Abstract
A reduced content of alveolar elastic fibers is a key feature of COPD lung. Despite continued elastogenic potential by alveolar fibroblasts in the lung affected by COPD, repair of elastic fibers does not take place, which is due to increased levels of the chondroitin sulfate proteoglycan versican that inhibits the assembly of tropoelastin into fibers. In this study, primary pulmonary fibroblast cell lines from COPD and non-COPD patients were treated with a small interfering RNA (siRNA) against versican to determine if knockdown of versican could restore the deposition of insoluble elastin. Versican siRNA treatment reduced versican expression and secretion by pulmonary fibroblasts from both COPD and non-COPD patients (P<0.01) and significantly increased deposition of insoluble elastin in the COPD cell cultures (P<0.05). The treatment, however, did not significantly affect production of soluble elastin (tropoelastin) in either the COPD or non-COPD cell cultures, supporting a role for versican in inhibiting assembly but not synthesis of tropoelastin. These results suggest that removal or knockdown of versican may be a possible therapeutic strategy for increasing deposition of insoluble elastin and stimulating repair of elastic fibers in COPD lung.
Keywords:
Introduction
COPD is characterized by irreversible airflow obstruction in the small airways,Citation1,Citation2 is thought to be primarily due to emphysematous changes in which there is loss of elastic fibers in alveolar walls and subsequent destruction of the alveoli, which in turn results in the loss of alveolar attachments to the small airways. This is believed to lead to the collapse of the small airways on expiration, which then leads to airflow obstruction.Citation3 The disease is ongoing, and repair mechanisms appear to be largely inhibited. Our previous research provides support for the idea that loss of repair mechanisms may be important in the development and progression of COPD. The lung tissues from patients undergoing lobectomy for bronchial carcinoma were analyzed immunohistochemically. Some of these individuals (who were current or ex-smokers) had normal lung function, while the remainder had mild-to-moderate COPD. We found that elastin content was significantly decreased in the lung tissue of patients with COPD and as a function of forced expiratory volume in 1 second (FEV1)Citation4 and, correspondingly and also as a function of FEV1, an increase in the matrix proteoglycan versican.Citation5 This is relevant because versican has been shown to inhibit the assembly of elastic fibers in cultures of fibroblastsCitation6 and smooth muscle cellsCitation7 and in vessels in vivo.Citation7,Citation8 Our findings on lung are consistent with the idea that patients with COPD have a reduced capacity to form new elastic fibers, despite the ability to synthesize the soluble elastin precursor tropoelastin.Citation9
Versican is a large chondroitin sulfate (CS) proteoglycan with a molecular mass of >1,000 kDa.Citation10 It is secreted by numerous cells, including lung fibroblasts, and interacts with various binding partners.Citation11 There are at least four isoforms, such as V0, V1, V2, V3; the central domain of V0 contains two GAG-binding domains, α and β; V1 has only the GAG-β domain; V2 has only the GAG-α domain; and V3 is void of both GAG attachment domains.Citation12 The three isoforms that possess GAG chains (V0, V1, and V2) inhibit the assembly of tropoelastin onto the microfibrillar scaffold of elastic fibers by binding to EBP. EBP is a receptor that chaperones tropoelastin through the golgi to the cell surface, but in the presence of the CS-containing versican isoforms or CS chains alone, the tropoelastin is prematurely released prior to assembly and cross-linking on the microfibrillar scaffold.Citation13
In a previous study, we reported that lung parenchyma from patients with mild-to-moderate COPD showed progressively increased immunostaining for versican and correspondingly decreased immunostaining for EBP, with decreasing FEV1.Citation5 In that study, we proposed that the elevated levels of the large CS-containing versican variants may explain the lack of repair of elastic fibers in the lungs of patients with moderate COPD.
