NAC group decreased 41%. In 2005, 523 patients were enrolled and followed for 3 years in the Bronchitis Randomized on NAC Cost-Utility Study (BRONCUS) study;Citation45 the results showed that NAC (600 mg/d) did not show any difference on acute exacerbation. However, for those who did not use inhaled corticosteroids (ICSs), acute exacerbation rate dropped 21% (P<0.05). The HIACECitation46 study, conducted in Hong Kong in 2013, showed that treatment with NAC (1,200 mg/d) for 12 months significantly reduced COPD acute exacerbation. A meta-analysis summarized 30 RCT studies with 7,436 COPD or chronic bronchitis patients. The results showed that NAC or carbocysteine reduced acute exacerbation by 17%,Citation47 suggesting that oral intake of mucoactive medicines may reduce acute exacerbation. The PANTHEONCitation6 study enrolled 1,297 COPD patients from 34 hospitals in the People’s Republic of China; among those patients, 1,006 were randomly assigned to control NAC (600 mg bid [twice a day]) and placebo groups. After 1 year follow-up, the results showed that NAC significantly reduced acute exacerbation, especially in moderate COPD patients with high tolerance to NAC.
Zheng et alCitation5 followed 709 COPD patients for 1 year (PEACE study) and found that there was a 24% decrease of acute exacerbation in COPD patients who received carbocysteine (0.5 g, tid [thrice a day]; treatment group) compared to the placebo group. Allegra et alCitation48 investigated 662 chronic bronchitis patients treated with carbocysteine lysine salt for 1 year. Among those patients, in the continuous treatment group (2.7 g/d for 6 months), only 66 patients (29%) had >1 acute exacerbations, while 100 patients in the intermittent treatment group (2.7 g/d, every other week) had >1 exacerbations.Citation48 The treatment group showed significantly delayed onset of first acute exacerbation. Compared to the placebo group, the continuous treatment group had fewer days of acute exacerbation, while no difference was found between the intermittent group and the placebo group in terms of acute exacerbations. These results suggest that continuous and long-term treatment with carbocysteine may provide meaningful clinical outcome for COPD acute exacerbation.
In 2004, a multiple-center double-blinded placebo control and long-term studyCitation49 using erdosteine in COPD patients showed that erdosteine (300 mg bid for 8 months) could significantly reduce acute exacerbation and hospitalization while increasing the lung function and quality of life compared to the placebo group.
COPD patients (n=242) with percent predicted FEV1 (FEV1%pred) between 60% and 80% were enrolled in a 1-year study with two arms; one arm used ambroxol (75 mg, bid) and one arm used placebo.Citation50 After 6 months, 63% patients from the treatment group and 60% patients from the placebo group did not have acute exacerbation, and the ratio decreased to 56% and 53% after 1-year follow-up, respectively. Among the patients who had the worst clinical score, 63% patients in the treatment group and 38% patients in the placebo group did not have acute exacerbation. This study showed that ambroxol application may have benefit in those COPD patients who had more severe symptoms. This evidence suggests that the 4 medicines listed in this review did reduce acute exacerbation in COPD to different extents, although dosing and duration are different.
Mucoactive/antioxidant drugs could improve symptoms and quality of life
In addition to reducing acute exacerbation in COPD, mucoactive/antioxidant therapy could also decrease cough with sputum and hospitalization. Results from the PANTHEON studyCitation6 showed significant improvement in clinical symptoms after 1-year treatment with NAC. In the PEACECitation5 study, the St George’s Respiratory Questionnaire (SGRQ) scores were significantly decreased in the carbocysteine group (4.06), compared to the placebo group, especially their symptoms and activity. In the EQUALIFECitation49 study, the SGRQ score was significantly improved in the erdosteine treatment group, while no SGRQ score changes were found in the placebo group. However, results from these studies were not consistent with each other. In the BRONCUSCitation45 study, there was no difference in the SGRQ scores between the treatment and control groups after 1-year treatment, and there was no improvement in the quality of life with NAC therapy in the second year. In the HIACECitation46 study (NAC 600 mg, bid), mMRC, SGRQ and 6 min walk distance did not show significant differences between treatment and placebo groups. In the PANTHEON study,Citation6 although NAC (600 mg, bid) could reduce SGRQ (-3.37, P=0.043), overall SGRQ and other scores were not different from the placebo group.
An RCT study conducted in Europe using erdosteine suggested that erdosteine could effectively reduce acute exacerbation in chronic bronchitis patients, improving clinical symptoms including cough, expectoration and dyspnea.Citation51 Acute exacerbated chronic bronchitis patients (n=226) were enrolled in this study, with the treatment group taking erdosteine 300 mg tid for 7–10 d while both treatment group and control group used amoxicillin 50 mg tid. Results showed that average clinical evaluation performances, including objective and subjective clinical symptom improvement, lung function and sputum properties, were improved in 60% of the treatment group, while only 41% in the control group showed improvement. Another double-blinded study conducted in France enrolled 170 stable chronic bronchitis patients, with the treatment group taking erdosteine 300 mg bid for 21 d. Results showed that erdosteine decreased the global effective index by 27%, while only 19.2% reduction was found in the placebo group.Citation52 The most prominent parameters with significant improvement were frequency and severity of cough. Within 10 d of treatment, erdosteine significantly decreased the sputum viscosity (−22.9% vs −10.8%; P<0.05) and improved the cough index (−19.3% vs −10.4%; P<0.05), respectively. Maximum ventilation capacity was also significantly improved in the treatment group.
Mucoactive/antioxidant drugs can decrease hospitalization and hospital time
In the HIACE study,Citation46 there was a trend showing declined hospitalization rate in the NAC group compared to placebo (0.5/year vs 0.8/year), as well as hospitalization days (1.8 d/year vs 4.2 d/year); however, there was no statistical difference. Gerrits et alCitation53 separated 1,219 COPD patients (>55 years old) into NAC group and non-NAC group, comparing their first acute exacerbation and hospitalization. Results showed that 30% rehospitalization was reduced in the NAC group and there was a reversed dose–response correlation between NAC dose and hospitalization, with less hospitalization at high doses of NAC (P<0.0001). Moretti et alCitation49 reported in the EQUALIFE study that hospitalization times and averaged hospitalization days were significantly reduced in the erdosteine group after 8-month treatment.
Mucoactive/antioxidant drugs can partially improve lung function
Flow limitation is mainly caused by small airway disease and lung parenchyma damage (emphysema). Chronic inflammation induces small airway structural changes, with reduction in the number of alveoli attached to the small airways, thus resulting in decreased lung elasticity. NAC has anti-inflammatory and antioxidant properties, decreasing distal space air retention and improving exercise endurance.Citation54 An RCT study by Stav and RazCitation55 found reduced hyperinflation after 6-week treatment with NAC (1,200 mg/d) in moderate-to-severe COPD patients (aged >40 years, FEV1 <58% pred, residual capacity to total lung capacity [RC/TLC] >137%, inspiratory capacity [IC] >2.2 L), including increased IC, free light chains (FLCs) after exercise, and decreasing RC/TLC after exercise. The decreased airway resistance and hyperinflation effects were also confirmed in the HIACE study,Citation46 suggesting that NAC could significantly improve small airway function.
In the BRONCUS study,Citation45 there was a 54 mL decrease of FEV1 after NAC treatment, with a 47 mL decrease in the placebo group; there was no statistical difference. FEV1, forced vital capacity (FVC), and FEV6 were not changed between NAC and placebo groups in the PANTHEONCitation6 study. No improvement in lung function and oxygen saturation was found in the PEACECitation5 study after carbocysteine treatment. Although MorettiCitation38 found that erdosteine treatment improved FEV1, the baseline of FEV1 was high in the treatment group (200 mL more). If this difference was subtracted, there was no FEV1 change after expectorant therapy in the EQUALIFECitation49 study. The possible explanation could be that expectorant/antioxidant medicines are not bronchodilators and FEV1 may not be the best indicator for COPD improvement; however, in moderate-to-severe COPD patients, reduced airway trapping and small airway function improvement may contribute to symptom improvement.
Effects of mucoactive/antioxidant drugs on the whole system and acute exacerbation
COPD is a traditional respiratory disease but with systemic involvement, including muscle atrophy, osteoporosis, exercise capacity, fat loss, etc.Citation56,Citation57 NAC reduces fatigue in healthy volunteers and delays fatigue duration.Citation58,Citation59 Stav and RazCitation55 found that treatment with NAC 1,200 mg for 6 weeks could increase exercise time, while in the HIACECitation46 study, such improvement was not confirmed. Zuin et alCitation60 found that both NAC 1,200 mg/d or 600 mg/d could improve symptoms related to acute exacerbation, such as cough with sputum, dyspnea, and lung function decline; the higher the dose used, the better was the outcome.