In a subsequent study, pulmonary fibroblasts were cultured from explants of lung tissue obtained from 20 patients undergoing surgery for resection of bronchial carcinoma. We found a significant increase in the expression of versican messenger RNA (mRNA) by the COPD fibroblasts compared with non-COPD controls. Secreted versican levels were also increased in the supernatants from the COPD fibroblasts compared with controls.Citation13 Soluble elastin was also increased in the COPD cultures, but there was, however, no difference between the COPD and control groups in the levels of insoluble elastin, indicating that the increased secretion of tropoelastin by COPD fibroblasts did not result in elastin deposition, consistent with the increase in versican expression and secretion. Versican may thus play a key role in maintaining the obstructive state and inhibiting repair of COPD lung by interfering with the assembly of elastic fibers. Removal of versican may offer a novel strategy for stimulating effective repair.Citation13
In this study, we have investigated the effect of a versican small interfering RNA (siRNA) on elastogenesis and show that knockdown of versican increases deposition of insoluble elastin by pulmonary fibroblasts from COPD patients.
Methods and materials
Pulmonary fibroblasts
A total of 12 lines of cryopreserved and characterized pulmonary fibroblasts were cultured from lung tissues of 12 patients obtained at the time of surgery for bronchial carcinoma. Six patients of the non-COPD group had normal lung function (FEV1 >80% and FEV1/vital capacity [VC] >0.7) and six patients of the COPD group had mild-to-moderate COPD (FEV1 <80% predicted and FEV1/VC <0.7). For each patient, fibroblasts were initially obtained by explant culture from peripheral portions of the resected lobe, remote from the tumor. All cell lines were evaluated immunohistochemically (vimentin) to confirm that >99% of the cells were fibroblasts. Cells of passages 3–5 were used for experiments. The study was approved by the Northern X Regional Ethics Committee, Ministry of Health. Written informed consent for permission to use the lung tissue for research was obtained preoperatively.
Culture and siRNA treatment
All cells lines were cultured to confluence over 7 days in 24-well plates of the culture medium Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal calf serum, penicillin (100 ng/mL), and streptomycin (100 ng/mL). Confluent cultures were treated with siRNA as follows. Stealth siRNA molecule–Lipofectamine® complexes were prepared just prior to transfection, based on manufacturer’s instructions (Stealth siRNAs for versican HSS175421, HSS175422, HSS175423 [Thermo Fisher Scientific], targeting all isoforms). Lipofectamine® (Thermo Fisher Scientific) was gently mixed before use and added in a ratio of 1:100 with Opti-MEM® Medium (Thermo Fisher Scientific) without serum. Stealth™ RNA interference was also added into the same medium at a ratio of 900 pmol/1.5 mL. The complex solution was mixed gently and incubated for 15 minutes at room temperature to allow for complex formation. In all, 0.5 mL of the complex solution and 1 mL of normal cultural medium (without serum) were added to each well. The plate was mixed gently by rocking. The cells were then incubated for 24 hours at 37°C in a CO2 incubator, and the complex medium replaced by normal cultural medium for these wells continued for subsequent treatments.
For each of the 12 cell lines, four wells were treated only once with the transfection solution (on day 8), four wells were treated on day 8 and again 1 week later (on day 15), and four wells were treated on days 8, 15 and 22. At each time point, two of the treated wells and duplicate untreated control wells were harvested 24 hours after each transfection (on days 9, 16 and 23) for gene expression analysis. At the same time, the medium was collected for determination of protein levels of versican and tropoelastin (soluble elastin). To determine the effect of siRNA treatment on insoluble elastin levels, duplicate wells for each of the 12 lines were treated three times on days 8, 15 and 22, with medium changes on days 9, 16 and 23 and cultured until day 28, to allow time for deposition of elastin.Citation7,Citation8 Both medium and cell layers were collected and pooled for analysis of insoluble elastin levels. Duplicate untreated control cultures for each line were also collected on day 28.