Wang et alCitation61 studied the effects of ambroxol on AECOPD. Eighty COPD patients were randomly assigned into 2 groups, with the treatment group using intravenous ambroxol at 120 mg daily for 10 d. Absolute changes in IL-8, IL-10, TNF-α, FEV1%pred and FEV1/FVC were significantly greater after ambroxol treatment, suggesting that large doses of ambroxol may have anti-inflammatory effects that facilitate lung function recovery.
Minimal side effects
Overall, there were few side effects in clinical studies using expectorant/antioxidant medicines. In the HIACECitation46 study, large doses of NAC did not bring about severe side effects. Long-term use of NAC (600 mg, 1 year) has been proved safe and tolerable, without significant difference in terms of side effects between treatment group and placebo group. In the PANTHEON study,Citation6 146 out of 495 (29%) in the NAC group showed mild side effects, not different from the control group (130 out of 495, 26%). In addition, the major side effects such as acute exacerbation should be irrelevant to NAC application. High doses of NAC (1,800 mg/d) in clinical trials on idiopathic pulmonary fibrosis also showed good tolerance to NAC.Citation62
Existing problems in these studies
There are several limitations that should be considered when interpreting these results. First, the major problem is the limited sample sizes. Except PEACE,Citation5 PANTHEONCitation6 and the BRONCUSCitation45 studies with sample size more than 500, most other studies only enrolled small number of patients, thus making the overall level of evidence lower. Second, the reviews or meta-analyses were mostly written in Chinese. Third, except the PEACE,Citation5 PANTHEON,Citation6 BRONCUSCitation45 and HIACECitation46 studies, treatment times were generally short, varying from few weeks to 6 months. The PEACECitation5 and PANTHEONCitation6 studies have shown that the longer the treatment duration, the better is the outcome that the patients would have. The dose–response profile of NAC suggested that a high dose (1,200 mg/d) is required for confirmed benefit. Fourth, the target population needs to be assessed in future studies. Lastly, the BRONCUSCitation45 study showed that COPD patients without ICS use gain more benefit from NAC than those who use ICS. In the PANTHEON study, moderate COPD patients gained more improvement than severe COPD patients, suggesting that long-term and regular treatment is critical to gaining benefit in the early stages of COPD.
Clinical application recommendations of mucoactive/antioxidant medicines
Expectorant/antioxidant medicine recommendation from COPD guidelines
GOLD 2013Citation63 indicated for expectorant/antioxidant medicine use for treatment of hypersecretion in COPD airway, as may induce recurrent infection and airway obstruction. Expectorants facilitate airway drainage and improve lung function but only work in patients who have mucus production. The frequently used medicines include ambroxol and NAC. The Chinese physician consensus on AECOPD (2014 revised version) proposed that NAC is effective in reducing acute exacerbation, especially in patients who do not use ICS.Citation64 GOLD 2015 cited an article by Zheng et alCitation6 and recommended the long-term use of NAC (1,200 mg/d) in moderate-to-severe COPD patients to reduce acute exacerbation with/without ICS inhalation. The American College of Chest Physicians (ACCP)/Canadian Thoracic Society (CTS)Citation65 recommend oral intake of NAC in moderate-to-severe COPD patients to prevent acute exacerbation. For clinically stable COPD patients, NAC or carbocysteine should be used regularly to reduce exacerbation and improve quality of life. GOLD 2016 particularly pointed cough with sputum as an independent factor associated with increased mortality in mild-to-moderate COPD patients; this statement strongly suggested the importance of antitussive and mucoactive therapy in COPD patients.Citation66
Recommendations based on the cited studies and clinical practice
Expectorant/antioxidant therapy in stable COPD
Long-term mucoactive/antioxidant therapy for chronic bronchitis or COPD patients should be initiated as long as patients complain of cough with sputum or dyspnea. If the patient with the evidence of COPD lung function complains of cough with sputum since childhood and if computed tomography (CT) scan shows evidence of bronchiectasis, patients do need mucoactive/antioxidant therapy. COPD patients whose FEV1% is more than 50% but who complain of cough with sputum, patients who have a problem sleeping due to expectoration symptom and asthma or allergy could be excluded. COPD patients with lung function classes 3 and 4, more than 2 clinical visits, and GOLD C or D group patients who do not have ICS inhalation or who show a combination with bronchiectasis need treatment.