mRNA expression and protein levels
mRNA expression of versican and elastin was measured by real-time reverse transcription polymerase chain reaction SYBR Green system as described previously.Citation13 The primer pairs used were as follows: elastin 5′-TCT GAG GTT CCC ATA GGT TAG GG-3′ (forward primer) and 5′-CTA AGC CTG CAG CAG CTC CT-3′ (reverse primer) and versican 1 5′-CCC AGT GTG GAG GTG GTC TAC-3′ (forward primer) and 5′-CGC TCA AAT CAC TCA TTC GAC GTT-3′ (reverse primer). Tropoelastin levels and versican levels in culture supernatant were measured by Human Versican ELISA Kit (Cusabio) and Fastin Elastin Assay (Biocolor), respectively. Briefly, for versican levels, pre-coated antibody specific for versican was provided onto a microplate. Standards and samples were pipetted into the wells, and versican present was bound by the immobilized antibody. After removing any unbound substances, a biotin-conjugated antibody specific for versican was added to the wells. After washing, avidin-conjugated HRP was added to the wells. Following a wash to remove any unbound avidin–enzyme reagent, a substrate solution was added to the wells with color developed in proportion to the amount of versican bound in the initial step. Color development was stopped, and the intensity of the color was measured. Insoluble elastin was determined by Fastin Elastin Assay according to the manufacturer’s instructions.
All statistical analyses were performed using GraphPad Prism v5.0, and one-way analysis of variance followed by Bonferroni post hoc test was performed to determine whether differences between groups were statistically significant. Duplicate values for each cell line at each time point for all determinations were averaged to provide an n=6 for the COPD lines and an n=6 for the non-COPD lines. Differences were considered significant at the level of P<0.05.
Results
Versican expression and levels
Cultures of pulmonary fibroblasts from COPD and non-COPD patients were cultured for up to 4 weeks and treated on days 8, 15, and 22 with versican siRNA. The cells were collected 24 hours after each treatment for the measurement of versican mRNA levels. Prior to the first treatment, versican expression was slightly (P>0.05) higher in fibroblasts from COPD patients compared to non-COPD control cultures. Following the first and second siRNA treatments, versican expression in the COPD group progressively decreased but did not reach significance until the third treatment (decreased by 24.1%; P<0.01; ). Similarly, versican expression in the non-COPD cultures was significantly reduced after the third treatment compared to before treatment (P<0.01; ).
Figure 1 Relative expression of versican mRNA by cultured lung fibroblasts from patients with COPD (n=6) and without COPD (non-COPD; n=6).
Abbreviations: siRNA, small interfering RNA; mRNA, messenger RNA.
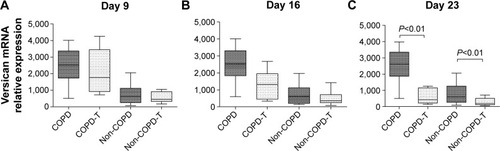
Versican levels in the supernatant of cultured primary pulmonary fibroblasts were measured by enzyme-linked immunosorbent assay on days 9, 16 and 23. Versican levels were progressively reduced after the first, second and third versican siRNA treatments compared to the before treatment level (), and for both groups, the decrease in the versican levels reached significance after the third treatment (P<0.05).
Figure 2 Versican levels in supernatants of cultured lung fibroblasts from patients with COPD (n=6) and without COPD (non-COPD; n=6).
Abbreviation: siRNA, small interfering RNA.
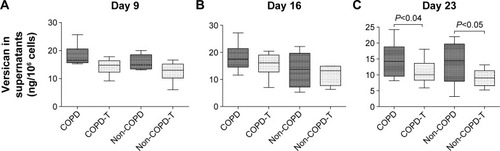
Tropoelastin expression and soluble and insoluble elastin levels
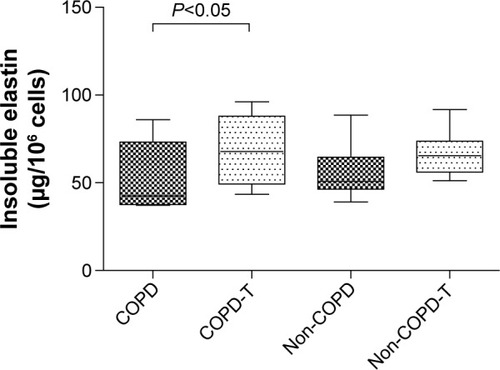
Versican siRNA treatment had no significant effect on tropoelastin mRNA expression by COPD and non-COPD cells (P>0.05; ) and similarly due to variability within groups had no significant effect on soluble elastin (tropoelastin) levels in either group (P>0.05; ) out to 23 days. COPD lines treated three times with siRNA, on days 8, 15, and 22, followed by culture out to 28 days showed a significant increase in insoluble elastin content compared to untreated controls (P<0.05). Insoluble elastin did not increase significantly in the treated non-COPD group (). The mean increase in insoluble elastin in the treated cultures of the COPD patients was 28.3% compared to the mean baseline value.
Figure 3 Relative expression of tropoelastin mRNA by lung fibroblasts from patients with COPD (n=6) and without COPD (non-COPD; n=6).
Notes: Message levels were determined 24 hours after the final siRNA treatment for each time period. The box plots are as for . Versican siRNA treatment did not significantly affect tropoelastin expression in either the COPD or non-COPD cell lines. (A) Untreated confluent cultures from COPD and non-COPD patients sampled on day 9 compared to versican siRNA-treated (COPD-T and non-COPD-T) cultures. Cultures were treated only once on day 8. (B) Untreated and treated COPD and non-COPD cultures treated twice on days 8 and 15 and sampled day 16. (C) Untreated and treated COPD and non-COPD cultures treated three times on days 8, 15 and 22 and sampled day 23.
Abbreviations: siRNA, small interfering RNA; mRNA, messenger RNA.
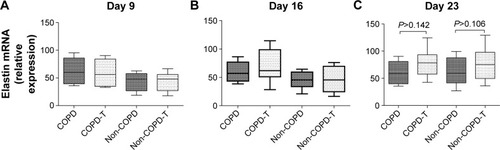
Figure 4 Soluble elastin levels in the supernatants of cultures of fibroblasts from patients with COPD (n=6) and without COPD (non-COPD; n=6).
Notes: Soluble elastin levels were determined in supernatants collected 24 hours after the final siRNA treatments. Versican siRNA treatment did not significantly affect soluble elastin levels in either the COPD or non-COPD cell lines. (A) Untreated confluent cultures from COPD and non-COPD patients sampled on day 9 compared to versican siRNA-treated (COPD-T and non-COPD-T) cultures. Cultures were treated only once on day 8. (B) Untreated and treated COPD and non-COPD cultures treated twice on days 8 and 15 and sampled day 16. (C) Untreated and treated COPD and non-COPD cultures treated three times on days 8, 15 and 22 and sampled day 23.
Abbreviation: siRNA, small interfering RNA.
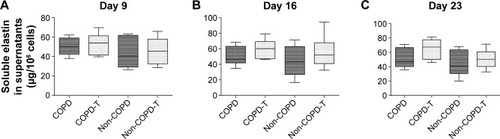
Figure 5 Insoluble elastin levels in cultures of lung fibroblasts from patients with COPD (n=6) and without COPD (non-COPD; n=6).
Abbreviation: siRNA, small interfering RNA.
Discussion
COPD is characterized by loss of elastic fibers in alveolar walls and small airways, and the decrease in elastin correlates with increase in disease severity as measured by FEV1.Citation5 Similarly, and reciprocal to elastin content, increasing disease severity is associated with increased versican content in COPD lung. Importantly, changes in the EBP track with elastin. Hallgren et alCitation14 and Larsson-Callerfelt et alCitation15 have also reported that (distally derived) fibroblasts from COPD patients have higher versican production and proposed that the elevated versican levels may have a negative influence on the elastic recoil in COPD lung. Numerous experimental studies have demonstrated the importance of versican, with its CS chains, as an inhibitor of the assembly of elastic fibers.Citation6–Citation9 High levels of versican likely play a key inhibitory role in the repair of COPD lung by interfering with the formation of new elastic fibers, and removal of versican may offer a new strategy for stimulating effective repair.