AECOPD
Few studies suggest that mucoactive/antioxidant therapy in AECOPD patients may provide additional benefit, and combining chest wall motion may propagate the airway clearance benefit.Citation49
Usage
There are many mucoactive medicines in clinical practice; we summarized a few of them that have relatively clearer therapeutic indications based on RCT results. Herein, NAC, carbocysteine, and erdosteine have been recommended for anti-inflammation therapy in COPD patients. Due to lack of RCT trials, ambroxol is not recommended for long-term therapy at large doses, while as mucoactive therapy, ambroxol 75 mg bid has been recommended in COPD and chronic bronchitis patients. Being an anti-inflammatory medicine, dose and duration are critical. NAC 1,200 mg/d, carbocysteine 1,500 mg/d and erdosteine 600 mg/d for 3–6 months are minimum regimes in COPD patients. For sole mucoactive therapy, this dose could be reduced in half. If patients cannot tolerate the 6-month regimen, they are recommended to try the treatment plan during the spring and winter, while persistent dosing in summer is recommended to reduce respiratory system symptom, CODP hospitalization and acute exacerbation.
Price is another concern during COPD treatment. The price of NAC in the People’s Republic of China is around 1 USD per capsule, roughly 60 USD/mo. Ambroxol costs around 0.15 USD per tablet in the People’s Republic of China, roughly 27 USD/mo; carbocysteine costs around 0.02 USD per capsule, roughly 3.6 USD/mo; for erdosteine, the price is around 0.9 USD per capsule, roughly 55 USD/mo. In general, the cost of antioxidant mucoactive therapy is roughly similar to or one-third to half the price of monthly used bronchodilators. Considering the fact that patients may need both bronchodilators and expectorants together, the cost-effectiveness should be borne in mind before prescription.
Conclusion
COPD is a heterogeneous disease with complicated pathogenesis and treatment responses. Current evidence suggests that antioxidant expectorants may reduce airway inflammation, decrease oxidative stress, reduce acute exacerbation and improve quality of life in COPD patients. Precise treatments in targeted COPD population need further investigation with stratification strategy.
Acknowledgments
The authors thank Quanlong Zhang, Linlin Wang, Xinxiu Liu, Jin Li, Jing Cao and Xiaowei Cao for providing the literature search and material organization, and Guiying Hu for organizing the consensus conference and revision of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- ZhongNWangCYaoWPrevalence of chronic obstructive pulmonary disease in China: a large, population-based surveyAm J Respir Crit Care Med2007176875376017575095
- GBD 2013 Mortality and Causes of Death CollaboratorsGlobal, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013Lancet2015385996311717125530442
- Soler-CataluñaJJMartínez-GarcíaMARomán SánchezPSalcedoENavarroMOchandoRSevere acute exacerbations and mortality in patients with chronic obstructive pulmonary diseaseThorax2005601192593116055622
- DonaldsonGCSeemungalTABhowmikAWedzichaJARelationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary diseaseThorax2002571084785212324669
- ZhengJPKangJHuangSGEffect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled studyLancet200837196292013201818555912
- ZhengJPWenFQBaiCXTwice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trialLancet Respir Med20142318719424621680
- LuWZhengJThe function of mucins in the COPD airwayCurr Respir Care Rep201323155166
- De MarcoRAccordiniSCerveriIIncidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegmAm J Respir Crit Care Med20071751323917008642
- KhuranaSRaviASutulaJClinical characteristics and airway inflammation profile of COPD persistent sputum producersRespir Med2014108121761177025459449
- VestboJPrescottELangePAssociation of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study GroupAm J Respir Crit Care Med19961535153015358630597
- VestboJEpidemiological studies in mucus hypersecretionNovartis Found Symp2002248312 Discussion 12–19, 277–28212568485
- BurgelPRNesme-MeyerPChanezPCough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjectsChest2009135497598219017866
- FischerBMVoynowJAGhioAJCOPD: balancing oxidants and antioxidantsInt J Chron Obstruct Pulmon Dis20151026127625673984
- SantusPCorsicoASolidoroPBraidoFDi MarcoFScichiloneNOxidative stress and respiratory system: pharmacological and clinical reappraisal of N-acetylcysteineCOPD201411670571724787454