In this study, we show that versican siRNA treatment reduces versican mRNA expression (P<0.01) and production (P<0.05) in pulmonary fibroblasts from COPD and non-COPD patients, does not significantly affect soluble elastin (tropoelastin) production in either the COPD or the non-COPD cell lines (P>0.05), and increases insoluble elastin levels in COPD (P<0.05) but not non-COPD cell lines.
While the increase in insoluble elastin was modest (~30%), as was the decrease in secreted versican into the culture medium (~24.1%), these findings are consistent with the inhibition of EBP-mediated assembly of elastic fibers by versican. In this model, pericellular CS, as a component of versican, binds to EBP, an inactive variant of β-galactosidase, and causes the premature release of tropoelastin prior to assembly onto the microfibrillar scaffold of developing fibers.Citation16 Knockdown of versican by siRNA reduces versican synthesis and secretion, as occurred for both COPD and non-COPD cell lines, and creates a more permissive environment at the cell surface for assembly of elastic fibers. The absence of a significant effect, notwithstanding variability, on tropoelastin mRNA and soluble elastin is not inconsistent with this model as these measures do not necessarily predict insoluble elastin content of tissues, which is determined by factors, including versican affecting assembly rather than synthesis. In some situations, notably in emphysematous lung tissueCitation9 and in neointima of vessels following balloon angioplasty,Citation17 tropoelastin message is significantly elevated without increased elastin deposition.
The relatively small decrease in secreted versican (~24.1%) is consistent with the increase in insoluble elastin and may reflect inefficiency of knockdown by siRNA. The decrease in versican message was notably greater than the decrease in secreted versican. Message levels, however, across a wide range of organs and cells are often not strongly correlated with protein concentrations.Citation18
Other studies have reported on the elastogenic effects of interference of versican expression. Huang et alCitation19 reported that retroviral overexpression of a versican antisense sequence by smooth muscle cells significantly reduced versican production, induced a flattened morphology, reduced cell proliferation and migration, increased tropoelastin synthesis, increased EBP, and most importantly increased deposition of elastic fibers in long-term cultures and in vivo in neointima in a balloon catheter model of injured artery wall.Citation19 Similarly, the same group found that overexpression of versican variant V3, the variant lacking CS chains and chain-less biglycan induces similar changesCitation7,Citation20 and in particular reduces the amount of versican V0/V1 in the cell coat of smooth muscle cells in culture and in arterial wall, a change found to facilitate the assembly of elastic fibers. A caveat to using elastogenic technologies to restore elastin to lung is that excessive deposition of insoluble elastin could have a deleterious effect on lung expansion during inspiration; thus, if shown to be effective, then treatments may need to be titrated.
A further caveat to the study is the source of cells from patients with bronchial carcinoma, at a stage sufficient to warrant surgical resection. While the lung fibroblasts were cultured from parenchyma sourced at a distance from tumors, tumor cells produce versicanCitation21 and versican may be elevated in adjacent parenchyma.Citation22 Fibroblasts, however, were sourced from subjects with and without COPD, but all with carcinomas. While we cannot discount differential effects between the groups due to variability in carcinoma morphology or metabolism between the two groups, the principal finding of increased insoluble elastin resulting from knockdown of versican remains regardless of the original stimuli to versican secretion by the parenchymal fibroblasts.
Conclusion
We report that siRNA knockdown of versican expression by primary pulmonary fibroblasts from patients with COPD inhibits production of versican and enhances the deposition of insoluble elastin. These results suggest that decreasing the amount of the large versican proteoglycans that are increased in COPD lung may offer a new strategy for stimulating effective repair of emphysematous lung.
Acknowledgments
This work was supported by Auckland Medical Research Foundation (Grant No 1110016). This article is dedicated to our close friend and mentor Professor Peter Black who died suddenly in January 2010. Peter was instrumental in the conception and design of this study. His death continues to be a huge loss to us personally and to the COPD research community.
Disclosure
The authors report no conflicts of interest in this work.
References
- PauwelsRABuistASCalverleyPMJenkinsCRHurdSSGOLD Scientific CommitteeGlobal strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summaryAm J Respir Crit Care Med200116351256127611316667
- HoggJCMacklemPTThurlbeckWMSite and nature of airway obstruction in chronic obstructive lung diseaseN Engl J Med196827825135513605650164
- JefferyPKRemodeling in asthma and chronic obstructive lung diseaseAm J Respir Crit Care Med200116410 pt 2S28S38 Review11734464
- BlackPNChingPSBeaumontBRanasingheSTaylorGMerrileesMJChanges in elastic fibres in the small airways and alveoli in COPDEur Respir J2008315998100418216063
- MerrileesMJChingPSBeaumontBHinekAWightTNBlackPNChanges in elastin, elastin binding protein and versican in alveoli in chronic obstructive pulmonary diseaseRespir Res200894118485243
- HinekAMechamRPKeeleyFRabinovitchMImpaired elastin fiber assembly related to reduced 67-kD elastin-binding protein in fetal lamb ductus arteriosus and in cultured aortic smooth muscle cells treated with chondroitin sulfateJ Clin Invest1991886208320941661296
- MerrileesMJLemireJMFischerJWRetrovirally mediated overexpression of versican v3 by arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointima after vascular injuryCirc Res200290448148711884379
- MerrileesMJBeaumontBWBraunKRNeointima formed by arterial smooth muscle cells expressing versican variant V3 is resistant to lipid and macrophage accumulationArterioscler Thromb Vasc Biol20113161309131621441139
- LuceyECGoldsteinRHStonePJSniderGLRemodeling of alveolar walls after elastase treatment of hamsters. Results of elastin and collagen mRNA in situ hybridizationAm J Respir Crit Care Med199815825555649700135
- ZimmermannDRRuoslahtiEMultiple domains of the large fibroblast proteoglycan, versicanEMBO J1989810297529812583089
- WightTNVersican: a versatile extracellular matrix proteoglycan in cell biologyCurr Opin Cell Biol2002145617623 Review12231358
- ItoKShinomuraTZakoMUjitaMKimataKMultiple forms of mouse PG-M, a large chondroitin sulfate proteoglycan generated by alternative splicingJ Biol Chem199527029589657822336
- ZhangJWuLQuJMBaiCXMerrileesMJBlackPNProinflammatory phenotype of COPD fibroblasts not compatible with repair in COPD lungJ Cell Mol Med20121671522153222117690
- HallgrenONihlbergKDahlbäckMAltered fibroblast proteoglycan production in COPDRespir Res2010115520459817
- Larsson-CallerfeltAKHallgrenOAndersson-SjölandADefective alterations in the collagen network to prostacyclin in COPD lung fibroblastsRespir Res2013142123406566
- HinekASmithACCutiongcoEMCallahanJWGrippKWWeksbergRDecreased elastin deposition and high proliferation of fibroblasts from Costello syndrome are related to functional deficiency in the 67-kD elastin-binding proteinAm J Hum Genet200066385987210712202
- NikkariSTJärveläinenHTWightTNFergusonMClowesAWSmooth muscle cell expression of extracellular matrix genes after arterial injuryAm J Pathol19941446134813568203472
- VogelCMarcotteEMInsights into the regulation of protein abundance from proteomic and transcriptomic analysesNat Rev Genet2012134227232 Review22411467
- HuangRMerrileesMJBraunKInhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injuryCirc Res200698337037716385080
- HwangJYJohnsonPYBraunKRRetrovirally mediated overexpression of glycosaminoglycan-deficient biglycan in arterial smooth muscle cells induces tropoelastin synthesis and elastic fiber formation in vitro and in neointimae after vascular injuryAm J Pathol200817361919192818988796
- PirinenRLeinonenTBöhmJVersican in nonsmall cell lung cancer: relation to hyaluronan, clinicopathologic factors, and prognosisHum Pathol2005361445015712181
- SoltermannATischlerVArbogastSPrognostic significance of epithelial-mesenchymal and mesenchymal-epithelial transition protein expression in non-small cell lung cancerClin Cancer Res200814227430743719010860