- KinnulaVLIlumetsHMyllarniemiMSovijärviARytiläP8-Isoprostane as a marker of oxidative stress in non symptomatic cigarette smokers and COPDEur Respir J2007291515517050565
- MontuschiPCollinsJVCiabattoniGExhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokersAm J Respir Crit Care Med20001623Pt 11175117710988150
- MakrisDParaskakisEKorakasPExhaled breathcondensate 8-isoprostane‚ clinical parameters‚ radiological indices and airway inflammation in COPDRespiration200875213814417641539
- García-RioFRomeroDLoresVDynamic hyperinflation, arterial blood oxygen, and airway oxidative stress in stable patients with COPDChest2011140496196921436248
- DavisSSScobieSInglisAThe effect of sulphydryl compounds and cross linking agents on the viscous and viscoelastic properties of mucusBiorheology1975123–42252321125
- SamuniYGoldsteinSDeanOMBerkMThe chemistry and biological activities of N-acetylcysteineBiochim Biophys Acta2013183084117412923618697
- MataMRuizACerdaMOral N-acetylcysteine reduces bleomycin-induced lung damage and mucin Muc5ac expression in ratsEur Respir J200322690090514680076
- RogersDFMucoactive agents for airway mucus hypersecretory diseasesRespir Care200752911761193 Discussion 1193–119717716385
- SadowskaAMVan OverveldFJGóreckaDThe interrelationship between markers of inflammation and oxidative stress in chronic obstructive pulmonary disease: modulation by inhaled steroids and antioxidantRespir Med200599224124915715193
- KasielskiMNowakDLong-term administration of N-acetylcysteine decreases hydrogen peroxide exhalation in subjects with chronic obstructive pulmonary diseaseRespir Med200195644845611421501
- SzkudlarekUZdziechowskiAWithkowskiKEffect of inhaled N-acetylcysteine on hydrogen peroxide exhalation in healthy subiectsPulm Pharmacol Ther200417315516215123225
- EklundAErikssonOHakanssonLOral N-acetylcysteine reduces selected humoral markers of inflammatory cell activity in BAL fluid from healthy smokers: correlation to effects on cellular variablesEur Respir J1988198328382852604
- Van OverveldFJVermeirePADe BackerWAInduced sputum of patients with chronic obstructive pulmonary disease (COPD) contains adhesion-promoting, therapy-sensitive factorsInflamm Res200049181310778915
- RiiseGCQvarfordtILarssonSInhibitory effect of N-acetycysteine on adherence of Streptococcus pneumoniae and Haemophilus influenzae to human orophryngeal epithelial cells in vitroRespiration200067555255811070462
- MataMMorcilloEGimenoCN-acetyl-L-cysteine (NAC) inhibits mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV)Biochem Pharmacol201182554855521635874
- MataMSarrionIArmengotMRespiratory syncytial virus inhibits ciliagenesis in differentiated normal human bronchial epithelial cells: effectiveness of N-acetylcysteinePLoS One2012710e4803723118923
- YaoHRahmanICurrent concepts on oxidative/carbonyl stress, inflammation and, epigenetics in pathogenesis of chronic obstructive pulmonary diseaseToxicol Appl Pharmacol20112542728521296096
- BragaPCAllegraLRampoldiCLong-lasting effects on rheology and clearance of bronchial mucus after short-term administration of high doses of carbocysteinelysine to patients with chronic bronchitisRespiration19905763533582099568
- MacciòAMadedduCPanzoneFMantovaniGCarbocysteine: clinical experience and new perspectives in the treatment of chronic inflammatory diseasesExpert Opin Pharmacother200910469370319239402
- CarpagnanoGERestaOFoschino-BarbaroMPExhaled interleukine-6 and 8-isoprostane in chronic obstructive pulmonary disease: effect of carbocysteine lysine salt monohydrate (SCMC-Lys)Eur J Pharmacol20045051–316917515556150
- IshibashiYOkamuraTMasumotoYTachiiriTMomoKEffects of carbocisteine on airway inflammation and related events in SO2-exposed ratsNihon Kokyuki Gakkai Zasshi20013911723 Article in Japanese11296380
- CakanGTurkozMTuranTAhmedKNagatakeTS-carboxymethylcysteine inhibits the attachment of Streptococcus pneumoniae to human pharyngeal epithelial cellsMicrob Pathog200334626126512782478
- MiyakeKKaiseTHosoeHAkutaKManabeHOhmoriKThe effect of erdosteine and its active metabolite on reactive oxygen species production by inflammatory cellsInflamm Res199948420520910344471
- MorettiMPharmacology and clinical efficacy of erdosteine in chronic obstructive pulmonary diseaseExpert Rev Respir Med20071330731620477170
- Dal NegroRWErdosteine: antitussive and anti-inflammatory effectsLung2008186suppl 1S70S7318185958
- FarkhutdinovURFarkhutdinovRRPetriakovWEffect of mucolytic therapy on the production of reactive oxygen species in the blood of patients with an exacerbation of chronic obstructive pulmonary diseaseTer Arkh20108232932
- YakootMSalemAOmarAMClinical efficacy of farcosolvin syrup (ambroxol-theophylline-guaiphenesin mixture) in the treatment of acute exacerbation of chronic bronchitisInt J Chron Obstruct Pulmon Dis201095251256
- RogemDFMucus hypersecretion in chronic obstructive pulmonary diseaseNovaflis Nund Syrup20012346572
- RubinBKSecretion properties, clearance, and therapy in airway diseaseTransl Respir Med201426 eCollection 201425505698
- PelaRCalcagniAMSubiacoSIsidoriPTubaldiASanguinettiCMN-acetylcysteine reduces the exacerbation rate in patients with moderate to severe COPDRespiration199966649550010575333
- DecramerMRutten-van MolkenMDekhuijzenPNEffects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trialLancet200536594701552156015866309
- TseHNRaiteriLWongKYHigh-dose N-acetylcysteine in stable COPD: the 1-year, double-blind, randomized, placebo-controlled HIACE studyChest2013144110611823348146
- PoolePBlackPNCatesCJMucolytic agents for chronic bronchitis or chronic obstructive pulmonary diseaseCochrane Database Syst Rev20128CD001287
- AllegraLCordaroCIGrassiCPrevention of acute exacerbations of chronic obstructive bronchitis with carbocysteine lysine salt monohydrate: a multicenter, double-blind, placebo controlled trailRespiration19966331741808739489
- MorettiMBottrighiPDallariRThe effect of long-term treatment with erdosteine on chronic obstructive pulmonary disease: the EQUALIFE StudyDrugs Exp Clin Res200430414315215553660
- MalerbaMPonticielloARadaeliAEffect of twelve-months therapy with oral ambroxol in preventing exacerbations in patients with COPD. Double-blind, randomized, multicenter, placebo-controlled study (the AMETHIST Trial)Pulm Pharmacol Ther2004171273414643168
- MarchioniCFPoluJMTaytardAEvaluation of efficacy and safety of erdosteine in patients affected by chronic bronchitis during an infective exacerbation phase and receiving amoxycillin as basic treatment (ECOBES, European Chronic Obstructive Bronchitis Erdosteine Study)Int J Clin Pharmacol Ther199533116126188688986
- AubierMBerdahLMulticenter, controlled, double – blind study of the efficacy and tolerance of Vectrine (erdostein) versus placebo in the treatment of stabilized chronic bronchitis with hypersecretionRev Mal Respir199916452152810549062
- GerritsCMHeringsRMLeufkensHGLammersJWN-acetylcysteine reduces the risk of re-hospitalisation among patients with chronic obstructive pulmonary diseaseEur Respir J200321579579812765423
- VestboJHurdSSAgustiAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summaryAm J Respir Crit Care Med2013187434736522878278
- StavDRazMEffect of N-acetylcysteine on air trapping in COPD: a randomized placebo-controlled studyChest2009136238138619447919
- LangenRCKornSHWoutersEFROS in the local and systemic pathogenesis of COPDFree Radic Biol Med200335322623512885585
- VestboJPrescottEAlmdalTBody mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart StudyAm J Respir Crit Care Med20061731798316368793
- MatuszczakYFaridMJonesJEffects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exerciseMuscle Nerve200532563363816025522
- CornSDBarstowTJEffects of oral N-acetylcysteine on fatigue, critical power, and W’ in exercising humansRespir Physiol Neurobiol2011178226126821740986
- ZuinRPalamideseANegrinRHigh dose N-Acetylcysteine in patients with exacerbations of chronic obstructive pulmonary diseasesClin Drug Invest2005256401408
- WangJBaoHChenXEffects of ambroxolon serum cytokine and pulmonary function in AECOPD patientsClin Pulm J2010152194195
- BehrJDemedtsMBuhlRLung function in idiopathic pulmonary fibrosis-extended analyses of the IFIGENIA trialRespir Res20091010119860915
- VestboJHurdSSAgustíAGGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2013187434736522878278
- YanXSongYShenCChinese experts consensus on AECOPD (revision)Int J Respir2014341111
- CrinerGJBourbeauJDiekemperRLPrevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society GuidelineChest2015147489494225321320
- Global Initiative for Chronic Obstructive Lung DiseaseGlobal Strategy for Diagnosis, Management, and Prevention of COPD – 2016 Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/Accessed June 1, 2016
